
A Meta-Analysis Taxonomizing Empathy in Schizophrenia
*Corresponding Author(s):
Sumayyah VarachhiaSchool Of Medicine, Division Of Psychiatry And Applied Psychology, University Of Nottingham, Nottingham, United Kingdom
Tel:+44 1157484331,
Email:Sumayyah.varachhia@nottingham.ac.uk
Abstract
Background
Trait empathy is integral to relationship development and maintenance. Therefore, impairment in this ability can have an adverse effect on many domains of life including social, sexual, and marital. Previous reviews show in schizophrenia, this ability to be impaired but with a high amount of heterogeneity that is yet to be explored more thoroughly.
Aim and method
Considering this, we aim to synthesise the extent literature using a meta-analytic approach and examine the source of the heterogeneity observed in previous reviews and develop taxonomy of empathy deficits in schizophrenia. Hedges’ g was calculated for cognitive and affective empathy using random effects models. Meta-regression models of key cognitive, clinical and demographic risk and protective factors were run. These included: Impact year of publication, age, gender, ethnicity, education, general IQ, verbal/pre-morbid IQ, global neuro-cognition, positive, negative and general symptoms of schizophrenia, age at schizophrenia diagnosis, duration of illness and medication has on cognitive and affective empathy.
Results
A literature search revealed 39 independent studies examining empathy in schizophrenia. Healthy controls scored higher than people with a diagnosis of schizophrenia, with a small effect size for affective empathy (Hedges’ g = 0.29) and a medium effect size for cognitive empathy (Hedges’ g = 0.53). Both components were heterogeneous. Analyses using meta-regression models found age at diagnosis and the duration of illness moderated the difference in effect size for cognitive empathy, such that those with an earlier diagnosis or a more chronic course exhibit greater difficulty in cognitive empathy compared to healthy controls.
Conclusion
We find a longer duration of illness and younger age at clinical diagnosis enhances impairments in cognitive empathy in severe and enduring schizophrenia. For affective empathy, we conclude, compared to healthy controls, some patients report having a deficit [i.e. experience lower affective empathy], others report comparable levels, and the remaining report to be experiencing higher emotional arousal. As an earlier diagnosis, prolonged illness course and dysfunctional emotional reactions are significant risk factors of poorer empathic interactions, it will be important to address the underlying mechanisms of this deficit in future work.
Keywords
INTRODUCTION
Measuring and defining empathy in schizophrenia disorders
What is known in relation to empathy and schizophrenia
EMPATHY AND CLINICAL CHARACTERISTICS OF SCHIZOPHRENIA
Clinical symptoms and empathy
In studies of empathy, the primary focus of some studies was not on examining symptom severity in schizophrenia [23-33]. For other studies, however, examining symptom severity in schizophrenia patients was included as part of secondary analyses, with mixed findings reported across individual studies. For example, Montag and colleagues [34] found in patients, the IRI Empathic Concern related negatively to PANSS negative and general symptoms, Thirioux, et al., [35] found, using the same sub-scales, only negative symptoms associated negatively with the IRI Perspective-Taking sub-scale. Lam, et al., [36] found a negative relationship between PANSS general symptoms and overall empathy score, and Shamay-Tsoory and colleagues [37] reported the degree of impaired empathy (total IRI score) depended on how severe negative symptoms were. However, several studies reported no significant relationship between PANSS and IRI sub-scales [38-43] and performance-based measures of cognitive and affective empathy [44,45]. These discrepancies further extend to the SANS and SAPS symptom measurements [46-48]. Critically, these in consistencies have prevented the field from gaining a more nuanced understanding of how coresymptoms of schizophrenia (i.e. positive, negative and general) relate to self-reported empathy. By examining this relationship in a meta-analytic framework, we can further our understanding of the mechanisms underlying empathy deficits in schizophrenia and develop, in a systematic manner, relevant clinical profiles.
Medication
INDIVIDUAL DIFFERENCES IN EMPATHY
Demographic variables and empathy in schizophrenia and related disorders
Empathy and neuro-cognition in schizophrenia and related disorders
In the current literature, we found only two studies to have used the MATRICS developed Consensus Cognitive Battery (MCCB) to assess all six neuro-cognitive domains [23,38]. More specifically, amongst the identified studies, authors have commonly produced a global/composite neuro-cognitive score, and examined its relation with empathy, with several studies finding no relationship between global cognition (i.e. the sixneuro-cognitive abilities) and self-reported empathy in schizophrenia [23,27,38]. Other studies however, reported a positive correlation between affective empathy and globalneuro-cognitive scores [67]. Two studies also reported having assessed neuro-cognitive domains proposed by the MATRICS panel (i.e. working memory (verbal and non-verbal) and attention) using measures closely aligning to the MCCB battery [31,74]. However, as neuro-cognition was not the focus of these studies, this ability was not examined in relation to empathy. Sincevery few studies have examined all six of the neuro-cognitive domains proposed, examining each domain separately may not be possible. However, we can gather studies which have assessed anyone, or more of the neuro cognitive domains identified by the MATRICS panel into a global neuro-cognitive score and assess its impact on cognitive and affective empathy. In this way, we would have sufficient studies to make provisional inferences relating to heterogeneity and taxonomize the role of neuro-cognition on empathy in schizophrenia.
As well as neuro cognition, findings relating to Intelligent Quotient (IQ) and empathy are also unclear. Two types of IQ’s have been measured in studies of empathy in schizophrenia: Verbal or pre-morbid IQ, and general IQ. Some studies have reported subtle impairments in pre-morbid/verbal IQ [29,30,50,66,74], while others have reported a more pronounced impairment in general IQ [36,59,67,68,75]. Since previous studies have found significant negative relationships between measures of IQ and empathetic responding in schizophrenia [23,68], it will be important to include this variable for the purposes of heterogeneity assessment and taxonomy development.
