
Virucidal Capacity of Novel ProtecTeaV Sanitizer Formulations Containing Lipophilic Epigallocatechin-3-Gallate (EGCG)
*Corresponding Author(s):
Stephen HsuDental College Of Georgia, Augusta University, Augusta, United States
Tel:+1 7067214816,
Email:shsu@augusta.edu
Abstract
Current non-toxic or “Generally Recognized as Safe” (GRAS) countermeasures against viral entry into cells in the human body are often inadequate. The objective of this study was to evaluate ProtecTeaV, a novel alcohol-based instant hand sanitizer formulation containing lipophilic EGCG (derived from green tea extract), in comparison to commonly used hand sanitizers either with or without alcohol. A Standard 50% Tissue Culture Infective Dose (TCID50) assay was used for determination of virucidal capacity against Poliovirus 1 (PV 1). Separately, neutralization by an ultra-filtration method was used to evaluate the mechanism of virucidal capacity. The results demonstrated that ProtecTeaV formulations (with and without gelling agent) reduced the TCID50 by a factor of 6 (mean log10–reduction of viral infectivity), 100-fold more than the reduction of viral infectivity (>4 log10) mandated by internationally accepted standards. In addition, the virucidal effect of ProtecTeaV formulations was associated with direct and irreversible inactivation of PV 1, rather than a reversible inhibition mechanism. In contrast, two commonly used instant hand sanitizers failed to reduce PV 1 viral infectivity by >4 log10. In conclusion, ProtecTeaV instant hand sanitizer formulations containing lipophilic EGCG possess effective virucidal capability with the potential for use in novel disinfectant and antiseptic approaches, pending additional research and development.
Keywords
INTRODUCTION
Alcohol-based instant sanitizers gained popularity due to their ease-of-use and effectiveness against actively growing bacteria, as well as some viruses [1,2]. They are used routinely in clinics, schools, institutions, families, and on cruise ships for the purpose of prevention of microbial infections. However, current alcohol-based hand sanitizers have some weaknesses regarding the prevention of viral infections, and thus far, an alcohol-based hand sanitizer with persistent virucidal capacity has not been described. Alcohol evaporates in a matter of seconds after each application on the skin or other surfaces, leaving the skin surface unprotected until the next application. For example, although the concept “alcohol-based sanitizer is effective to inactivate influenza virus” has been well-accepted, a recent case-control human study indicates that alcohol-based hand sanitizer is not effective in school-aged children during outbreaks of influenza infection in community settings [3]. This result could be due to the short life of alcohol activity. More importantly, many non-enveloped pathogenic viruses such as norovirus, poliovirus, adenovirus and hepatitis A virus are resistant to alcohol [4-7]. Conliffe et al., reported that three of these - norovirus, rotavirus and adenovirus were responsible for the majority of healthcare-associated viral gastroenteritis cases among children in a large pediatric hospital setting [8]. Gastroenteritis caused by these viruses can be severe and costly to patients and healthcare providers [9]. In the US, norovirus alone is responsible for approximately 20 million acute gastroenteritis cases, 400,000 emergency visits, and 70,000 hospitalizations, with up to 800 deaths annually (US CDC, Norovirus: US Trends and Outbreaks). Another weakness of alcohol-based hand sanitizers is that alcohols do not inactivate bacterial endospores effectively [10,11]. Due to the weak virucidal capacity of alcohols against enteric viruses, and inability to inactivate bacterial spores and hydrophilic (non-enveloped) viruses, the US CDC 2008 Guidelines for Disinfection and Sterilization in Healthcare Facilities removed isopropyl alcohol and ethyl alcohol from the list of high-level disinfectants. In 2011, the CDC updated the guidelines for the Prevention and Control of Norovirus Gastroenteritis Outbreaks in Healthcare Settings. The new guideline for hand hygiene is: “during outbreaks, use soap and water for hand hygiene after providing care or having contact with patients suspected or confirmed with norovirus gastroenteritis”, because “very low-quality evidence was available to suggest that hand hygiene using alcohol-based hand sanitizers may reduce the likelihood of symptomatic norovirus infection”. In fact, the use of alcohol-based hand sanitizer was found to be a risk factor for norovirus spread vs soap and water wash in long-term care facilities [12]. In a human study, a 30-second incubation with alcohol (3% - 95%) was ineffective in reducing the infectivity of norovirus (genomic copy reduction <0.5 log10); this was less effective than liquid soap or water rinse [13]. Although various chemicals inactivate non-enveloped viruses effectively, they are not feasible for use in hand sanitizers due to toxicity.