The goal of current research
A meta-analytic framework was chosen as it enabled us to gather data systematically and provide us with a large schizophrenia sample, which to some extent helped us in overcoming some of the problems associated with small sample sizes (a common issue in this area of research). This meta-analysis aimed to: (1) Synthesise the extant literature on self-reported cognitive and affective empathy in schizophrenia using a meta-analytic approach. (2) Examine in detail the heterogeneity observed in previous reviews by examining for the first time, the moderating effect of several important variables to create taxonomy. These included: Severity of positive, negative, and general symptoms, duration of illness, age at diagnosis, medication dosages, age, gender, ethnicity, education, global neuro-cognition, verbal/pre-morbid IQ, general IQ and year of publication on the difference in performance on self-reported empathy between schizophrenia patients and healthy controls. Consistent with Bonfils and colleagues reviews [11,12] we hypothesised that healthy controls would report higher levels of cognitive and affective empathy than people with a diagnosis of schizophrenia. Due to mixed findings in the literature for clinical, demographic and cognitive variables, these moderators were examined in an exploratory manner.
METHODS
Database search
Study selection criteria
Data extraction
Effect sizes
ANALYSES
Preliminary analyses
Main analyses
Heterogeneity and moderator analyses
Barring descriptive statistics, all other meta-analytic analyses were conducted on Comprehensive Meta-Analysis Version 2 (CMA) [86].
RESULTS
Figure 1: Literature search diagram using PRISMA.
Study characteristics
|
Citation (K = 39) |
Country (City) |
SSD N |
HC N |
% Patients in study with a Schizophrenia Diagnosis |
M Age |
M Age HC |
% Male |
% Male |
M Years in Education |
M Years |
% Ethnicity |
% Ethnicity |
|
Achim, et al., [10] |
Canada (Québec) |
31 |
31 |
74.2 |
24.9 |
25.2 |
83.9 |
83.8 |
|
|
|
|
|
Andrews, et al., [87] |
Australia (Victoria) |
18 |
18 |
61.1 |
44.1 |
38.4 |
61.1 |
44.4 |
13.3 |
15.6 |
|
|
|
Berrada-Baby, et al., [88] |
France (Versailles) |
20 |
20 |
100 |
46.3 |
41.5 |
54.0 |
54.0 |
12.2 |
12.5 |
|
|
|
Brown, et al., [24] |
USA (Baltimore) |
17 |
17 |
100 |
41.7 |
38.2 |
52.9 |
52.9 |
|
|
|
|
|
Chiang, et al., [68] |
Taiwan (Hualien County) |
70 |
35 |
100 |
44.5 |
46.0 |
47.1 |
48.0 |
10.9 |
13.0 |
|
|
|
Corbera, et al., [58] |
USA (New Haven) |
30 |
24 |
66.7 |
46.5 |
39.7 |
46.7 |
62.5 |
13.1 |
16.2 |
43.3-Caucasian 56.5 - Non- Caucasian |
54.2-Caucasian 45.8 - |
|
Corbera, et al., [50] |
USA (New Haven) |
21 |
26 |
100 |
32.2 |
30.1 |
61.9 |
57.7 |
14.9 |
14.9 |
45.0 -Caucasian 55.0-Non- Caucasian |
42.3-Caucasian 57.7 - Non- Caucasian |
|
Derntl, et al., (2012a) t[44] |
Germany (Aachen) |
15 |
15 |
100 |
34.2 |
30.4 |
66.7 |
66.7 |
|
|
100-Caucasian |
100-Caucasian |
|
Derntl, et al., [70] |
Germany (Aachen) |
24 |
24 |
100 |
40.1 |
39.9 |
50 |
50.0 |
|
|
100-Caucasian |
100-Caucasian |
|
Didehnani, et al., [75] |
USA (Dallas) |
19 |
21 |
63.2 |
32.4 |
27.1 |
88.5 |
|
12.6 |
14.6 |
|
|
|
Fischer-Shofty, et al., [25] |
Israel (Haifa) |
35 |
48 |
100 |
|
30.0 |
|
69.9 |
12.2 |
15.0 |
100-Non-Caucasian |
100-Non -Caucasian |
|
Fujino, et al., [39] |
Japan (Kyoto) |
69 |
69 |
100 |
36.6 |
24.2 |
57.9 |
57.9 |
13.9 |
14.5 |
|
|
|
Fujiwara, et al [40] |
Japan (Kyoto) |
24 |
20 |
100 |
37 |
34.6 |
50 |
50.0 |
13.7 |
14.3 |
|
|
|
Gizewski, et al., [26] |
Germany (Essen) |
12 |
12 |
100 |
37.8 |
36.6 |
100 |
100 |
9.3 |
9.8 |
|
|
|
Haker, et al., [65] |
Switzerland (Zurich) |
43 |
45 |
100 |
34 |
35.0 |
39.5 |
73.0 |
13 |
14.0 |
|
|
|
Hooker, et al., [23] |
USA (Berkeley/San Francisco) |
21 |
17 |
52 |
44.3 |
43.7 |
80.9 |
76.4 |
13.0 |
15.0 |
|
|
|
Horon, et al., [51] |
USA (Los Angeles) |
32 |
26 |
100 |
47.9 |
44.4 |
81.3 |
73.1 |
12.9 |
14.9 |
56.3 -Caucasian |
76.0 -Caucasian |
|
Horon, et al., [27] |
USA (Los Angeles and Chapel Hill) |
145 |
45 |
100 |
40.9 |
43.3 |
75 |
71.0 |
12.5 |
14.2 |
48.3-Caucasian 51.7-Non-Caucasian |