In recent years, plant-derived phytochemicals, such as extracts from grape seed, pomegranate, mulberry, cranberry, ginseng, persimmon, green tea and herbal essential oils, have been reported to exert antiviral properties against non-enveloped viruses, including enteric viruses [14]. Epigallocatechin-3-Gallate (EGCG) is the major polyphenol present in green tea leaves and its antiviral activities have been studied extensively. The most suitable derivatives of EGCG for topical applications are lipophilic EGCG compounds due to their global antiviral properties, high potency, persistency, and absence of associated toxicity [15]. Results from a recent phase II clinical trial of the proprietary AverTeaX formula containing lipophilic EGCG for treating herpes labialis demonstrated that topical application of the AverTeaX formula provides high efficacy in treating herpes labialis [16]. Due to its unique antiviral activities, lipophilic EGCG could be used in a novel approach to a new generation of sanitizing products, either with or without alcohol. We hypothesized that lipophilic EGCG in surface-sanitizing formulations would be effective against human pathogenic viruses, with the potential for use as a global virucidal agent to prevent viral infections, particularly from alcohol-resistant non-enveloped viruses.
To test our hypothesis, the current study evaluated two proprietary alcohol-based instant hand sanitizer formulations, ProtecTeaV+cb and ProtecTeaV-cb both containing lipophilic EGCG (with (+) or without (-) gelling thickener), in comparison to two widely used alcohol-based hand sanitizers, one containing alcohol and the other a mixture of iodine and polyvinyl pyrrolidone. The internationally accepted method to test virucidal activity in vitro for disinfectants and cleansing agents was used [EN 14476-2013: Chemical and Antiseptics - Quantitative Suspension Test for the evaluation of virucidal activity in the medical area - test method and requirements (Phase 2/Step 1); Guidance Document Human-Use Antiseptic Drugs 2009, Minister of Health of Canada; and Technical Standard For Disinfection 2002 edition, Department of Health of People’s Republic of China] to determine the efficacy (measured by TCID50 assays) against Poliovirus 1 (PV 1, a non-enveloped virus resistant to alcohol) as a representative of virucidal activity. This virus is considered the most difficult to eradicate. To the best of our knowledge, this was the first attempt to test the virucidal capacity of lipophilic EGCG against a non-enveloped virus in alcohol-based hand sanitizer formulations.
MATERIALS & METHODS
VERO cells were cultured in T75 flasks (≥90% confluent monolayer) and infected with PV1 at a Multiplicity of Infection (MOI) of 0.1-1 for 2~3 days until more than 80% cells showed a Cytopathic Effect (CPE). Stock virus suspensions were made by collecting and filtering the supernatant and the viral titer was measured and expressed as 108 TCID50/0.1 ml by the TCID50 protocol [17]. Aliquots were stored at -80?C until use.
Viral titration
Testing sanitizer samples
Testing procedures
Physical neutralization by sanitizer assay
As a control to normalize the virus that might be lost during the ultra-filtration process (without sanitizer removal), sanitizer formulation was mixed with PV1 stock suspension prior to mock ultra-filtration. Sanitizer (450 µl) was initially incubated in a 20?C (±1?C) water bath for 5 min, mixed with 50 µl PV 1 suspension, incubated for 1 min before a series of 10-fold dilution, then transferred to 30 kDa ultra filter tubes (with an inner tube/filter and sleeve tube). The tubes were centrifuged at 7500 g for 20 min. Then the inner tubes were inverted and placed in the same sleeve tubes prior to centrifugation at 13,000 g for 5 min. The supernatants were collected and brought to 500 µl with cell culture medium. As a positive control without sanitizer incubation, PV1 suspension was ultra-filtered. PV1 suspension (50 µl) was mixed with 450 µl serum-free cell culture medium, and then transferred to 30 kDa ultra filter tubes. The tubes were centrifuged at 7500 g for 20 min. Then the inner tubes were inverted in new sleeve tubes prior to centrifugation at 13,000 g for 5 min. The supernatants were collected and brought to 500 µl with cell culture medium.