31.0 -Caucasian |
|
Kucharska-Pietura, et al., [61] |
Poland (Lublin) |
100 |
50 |
100 |
31.3 |
29.6 |
|
|
12.8 |
13.7 |
|
|
|
Lam, et al., [36] |
China (Hong Kong) |
58 |
61 |
100 |
40.1 |
41.3 |
50 |
50.8 |
10.4 |
11.3 |
100-Non- Caucasian |
100-Non-Caucasian |
|
Lee, et al., [28] |
South Korea (Seoul) |
15 |
18 |
100 |
26.0 |
25.8 |
46.6 |
50.0 |
15.1 |
15.4 |
|
|
|
Lee, et al., [69] |
USA (Los Angeles) |
30 |
22 |
100 |
46.1 |
44.3 |
83.3 |
77.2 |
12.8 |
14.7 |
|
|
|
Lehmann, et al., [29] |
Germany (Berlin) |
55 |
55 |
100 |
39.8 |
38.9 |
58.1 |
54.5 |
14.0 |
15.0 |
|
|
|
Matsumoto, et al., [30] |
Japan (Kyoto) |
17 |
18 |
100 |
40.0 |
35.0 |
35.0 |
66.6 |
|
|
|
|
|
McCormick, et al., [48] |
USA (Iowa City) |
16 |
16 |
88 |
37.0 |
36.6 |
88 |
87.5 |
13.9 |
15.4 |
|
|
|
McGuire, et al., [31] |
Australia (Sydney) |
24 |
20 |
83 |
46.6 |
38.6 |
|
|
|
|
|
|
|
McGuire, et al., [74] |
Australia (Sydney) |
45 |
27 |
|
43.7 |
40.7 |
82.2 |
62.9 |
|
|
|
|
|
Montag, et al., [42] |
Germany (Berlin) |
45 |
45 |
100 |
37.5 |
38.8 |
77.7 |
77.7 |
12.6 |
15.0 |
|
|
|
Montag, et al., [58] |
Germany (Berlin) |
145 |
145 |
97 |
36.9 |
37.2 |
62.7 |
54.4 |
13.0 |
15.1 |
100-Caucasian |
100-Caucasian |
|
Pijnenborg, et al., [60] |
The Netherlands (Groningen) |
46 |
53 |
100 |
27.4 |
31.1 |
73.0 |
46.0 |
|
|
|
|
|
Ramos-Loyo, et al., [62] |
Mexico (Guadalajara) |
38 |
38 |
100 |
36.1 |
34.2 |
47.3 |
47.3 |
13.1 |
13.8 |
|
|
|
Regenbogen, et al., [43] |
Germany (Aachen) |
20 |
24 |
100 |
37.3 |
35.2 |
54.1 |
65.0 |
11.5 |
12.3
|
|
|
|
Smith, et al., [47] |
USA (Chicago) |
45 |
60 |
100 |
35.3 |
33.0 |
63.3 |
53.3 |
|
|
43.3- Caucasian 56.7 - Non-Caucasian |
48.9- Caucasian 51.1 - Non-Caucasian |
|
Singh, et al., [32] |
India (New Delhi) |
14 |
14 |
100 |
31.5 |
27.2 |
78.5 |
71.4 |
9.6 |
11.3 |
100-Non-Caucasian |
100-Non-Caucasian |
|
Shamay-Tsoory, et al., [37] |
Israel (Haifa) |
26 |
31 |
100 |
|
26.6 |
69.2 |
51.6 |
|
|
|
|
|
Sparks, et al., [46] |
Australia (Sydney) |
28 |
25 |
89 |
45.9 |
35.7 |
57.1 |
40.0 |
13.2 |
16.7 |
|
|
|
Thirioux, et al., [35] |
France (Paris) |
10 |
10 |
100 |
33.3 |
32.5 |
100 |
100 |
11.8 |
|
|
|
|
Vistoli, et al., [59] |
Canada (Québec) |
27 |
21 |
67 |
29.7 |
29.2 |
85.1 |
80.9 |
|
|
|
|
|
Wojakiewicz, et al., [33] |
France (Paris) |
29 |
27 |
100 |
36.5 |
31.0 |
68.9 |
74.0 |
10.3 |
10.5 |
|
|
Note: SSD = Schizophrenia-Spectrum Disorder Sample. HC = Healthy Control Sample. M = Mean. tSupplemental information was provided by authors to assist in the coding of these studies.
|
Country (City) |
M Age at Diagnosis |
M Duration of Illness |
M Medication Dosage (Chlorpromazine equivalents) – mg/day |
M Positive Symptom Severity Score |
M Negative Symptom Severity |
M General |
|
|
Achim, et al., [10] |
Canada (Québec) |
- |
1.7 |
- |
15.1 |
16.0 |
32.0 |
|
Andrews, et al., t[87] |
Australia (Victoria) |
21.4 |
21.1 |
- |
- |
- |
- |
|
Berrada-Baby, et al., [88] |
France (Versailles) |
- |
- |
406.0 |
- |
- |
- |
|
Brown, et al., [24] |
USA (Baltimore) |
30.5 |
9.3 |
- |
19.1 |
24.9 |
45.4 |
|
Corbera, et al., [58] |
USA (New Haven) |
24.3 |
22.2 |
654.0 |
18.9 |
N - 17.0 |
30.2 |
|
Corbera, et al., [50] |
USA (New Haven) |
- |
- |
181.3 |
15.2 |
15.2 |
29.2 |
|
Derntl, et al., [44] |
Germany (Aachen) |
26.8 |
7.3 |
329.9 |
12.3 |
14.6 |
24.5 |
|
Derntl, et al., [70] |
Germany (Aachen) |
28.9 |
11.5 |
601.4 |
14.0 |
14.2 |
28.9 |
|
Didehnani, et al., [75] |
USA (Dallas) |
- |
- |
- |
18.9 |
16.5 |
37.1 |
|
Fischer-Shofty, et al., [25] |
Israel (Haifa) |
- |
11.8 |
- |
15.4 |
19.3
|
34.5
|
|
Fujino, et al., [39] |
Japan (Kyoto) |
24.2 |
13.1 |
- |
14.2 |
15.9 |
31.4 |
|
Fujiwara, et al., [40] |
Japan (Kyoto) |
26.9 |
10.4 |
- |
14.3 |
13.9 |
32.7 |
|
Gizewski, et al., [26] |
Germany (Essen) |
21.0 |
16.8 |
672.3 |
14.6 |
20.5 |
31.9 |
|
Haker, et al., [65] |
Switzerland (Zurich) |
24.0 |
11.0 |
297.0 |
12.2 |
14.6 |
26.3 |
|
Hooker, et al., [23] |
USA (Berkeley/San Francisco) |
- |
24.5 |
- |
11.7 |
16.5 |
- |
|
Horon, et al., [51] |
USA (Los Angeles) |
20.8 |
26.8 |
282.5 |
1.5 |
1.7 |
- |
|
Horon, et al., [27] |
USA (Los Angeles and Chapel Hill) |