To normalize for the virus that might be lost during the ultra-filtration process (without sanitizer present), mock ultra-filtration was performed for PV1 without sanitizer. PV1 suspension (50 µl) was mixed with 450 µl serum-free cell culture medium prior to a series of 10-fold dilution and then transferred to 30 kDa ultra filter tubes. The tubes were centrifuged at 7500 g for 20 min. Then the inner tubes were inverted in the same sleeve tubes prior to centrifugation at 13,000 g for 5 min. The supernatants were collected and brought to 500 µl with cell culture medium.
The negative control was VERO cell culture without PV1 infection in duplicate wells. The supernatants from various treatments were used for the TCID50 assay according to the method described above. The experiments were repeated three times independently.
Statistical analysis
| Product | Active ingredients | Mean log10–reduction of viral infectivity | ||
| 30 s (SD) | 60 s (SD) | 90 s (SD) | ||
| ProtecTeaV- with L - EGCG | Ethanol (70%) | 6.65 (±0) | 6.65 (±0) | 6.62 (±0.58) |
| ProtecTeaV+ with L - EGCG | Ethanol (70%) | 5.98 (±0.49) | 6.02 (±0.42) | 6.53 (±0.40) |
| Purell | Ethanol (62%) | 3.28 (±1.1) | 2.85 (±0.61) | 3.24 (±0.51) |
| adf | PVP-Iodine | 2.88 (±1.07) | 3.12 (±1.31) | 2.75 (±0.98) |
Table 1: Efficacy of four hand sanitizers either with alcohol (ProtecTeaV, Purell) or without alcohol (adf) against PV-1 using TCID50 assays. Data are from three independent experiments.
ProtecTeaV-: without thickener agent.
ProtecTeaV+: with thickener agent.
L-EGCG: Lipophilic Epigallocatechin-3-Gallate.
PV-1: poliovirus 1.
SD: Standard Deviation of 3 independent experiments (n=3).
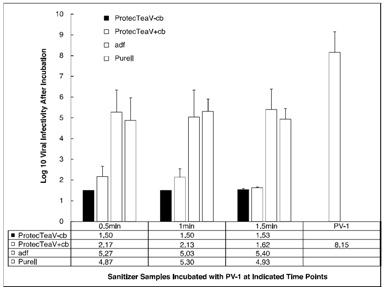 Figure 1: TCID50 assay results comparing virucidal capacity against PV-1 after incubation with different sanitizer samples for 30, 60 and 90 seconds. Data is from three independent experiments. TCID50 infectivity is presented as log10 scales with standard deviations (n=3).
Figure 1: TCID50 assay results comparing virucidal capacity against PV-1 after incubation with different sanitizer samples for 30, 60 and 90 seconds. Data is from three independent experiments. TCID50 infectivity is presented as log10 scales with standard deviations (n=3).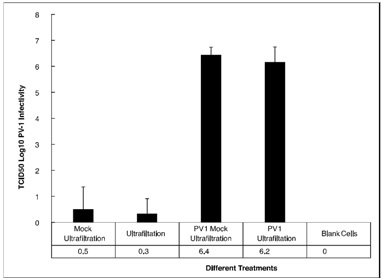 Figure 2: Mean log10 of PV 1 infectivity (TCID50) after ultra-filtration of ProtecTeaV+ sanitizer formulation in comparison to mock ultra-filtration and untreated PV 1 with standard deviations. From left, lane 1: Mock ultra-filtration collected both virus and sanitizer after incubation and ultra-filtration; lane 2: Ultra-filtration only collected virus after incubation and ultra-filtration; lane 3: PV 1 mock ultra-filtration collected medium and virus after incubation with cell culture medium; lane 4: PV 1 ultra-filtration only collected virus after incubation with cell culture medium; lane 5: blank control using VERO cells only.
Figure 2: Mean log10 of PV 1 infectivity (TCID50) after ultra-filtration of ProtecTeaV+ sanitizer formulation in comparison to mock ultra-filtration and untreated PV 1 with standard deviations. From left, lane 1: Mock ultra-filtration collected both virus and sanitizer after incubation and ultra-filtration; lane 2: Ultra-filtration only collected virus after incubation and ultra-filtration; lane 3: PV 1 mock ultra-filtration collected medium and virus after incubation with cell culture medium; lane 4: PV 1 ultra-filtration only collected virus after incubation with cell culture medium; lane 5: blank control using VERO cells only.RESULTS
Virucidal activities of different hand sanitizers
In summary, both ProtecTeaV sanitizers reduced PV 1 infectivity by approximately 6 log10 (from 8.15 to a range of 1.5 to 2.16). The adf and Purell sanitizers only reduced PV 1 infectivity by approximately 3 log10(from 8.15 to a range of 4.9 to 5.3). That is, the ProtecTeaV sanitizers possessed significant higher virucidal capacity than currently used hand sanitizers Purell and adf (Table 1).