21.0 |
20.0 |
- |
2.5 |
2.1 |
- |
|
Kucharska-Pietura, et al., [67] |
Poland (Lublin) |
- |
8.6 |
408.1 |
39.4 |
59.8 |
- |
|
Lam, et al., [36] |
China (Hong Kong) |
25.9 |
13.4 |
- |
9.7 |
13.3 |
20.6 |
|
Lee, et al., [28] |
South Korea (Seoul) |
21.7 |
4.6 |
422.1 |
13.1 |
15.4 |
30.6 |
|
Lee, et al., [68] |
USA (Los Angeles) |
- |
- |
- |
5.9 |
5.3 |
- |
|
Lehmann, et al., [29] |
Germany (Berlin) |
29.8 |
10.0 |
407.8 |
13.2 |
15.9 |
- |
|
Matsumoto, et al., [30] |
Japan (Kyoto) |
- |
15.2 |
- |
16.1 |
6.6 |
10.6 |
|
McCormick, et al., [48] |
USA (Iowa City) |
20.7 |
15.8 |
- |
4.6 |
9.2 |
- |
|
McGuire, et al., [31] |
Australia (Sydney) |
22.5 |
22.7 |
- |
1.3 |
2.1 |
- |
|
McGuire, et al., [74] |
Australia (Sydney) |
21.6 |
21.80 |
- |
1.7 |
2.1 |
- |
|
Montag, et al., [42] |
Germany (Berlin) |
25.8 |
11.6 |
- |
19.7 |
19.7 |
- |
|
Montag, et al., [58] |
Germany (Berlin) |
26.5 |
10.4 |
453.8 |
17.0 |
19.4 |
35.6 |
|
Pijnenborg, et al., [60] |
The Netherlands (Groningen) |
24.2 |
7.0 |
- |
12.8 |
15.3 |
29.2 |
|
Ramos-Loyo, et al., [62] |
Mexico (Guadalajara) |
- |
23.4 |
200.0 |
16.9 |
17.8 |
28.9 |
|
Regenbogen, et al., [43] |
Germany (Aachen) |
27.8 |
9.5 |
- |
14.2 |
23.1 |
- |
|
Smith, et al., [47] |
USA (Chicago) |
- |
14.4 |
360.9 |
0.6 |
0.6 |
- |
|
Singh, et al., [32] |
India (New Delhi) |
23.7 |
9.3 |
389.3 |
8.6 |
12.6 |
- |
|
Shamay-Tsoory, et al., [37] |
Israel (Haifa) |
- |
- |
- |
16.5 |
21.0 |
- |
|
Sparks, et al., [46] |
Australia (Sydney) |
- |
- |
300.9 |
38.8 |
39.3 |
-- |
|
Thirioux, et al., [35] |
France (Paris) |
- |
11.8 |
664.4 |
21.6 |
32.2 |
- |
|
Vistoli, et al., [59] |
Canada (Québec) |
- |
7.6 |
547.7 |
16.0 |
9.6 |
- |
|
Wojakiewicz, et al., [33] |
France (Paris) |
- |
8.0 |
- |
14.2 |
19.2 |
- |
Note: M = Mean. CPZ-equivalent-mg/day = Chlorpromazine Equivalent in milligram per day. tSupplemental data was provided by authors. M = Mean.
In this table, only that study for which data was available is included.
|
|
M General IQ Score in SSD |
M General IQ Score in HC |
M Pre-morbid/Verbal IQ Score in SSD |
M Pre-morbid/Verbal IQ Score in HC |
M Global Neuro-cognition Score in SSD |
M Global Neuro-cognition Score in HC |
|
Achim, et al., [10] |
100.4 |
101.8 |
- |
- |
- |
- |
|
Berrada-Baby, et al., [87] |
- |
- |
26.5 |
28.9 |
- |
- |
|
Chiang, et al., [68] |
83.9 |
100.4 |
- |
- |
- |
- |
|
Corbera, et al., [58] |
- |
- |
- |
- |
39.6 |
52.0 |
|
Corbera, et al., [50] |
89.5 |
- |
- |
- |
32.1 |
32.0 |
|
Derntl, et al., t[44] |
- |
114.2 |
30.2 |
32.0 |
- |
- |
|
Derntl, et al., [70] |
- |
- |
107.7 |
111.3 |
- |
- |
|
Didehnani, et al., [75] |
102.2 |
112.1 |
- |
- |
- |
- |
|
Fujino, et al., [39] |
- |
- |
103.1 |
105.3 |
- |
- |
|
Fujiwara, et al., [40] |
104.0 |
109.0 |
104.0 |
107.0 |
- |
- |
|
Gizewski, et al., [26] |
- |
- |
102.2 |
109.8 |
- |
- |
|
Haker, et al., [65] |
- |
- |
24.4 |
33.1 |
16.8 |
22.5 |
|
Hooker, et al., [23] |
101.1 |
- |
- |
- |
-0.32 |
0.4 |
|
Kucharska-Pietura, et al., [61] |
- |
- |
- |
- |
24.4 |
37.4 |
|
Lam, et al., [36] |
34.9 |
49.8 |
- |
- |
16.6 |
20.4 |
|
Lee, et al., [28] |
- |
- |
11.4 |
12.7 |
14.5 |
10.4 |
|
Lehmann, et al., [29] |
- |
- |
108.5 |
118.8 |
25.0 |
21.9 |
|
Matsumoto, et al., [30] |
- |
- |
101.7 |
107.9 |
- |
- |
|
McGuire, et al., [31] |
- |
- |
105.6 |
107.7 |
35.1 |
37.5 |
|
McGuire, et al., [73] |
- |
- |
103.0 |
109.5 |
27.9 |
33.5 |
|
Montag, et al., [42] |
- |
- |
25.8 |
29.6 |
- |
- |
|
Montag, et al., [58] |
- |
- |
103.9 |
108.9 |
- |
- |
|
Pijnenborg, et al., [60] |
90.2 |
103.4 |
41.9 |
52.1 |
36.3 |
31.3 |
|
Regenbogen, et al., [43] |
- |
|
71.4 |
82.1 |
22.3 |
17.7 |
|
Smith, et al., [47] |
- |
- |
- |
- |
0.32 |
0.4 |
|
Sparks. et al., [46] |
- |
- |
104.9 |
110.0 |
- |
- |
|
Thirioux, et al., [35] |
- |
- |
- |
- |
- |
- |
|
Vistoli, et al., [59] |
99.9 |
109.3 |
- |
- |
- |
- |
|
Wojakiewicz, et al., [33] |
90.3 |
93.6 |
- |
- |
-- |
- |
Table 3: Mean general IQ, pre-morbid/verbal IQ and global neuro cognitive scores for schizophrenia spectrum disorders and healthy controls coded from individual studies included in the meta-analysis.
Note: M = Mean. SSD = Schizophrenia Spectrum Disorder. HC = Healthy Controls. tSupplemental data was provided by authors.In this table, only studies for which was available is included. tSupplemental data was provided by authors.
|
|
Mean (SD)/Mean Percent (SD) |
Range |
K |
|
Sample Type |
|
|
|
|
Published Article |
94.9 |
- |
37 |
|
Poster (data from authors) |
5.1 |
- |
2 |
|
Year |
2012 |
2007-2017 |
39 |
|
SPD Sample Size |
37.9 (31.4) |
10-145 |
39 |
|
HC Sample Size |
33.2 (23.9) |
10-145 |
39 |
|
Location |
|
|
|
|
Europe |
33.3 |
- |
13 |
|
United States |
33.3 |
- |
13 |
|
Asia |
23.2 |
- |
9 |
|
Oceania |
10.3 |
- |
4 |
Table 4: Study characteristics of included studies in the meta-analysis.
|
|
Mean (SD)/Mean Percent (SD) |
Range |
K |
|
Diagnosis |
|
|
|
|
Schizophrenia |
93.2 (13.6) |
52.4-100 |
38 |
|
Shizo-affective |
4.65 (15.6) |
2.7-42.9 |
10 |
|
Other Psychoses |
1.86 (9.2) |
6.3-19.3 |
2 |
|
Age at Diagnosis |
24.6 (3.1) |
20.7-30.5 |
22 |
|
Duration of Schizophrenia |
13.5 (6.3) |
1.7-26.8 |
32 |
|
Symptom Severity |
|
|
|
|
Positive Symptoms |
13.9 (8.4) |
0.61-39.40 |
36 |
|
Negative Symptoms |
16.6 (10.9) |
0.66-59.80 |
36 |
|
General Symptoms |
31.0 (5.3) |
20.6-45.4 |
18 |
|
Medication Dosage (Chlorpromazine equivalents) - mg/day |
414.3 (38.02) |
162.1-642.3 |
18 |
Note: SD = Standard Deviation. K = Number of studies included.
|
|
Mean (SD)/Mean Percent (SD) |
Range |
K |
|
General IQ, Healthy Controls |
99.29 (19.6) |
49.8-14.2 |
9 |
|
General IQ, Schizophrenia Spectrum Disorder |
89.6 (20.4) |
34.9-04.0 |
10 |
|
Verbal/Pre-morbid IQ, Healthy Controls |
79.9 (40.7) |
12.7-18.8 |
15 |
|
Verbal/Pre-morbid IQ, Schizophrenia Spectrum Disorder |
74.9 (39.7) |
11.4-08.5 |
15 |
|
Global Neuro-cognition, Healthy Controls |
24.4 (15.03) |
0.43-52.1 |
13 |
|
Global Neuro-cognition, Schizophrenia Spectrum Disorder |
22.3 (12.6)
|
-0.32-39.5
|
13 |
|
Demographic variables |
Mean (SD)/Mean Percent (SD) |
Range |
K |
|
Age, Healthy Controls |
35.2 (5.9) |
24-46 |
39 |
|
Age, Schizophrenia Spectrum Disorders |
37.8 (6.1) |
25-48 |
38 |
|
Education, Healthy Controls |
14.0 (1.7) |
9.8-16.7 |
27 |
|
Education, Schizophrenia Spectrum Disorders |
12.5 (1.3) |
9.3-15.1 |
28 |
|
Male, Healthy Controls |
63.9 (15.2) |
40-100 |
36 |
|
Male, Schizophrenia Spectrum Disorder |
67.5 (15.6) |
47-100 |
36 |
|
Ethnicity, Healthy Controls |
Caucasian-69.1 (28.6) |
31-100 24-100 |
8 7 |
|
Ethnicity, Schizophrenia Spectrum Disorder |
Caucasian-67.0 (27.6) |
43.3-100 43.7-100 |
8 8 |
Table 7: Demographic characteristic of samples included in the meta-analysis.
Empathy measures
Symptom assessment
Assessment of neuro-cognition
One study [58] measured all six neuro-cognitive domains (i.e. attention, speed of processing, working memory (verbal and non-verbal), visual learning, verbal learning, reasoning and problem solving) using the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB) [94]). Barring the attention domain, Hooker, et al., [23] also measured the above-mentioned domains using the MCCB. Although Smith and colleagues [47] reported not to have used the MCCB battery, nonetheless assessed the six neuro-cognitive domains using approximate measures representing the MCCB assessment battery [94]. Across the remaining ten studies [29,31,36,43,51,60,61,64,68,73] few, but not all six neuro-cognitive domains were assessed using tests that differed from but comparable to the MCCB. For example, Lam, et al., [36] assessed the reasoning and problem solving, and visual learning domain, whereasother studies (see for example, [61]), assessed the attention and working memory (non-verbal) domain. We also found few studies [31,51,64] to have examined cognitive flexibility, which we additionally included within the global neuro-cognitive moderator (( Supplementary Table S3 for a description of the neuro-cognitive measures used in individual studiesand the corresponding neurocognitive domain examined).
In total, 15 studies measured pre-morbid/verbal IQ. Pre-morbid/verbal IQ was measuredin 13 of these studies using either the Multiple-choice vocabulary test (German version) (MCVT; [95]) or National Adult Reading Test (NART; [96]). MCVT was included by seven studies [58,26,29,42-44,69] and NART by six studies [30,31,39,46,72,73,87]. The remaining two studies [28,40] used the verbal sub-set from the Wechsler’s Adult Intelligence Scale - III [97] (( Supplementary Table S4 for a short description of each of the verbal task used by the included studies).
Data for general IQ was available for ten of the included studies. Eight of these studies [10,23,33,40,50,59,67,74] used several versions of the Wechsler’s Adult Intelligence Scales (e.g. [97]). The remaining two studies [36,60] used the Raven’s Progressive Matrices Test (120) and Groninger Intelligence Test [98] respectively (( Supplementary Table S5 for a description of the general IQ measures used by the included studies).