Physical neutralization by sanitizer assay
For the filtration control on untreated virus, PV 1 without sanitizer treatment after mock ultra-filtration showed a TCID50 value of 6.4 ± 0.31, while PV 1 without sanitizer treatment but underwent ultra-filtration had a TCID50 value of 6.2 ± 0.58. These values were not significantly different (one-way ANOVA, p=0.949). After PV 1 was incubated with the ProtecTeaV+ formulation for 60 second removal of the sanitizer by ultra-filtration, the mean TCID50 value was reduced to 0.3 ± 0.58, significantly lower than the untreated control (p<0.0001), but not significantly different from the treatment followed by mock ultra-filtration control (one-way ANOVA, p=0.99), which was also significantly lower than the untreated controls (p<0.0001).
Thus, after removal of sanitizer via ultra-filtration, ProtecTeaV treatment of PV 1 still resulted in a mean of 6.1 log10-reduction of viral infectivity in VERO cells.
DISCUSSION
Due to the increasing evidence indicating a weak efficacy of alcohol-based hand sanitizer against non-enveloped viruses, especially norovirus and poliovirus [5], the 2011 CDC guidelines suggested using “proper hand washing with soap and running water for at least 20 seconds” as the most effective way to reduce norovirus contamination on the hands [18]. However, the implementation of new guidelines has not been associated with a reduction in norovirus outbreaks. Since 2011, rather than a decline in the number of norovirus outbreaks reported to the CDC, there was a spike during 2013-2014 (CDC NoroSTAT, 2009-2015). It is clear that in order to reduce outbreaks and infections from norovirus and other non-enveloped or enveloped viruses, novel hand hygiene approaches that are more effective than hand washing with soap and water are in urgent need.
Results from the current study have demonstrated for the first time that alcohol-based sanitizer formulations containing lipophilic EGCG exert potent virucidal capacity. According to international standards (i.e. European standard for testing EN 14476 and Chinese CDC standard), virucidal disinfectants and antiseptics require a mandatory >4 log10 virus infectivity reduction by 30s or 60s to be classified as such [19]. Our results from TCID50 assays demonstrated that both ProtecTeaV hand sanitizer formulations, either with or without carbomer thickening agent, exceeded this mandatory requirement by 100-fold (>6 log10) within the requisite time frame of 30-90 sec (Figure 1 and Table 1). In contrast, both the conventional alcohol-based hand sanitizer and non-alcohol-based hand sanitizer tested here failed to meet the internationally accepted standards. In addition, results from the neutralization assay indicated that the inactivation of PV 1 by ProtecTeaV formulations was irreversible, as virus incubated with ProtecTeaV formulations for 60s lost infectivity even after the sanitizer formulations were removed by microfiltration (Figure 2). The reduction of PV 1 infectivity in these neutralization assays was consistent with the TCID50 assays (6.1 log10 reduction, Figure 2).
The high potency of the ProtecTeaV hand sanitizer formulations demonstrated here was consistent with the antiviral properties of lipophilic EGCG, and previous studies showing antiviral activities of EGCG or its derivatives that did not use alcohol as the primary solvent. Although a synergistic effect might occur between alcohol and lipophilic EGCG, the contribution of alcohol is likely to be rather small, based on prior measures of EGCG effectiveness. Medications such as Veregen ointment against genital warts primarily induced by human papilloma virus and AverTeaX ointment for treating herpes labialis, either have EGCG (Veregen) or lipophilic EGCG (AverTeaX), but do not contain alcohol [16,20]. Previous studies using Dimethyl Sulfoxide (DMSO) as a solvent demonstrated that lipophilic EGCG is 24-fold more effective than native EGCG against influenza virus (H1N1) [21]. Another study using ethanol as a solvent showed lipophilic EGCG is 8.7 fold more effective against Herpes Simplex Virus 1 (HSV-1), in comparison to native hydrophilic EGCG [22]. In both studies, 50 µM of EGCG-mono-palmitate was sufficient to completely inhibit plaque formation by H1N1 and HSV-1 [21,22]. Although native green tea extract and EGCG exert significant antiviral activities against a wide spectrum of viral species, including Ebola virus, native EGCG is not able to effectively inactivate poliovirus within a concentration range (µM) that is active against other types of viruses [23,24]. The current study indicates that lipophilic EGCG in alcohol-based ProtecTeaV hand sanitizer formulations possesses high efficacy against poliovirus (Figures 1 and 2 & Table 1). It is interesting to note that Veregen ointment contains 15% of tea polyphenols (sinecatechins) with the majority being native EGCG (>55%). That is, the EGCG concentration in this prescription drug is >8.25%. In contrast, the lipophilic EGCG in AverTeaX ointment is significantly less than 1% (undisclosed proprietary formula). In summary, alcohol is not an important contributor to the virucidal capacity of lipophilic EGCG in the tested formulations, and the antiviral efficacy of lipophilic EGCG is significantly higher than native EGCG.