Sensitivity analysis
Meta-analyses
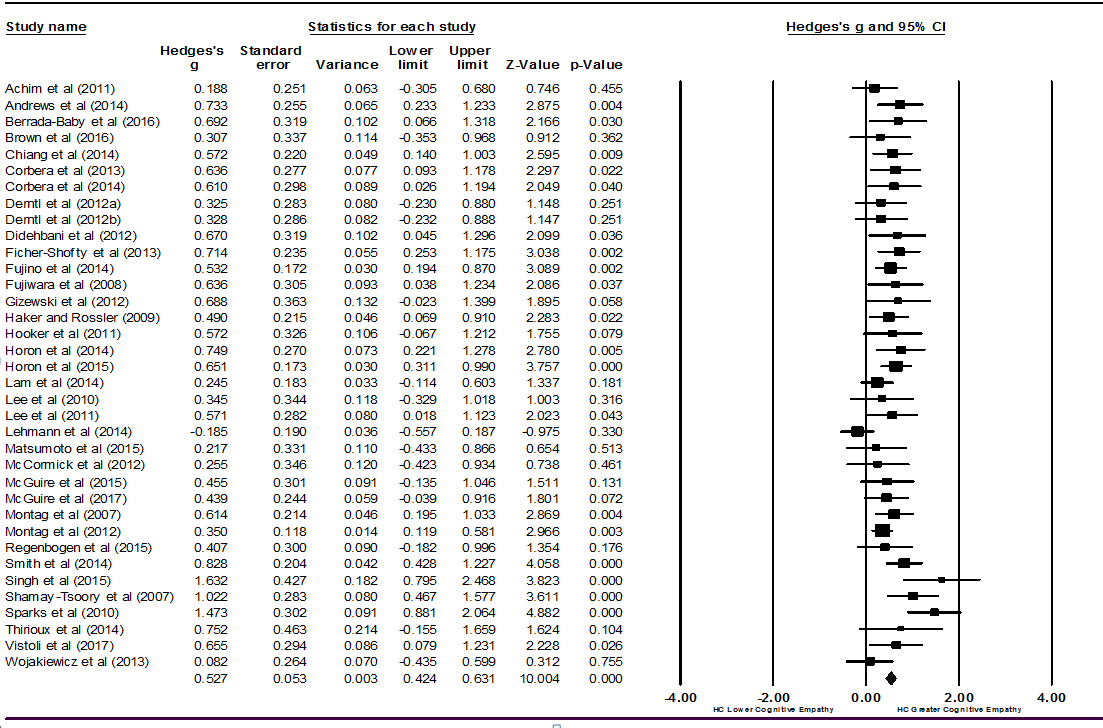
Figure 2: Forest plot of studies included in the cognitive empathy meta-analysis (k = 36).
For affective empathy, a small effect size was found (k = 39, Hedges’ g = 0.29, 95% CI [0.16, 0.42], p<0.001) (Figure 3). This indicated the healthy control group had better affective empathic ability than the schizophrenia group. The Q-statistic was significant (Q-statistic = 98.21, df = 38, p<0.001) with an I2index of 61.31%.

Meta-analyses examining impact of co-morbid psychiatric condition on empathy
Moderator analyses
Cognitive empathy
DISCUSSION
MODERATING EFFECT OF CLINICAL VARIABLES ON EMPATHY
Duration of illness and empathy
Age at clinical diagnosis also had a moderating effect on cognitive empathy. As the age at diagnosis decreased, the difference in performance between patients and controls on self-reported cognitive empathy increased. This means that those with an earlier diagnosisreported havinggreater difficulties in perspective-taking then those whose symptom onset was at a later age. Duration of illness and age at diagnosis are related. Both are reliable indicators of severity of illness in schizophrenia (i.e. the earlier the onset, the worse it is regarding functional outcome, and the longer it persists without remission, the less likely you are to improve) [103]. Therefore, it will be important to address the underlying mechanisms of this deficit in future work.
Clinical symptoms and empathy
We found no moderating effect of chlorpromazine equivalents (mg/day) on self-reported cognitive and affective empathy. These findings are consistent with studies that directly compared the effects of chlorpromazine equivalent on self-reported empathy [29,32,44,46,47]. These findings also extend to haloperidol equivalents [32,39,40]. Singh, et al., [32] also reported having found no effect of duration of antipsychotic drug taken on any of the IRI scores in an enduring schizophrenia sample. Also, in one of the largest sample study comparing patients treated on conventional versus atypical antipsychotic drugs on social cognitive abilities, Kucharska-Pietura and colleagues [61] found no clear advantage of atypical antipsychotics over typical antipsychotics on emotional functioning in patients with schizophrenia. Results from several longitudinal studies [104] have also indicated no significant effect of antipsychotic drug treatment on several other related social-cognitive domains (e.g. facial affect perception). Thus, it appears that while antipsychotic drugs are useful in treating core symptoms of schizophrenia, deficits in empathy may perhaps be resistant to pharmacological intervention.
Demographic variables and empathy
Neuro-cognition and empathy
In this study, instead of examining individual neuro-cognitive domains, we examined what we termed ‘global neuro-cognitive abilities’ by including studies that assessed all, few or one of the six neuro-cognitive domains defined and recommended by the MATRICS panel [71,72] as well as an additional, cognitive flexibility/inhibitory control domain. Overall, we did not find any impact of this variable on cognitive or affective empathy which is consistent with several of the published studies in the field [23,27,58]. However, as it is well established that like IQ, neuro-cognitive deficits do exist in patients with more severe and enduring schizophrenia [106] and is an essential component of empathy [31,36,47,51,73]. Therefore, the lack of association is somewhat surprising. It may be that this moderator was somewhat underpowered, or there was a lack of dispersion in the neuro cognitive scores. Alternatively, it may have been that for neuro-cognitive abilities to relate to empathy; tasks need tapping into specific cognitive abilities. In other words, specific executive function tasks (e.g. emotion-regulation) relating to empathy [77,101] is perhaps necessary to find a significant effect.
Affective empathy and heterogeneity
LIMITATIONS
Publication bias
For cognitive empathy the opposite held. In total, nine studies were identified as missing (Figure 5) and including them would have reduced the effect size from Hedges’ g = 0.53 to Hedges’ g = 0.41. This observation is consistent with a previous meta-analysis in the field [12] and together highlight two important issues: (1) The need to also publish nil findings and (2) where possible, include schizophrenia samples at different stages of the illness course, particularly at the earlier phase, where deficits in perspective-taking are likely to be less pronouncedthen in the more severe and enduring phase.
Measures of empathy: Self-report
Impact of additional variables
Generalisability of current findings
Also, over 90 percent of the studies included were conducted in developed countries (Table 1). Better outcomes have been found in many developing compared to developed countries [101]. Thus, findings from this study may not be fully general is able to those recovering in developing countries.
CONCLUSION
SUPPLEMENTARY MATERIAL
Funding
Acknowledgement
REFERENCES
- Cheng Y, Chena C, Decety J (2014) An EEG/ERP investigation of the development of empathy in early and middle childhood. Dev cogn neurosci 10: 160-169.
- Decety J, Cowell JM (2014) The complex relation between morality and empathy. Trends cogn Sci 18: 337-339.
- Ferguson E (2016) Empathy “The good, the bad and the ugly”. In: Wood A, et al., (Eds.) Posi clinic psychol: An integrative approach to studying and improving well-being. Wiley-Blackwell, Chichester, UK. Pg no: 103-124.
- Batson CD (1991) The altruism question: Towards a social-psychological answer. Lawrence Erlbaum Associates, Inc, Routledge, Hillsdale, New Jersey, USA, Pg no: 268.
- Reniers RL, Corcoran R, Drake R, Shryane NM, Völlm BA (2011) The QCAE: A questionnaire of cognitive and affective empathy. J Personali Asses 93: 84-95.
- Blair RJ (2005) Responding to the emotions of others: Dissociating form of empathy through the study of typical and psychiatric populations. Conscious Cogn 14: 698-718.
- Spinella M (2005) Prefrontal substrates of empathy: Psychometric evidence in a community sample. Send to Biol Psychol 70: 175-181.
- Lee KH, Farrow TF, Spence SA, Woodruff PW (2004) Social cognition, brain networks and schizophrenia. Psychol Med 34: 391-400.
- Bleuler E (1950) Dementia Praecox or the Group of Schizophrenias. International Universities Press, Madison, Connecticut, USA.
- Achim AM, Ouellet R, Roy MA, Jackson PL (2011) Assessment of empathy in first-episode psychosis and meta-analytic comparison with previous studies in schizophrenia. Psychiatry Res 190: 3-8.
- Bonfils KA, Lysaker PH, Minor KS, Salyers MP (2016) Affective empathy in schizophrenia: A meta-analysis. Schizophr Res 175: 109-117.
- Bonfils KA, Lysaker PH, Minor KS, Salyers MP (2017) Empathy in schizophrenia: A meta-analysis of the Interpersonal Reactivity Index. Psychiatry Res 249: 293-303.
- Bleuler E (1911) Dementia Praecox: Or the Group of Schizophrenias. International Universities Press, New York, USA.
- Kraepelin E (1919) Dementia praecox and paraphrenia. Chicago Medical Book Co, Chicago, USA.
- Davis MH (1983) Measuring individual differences in empathy: Evidence for a multidimensional approach. J Pers Soc Psychol 44: 113-126.
- Decety J (2011) The neuroevolution of empathy. Ann NY Acad Sci 1231: 35-45.
- Eisenberg N, Strayer J (1987) Critical issues in the Study of empathy. In: Eisenberg N, et al. (Eds.) Empath and its Develop, Cambridge University Press, Cambridge, UK, Pg no: 13.
- Jolliffe D, Farrington DP (2004) Empathy and offending: A systematic review and meta-analysis. Aggress Violent Behav 9: 441-476.
- Davis MH (1980) A multidimensional approach to individual difference in empathy. American Psychological Association Massachusetts, USA.
- Harrington L, Siegert RJ, McClure J (2005) Theory of mind in schizophrenia: a critical review. Cogn Neuropsychiatry 10: 249-286.
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, (5th edn), APA, Washington, DC, USA.
- Kay SR, Fisbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261-276.
- Hooker CI, Bruce L, Lincoln SH, Fisher M, Vinogradov S (2011) Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biol Psychiatry 70: 1169-1178.
- Brown EC, Gonzalez-Liencres C, Tas C, Brune M (2016) Reward modulates the mirror neuron system in schizophrenia: A study into the mu rhythm suppression, empathy, and mental state attribution. Soc Neurosci 11: 175-186.
- Fischer-Shofty M, Brüne M, Ebert A, Shefet D, Levkovitz Y, et al. (2013) Improving social perception in schizophrenia: The role of oxytocin. Schizophr Res: 146: 357-362.
- Gizewski ER, Müller BW, Scherbaum N, Lieb B, Forsting M, et al. (2013) The impact of alcohol dependence on social brain function. Addict Biol 18: 109-120.
- Horan WP, Reise SP, Kern RS, Lee J, Penn DL, et al. (2015) Structure and correlates of self-reported empathy in schizophrenia. J Psychiatr Res 66-67: 60-66.
- Lee SJ, Kang DH, Kim CW, Gu BM, Park JY, et al. (2010) Multi-level comparison of empathy in schizophrenia: an fMRI study of a cartoon task. Psychiatry Res 181: 121-129.
- Lehmann A, Bahcesular K, Brockmann EM, Biederbick, SE, Dziobek I, et al. (2014) Subjective experience of emotions and emotional empathy in paranoid schizophrenia. Psychiatry Res 220: 825-833.
- Matsumoto Y, Takahashi H, Murai T, Takahashi H (2015) Visual processing and social cognition in schizophrenia: Relationships among eye movements, biological motion perception, and empathy. Neurosci Res 90: 95-100.
- McGuire J, Barbanel L, Brüne M, Langdon R (2015) Re-examining Kohlberg's conception of morality in schizophrenia. Cogn Neuropsychiatry 20: 377-381.
- Singh S, Modi S, Goyal S, Kaur P, Singh N, et al. (2015) Functional and structural abnormalities associated with empathy in patients with schizophrenia: An fMRI and VBM study. J Biosci 40: 355-364.
- Wojakiewicz A, Januel D, Braha S, Prkachin K, Danziger N, et al. (2013) Alteration of pain recognition in schizophrenia. Eur J Pain 17: 1385-1392.
- Montag C, Brockmann EM, Lehmann A, Müller DJ, Rujescu D, et al. (2012) Association between oxytocin receptor gene polymorphisms and self-rated 'empathic concern' in schizophrenia. PLoS One 7: 51882.