It is known that auto-oxidation is the major cause of instability of EGCG due to its antioxidant activity in aqueous preparations or beverages [25,26]. Acylation of EGCG not only enhanced the antiviral activity but also increased stability by 10-fold [27]. Matsumoto et al., reported that EGCG-palmitate exhibits antibacterial and antifungal activities, with rapid bactericidal activity against MRSA ATCC43300 at > 16 µg/ml [27]. Thus, lipophilic EGCG in an alcohol-based hand sanitizer (and other forms of surface disinfectants) is expected to have both strong virucidal and bactericidal activity. Future studies should include testing the persistency of the virucidal activity after application to determine the duration of protection by lipophilic EGCG on skin or other surfaces. In addition, lipophilic EGCG sanitizer formulations need to be tested against other non enveloped viruses, such as norovirus, adenovirus and enteric virus 71 (EV71), which work is underway in our laboratories (manuscript in preparation).
In conclusion, the ProtecTeaV alcohol-based hand sanitizer formulations containing lipophilic EGCG irreversibly inactivate non-enveloped polio virus 1 and exceeded the international standard for virucidal disinfectants and antiseptics. With superior antiviral activity and stability in comparison to native EGCG, lipophilic EGCG has the potential for use as a novel, effective and nontoxic component for the next generation of approaches to prevention of pathogenic viral infections.
DISCLAIMER
The views expressed in this manuscript are those of the author(s) and do not reflect official policy or position of the Department of the Army, Department of Defense or the US Government.
ACKNOWLEDGEMENT
The authors thank Ms. Rhonda Powell for her work on the graphs.
REFERENCES
- de Aceituno AF, Bartz FE, Hodge DW, Shumaker DJ, Grubb JE , et al. (2015) Ability of Hand Hygiene Interventions Using Alcohol-Based Hand Sanitizers and Soap To Reduce Microbial Load on Farmworker Hands Soiled during Harvest. J Food Prot 78: 2024-2032.
- Mott PJ, Sisk BW, Arbogast JW, Ferrazzano-Yaussy C, Bondi CA, et al. (2007) Alcohol-based instant hand sanitizer use in military settings: a prospective cohort study of Army basic trainees. Mil Med 172: 1170-1176.
- Torner N, Soldevila N, Garcia JJ, Launes C, Godoy P, et al. (2015) Effectiveness of non-pharmaceutical measures in preventing pediatric influenza: a case-control study. BMC Public Health 15: 543.
- Macinga DR, Sattar SA, Jaykus LA, Arbogast JW (2008) Improved Inactivation of Non-enveloped Enteric Viruses and Their Surrogates by a Novel Alcohol-Based Hand Sanitizer. Appl Environ Microbiol 74: 5047-5052.
- Tuladhar E, Hazeleger WC, Koopmans M, Zwietering MH, Duizer E, et al. (2015) Reducing viral contamination from finger pads: handwashing is more effective than alcohol-based hand disinfectants. J Hosp Infect 90: 226-234.
- Rutala WA, Peacock JE, Gergen MF, Sobsey MD, Weber DJ (2006) Efficacy of hospital germicides against adenovirus 8, a common cause of epidemic keratoconjunctivitis in health care facilities. Antimicrob Agents Chemother 50: 1419-1424.
- Okunishi J, Okamoto K, Nishihara Y, Tsujitani K, Miura T, et al. (2010) [Investigation of in vitro and in vivo efficacy of a novel alcohol based hand rub, MR06B7]. Yakugaku Zasshi 130: 747-754.
- Cunliffe NA, Booth JA, Elliot C, Lowe SJ, Sopwith W, et al. (2010) Healthcare-associated Viral Gastroenteritis among Children in a Large Pediatric Hospital, United Kingdom. Emerging Infectious Diseases 16: 55-62.
- Piednoir E, Bessaci K, Bureau-Chalot F, Sabouraud P, Brodard V, et al. (2003) Economic impact of healthcare-associated rotavirus infection in a paediatric hospital. J Hosp Infect 55: 190-195.
- Sasahara T, Hayashi S, Hosoda K, Morisawa Y, Hirai Y (2014) Comparison of hand hygiene procedures for removing Bacillus cereus spores. Biocontrol Sci 19: 129-134.
- Nerandzic MM, Sunkesula VC, C TS, Setlow P, Donskey CJ (2015) Unlocking the Sporicidal Potential of Ethanol: Induced Sporicidal Activity of Ethanol against Clostridium difficile and Bacillus Spores under Altered Physical and Chemical Conditions. PLoS One 10: 0132805
- Blaney DD, Daly ER, Kirkland KB, Tongren JE, Kelso PT, et al. (2011) Use of alcohol-based hand sanitizers as a risk factor for norovirus outbreaks in long-term care facilities in northern New England: December 2006 to March 2007. Am J Infect Control 39: 296-301.
- Liu P, Yuen Y, Hsiao HM, Jaykus LA, Moe C (2010) Effectiveness of liquid soap and hand sanitizer against Norwalk virus on contaminated hands. Appl Environ Microbiol 76: 394-399.
- Ryu S, You HJ, Kim YW, Lee A, Ko GP, et al. (2015) Inactivation of norovirus and surrogates by natural phytochemicals and bioactive substances. Mol Nutr Food Res 59: 65-74.
- Hsu S (2015) Compounds Derived from Epigallocatechin-3-Gallate (EGCG) as a Novel Approach to the Prevention of Viral Infections. Inflamm Allergy Drug Targets 14: 13-18.
- Zhao M, Zheng R, Jiang J, Dickinson D, Fu B, et al. (2015) Topical lipophilic epigallocatechin-3-gallate on herpes labialis: a phase II clinical trial of AverTeaX formula. Oral Surg Oral Med Oral Pathol Oral Radiol 120: 717-724.
- Ministry of Health (2002) Technical Standard For Disinfection. Department of Health, Ministry of Health, People’s Republic of China.
- Vogel L (2011) Hand sanitizers may increase norovirus risk. CMAJ 183: 799-800.
- European Standard for Testing (2013) Chemical disinfectants and antiseptics-Virucidal quantitative suspension test for chemical disinfectants and antiseptics used in human medicine -Test methods and requirements (phase 2/step 1). European Standard NEN-EN 14476, London, UK.
- Tatti S, Swinehart JM, Thielert C, Tawfik H, Mescheder A, et al. (2008) Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts: a randomized controlled trial. Obstet Gynecol 111: 1371-1379.
- Mori S, Miyake S, Kobe T, Nakaya T, Fuller SD, et al. (2008) Enhanced anti-influenza A virus activity of (-)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: effect of alkyl chain length. Bioorg Med Chem Lett 18: 4249-4252.
- de Oliveira A, Adams SD, Lee LH, Murray SR, Hsu SD, et al. (2012) Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem Toxicol 52: 207-215.
- Colpitts CC, Schang LM (2014) A small molecule inhibits virion attachment to heparan sulfate- or sialic acid-containing glycans. J Virol 88: 7806-7817.
- Reid SP, Shurtleff AC, Costantino JA, Tritsch SR, Retterer C, et al. (2014) HSPA5 is an essential host factor for Ebola virus infection. Antiviral Res 109: 171-174.
- Mochizuki M, Yamazaki S, Kano K, Ikeda T (2002) Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim Biophys Acta 1569: 35-44.
- Chen Z, Zhu QY, Tsang D, Huang Y (2001) Degradation of green tea catechins in tea drinks. J Agric Food Chem 49: 477-482.
- Matsumoto Y, Kaihatsu K, Nishino K, Ogawa M, Kato N, et al. (2012) Antibacterial and antifungal activities of new acylated derivatives of epigallocatechin gallate. Front Microbiol 16: 53.
Citation: Zhang Q, Yang Q, Yang W, Wu K, Wu J, et al. (2016) Virucidal Capacity of Novel ProtecTeaV Sanitizer Formulations Containing Lipophilic Epigallocatechin-3-Gallate (EGCG). J Antivir Antiretrovir Res Ther 1: 002.
Copyright: © 2016 Qi Zhang, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