- Thirioux B, Tandonnet L, Jaafari N, Berthoz A (2014) Disturbances of spontaneous empathic processing relate with the severity of the negative symptoms in patients with schizophrenia: A behavioural pilot-study using virtual reality technology. Brain Cogn 90: 87-99.
- Lam BY, Raine A, Lee TM (2014) The relationship between neurocognition and symptomatology in people with schizophrenia: Social cognition as the mediator. BMC Psychiatry 14: 138.
- Shamay-Tsoory SG, Shur S, Harari H, Levkovitz Y (2007) Neurocognitive basis of impaired empathy in schizophrenia. Neuropsychology 21: 431-438.
- Corbera S, Wexler BE, Ikezawa S, Bell MD (2013) Factor structure of social cognition in schizophrenia: Is empathy preserved? Schizophrenia Research and Treatment 1-13.
- Fujino J, Takahashi H, Miyata J, Sugihara G, Kubota M, et al. (2014) Impaired empathic abilities and reduced white matter integrity in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 48: 117-123.
- Fujiwara H, Shimizu M, Hirao K, Miyata J, Namiki C, et al. (2008) Female specific anterior cingulate abnormality and its association with empathic disability in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 32: 1728-1734.
- Haker H, Rössler W (2009) Empathy in schizophrenia: Impaired resonance. Eur Arch Psychiatry Clin Neurosci 259: 352-361.
- Montag C, Heinz A, Kunz D, Gallinat J (2007) Self-reported empathic abilities in schizophrenia. Schizophr Res 92: 85-89.
- Regenbogen C, Kellermann T, Seubert J, Schneider DA, Gur RE, et al. (2015) Neural responses to dynamic multimodal stimuli and pathology-specific impairments of social cognition in schizophrenia and depression. Br J Psychiatry 206: 198-205.
- Derntl B, Finkelmeyer A, Voss B, Eickhoff SB, Kellermann T, et al. (2012) Neural correlates of the core facets of empathy in schizophrenia. Schizophr Res 136: 70-81.
- Derntl B, Seidel EM, Schneider F, Habel U (2012) How specific are emotional deficits? A comparison of empathic abilities in schizophrenia, bipolar and depressed patients. Schizophr Res 142: 58-64.
- Sparks A, McDonald S, Lino B, O'Donnell M, Green MJ (2010) Social cognition, empathy and functional outcome in schizophrenia. Schizophr Res 122: 172-178.
- Smith MJ, Horan WP, Cobia DJ, Karpouzian TM, Fox JM, et al. (2014) Performance-based empathy mediates the influence of working memory on social competence in schizophrenia. Schizophr Bull 40: 824-834.
- McCormick LM, Brumm MC, Beadle JN, Paradiso S, Yamada T, et al. (2012) Mirror neuron function, psychosis, and empathy in schizophrenia. Psychiatry Res 201: 233-239.
- National Institute for Health and Care Excellence (NICE) (2014) Psychosis and schizophrenia in adults: Prevention and management. Clinical guideline, London.
- Corbera S, Cook K, Brocke S, Dunn S, Wexler BE, et al. (2014) The relationship between functional deficits and empathy for emotional pain in schizophrenia. Biol Psychiatry 75: 200.
- Horan WP, Pineda JA, Wynn JK, Iacoboni M, Green MF (2014) Some markers of mirroring appear intact in schizophrenia: Evidence from mu suppression. Cogn Affect Behav Neurosci 14: 1049-1060.
- Bardenstein KK, McGlashan TH (1990) Gender differences in affective, schizoaffective, and schizophrenic disorders: A review. Schizophr Res 3: 159-172.
- Cernovsky ZZ, Landmark JA, O’Reilly RL (1997) Symptom patterns in schizophrenia for men and women. Psychol Rep 80: 1267-1271.
- Meltzer HY, Rabinowitz J, Lee MA, Cola PA, Ranjan R, et al. (1997) Age at onset and gender of schizophrenic patients in relation to neuroleptic resistance. Am J Psychiatry 154: 475-482.
- Halari R, Kumari V, Mehrotra R, Wheeler M, Hines M, et al. (2004) The relationship of sex hormones and cortisol with cognitive functioning in schizophrenia. J Psychopharmacol 18: 366-374.
- Lindamer LA, Bailey A, Hawthorne W, Folsom DP, Gilmer TP, et al. (2003) Gender differences in characteristics and service use of public mental health patients with schizophrenia. Psychiat Serv 54: 1407-1409.
- Goldstein JM, Link BG (1988) Gender and the expression of Schizophrenia. J Psychiat Res 22: 141-155.
- Montag C, Brockmann EM, Lehmann A, Muller DJ, Rujescu D, et al. (2012) Association between oxytocin receptor gene polymorphisms and self-rated 'empathic concern' in schizophrenia. PLoS One 7: 51882.
- 59. Vistoli D, Lovoie, MA, Sutliff S, Jackson PL, Achim AM (2017) Functional MRI examination of empathy for pain in people with schizophrenia reveals abnormal activation related to cognitive perspective-taking but typical activation linked to affective sharing. J Psychiatry Neurosci 42: 263-272.
- Pijnenborg GH, Spikman JM, Jeronimus BF, Aleman A (2013) Insight in schizophrenia: associations with empathy. Eur Arch Psychiatry Clin Neurosci 26: 299-307.
- Kucharska-Pietura K, Tylec A, Czernikiewicz A, Mortimer A (2012) Attentional and emotional functioning in schizophrenia patients treated with conventional and atypical antipsychotic drugs. Med Sci Monit 18: 44-49.
- Ramos Loyo J, Mora Reynoso L, Sanchez Loyo LM, Medina Hernandez V (2012) Sex differences in facial, prosodic and social context emotional recognition in early-onset schizophrenia. Schizophrenia Research and Treatment 1-12.
- Sparks A, McDonald S, Lino B, O'Donnell M, Green MJ (2010) Social cognition, empathy and functional outcome in schizophrenia. Schizophr Res 122: 172-178.
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I (2001) The "Reading the Mind in the Eyes" test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry 42: 241-251.
- Haker H, Rössler W (2009) Empathy in schizophrenia: Impaired resonance. Eur Arch Psychiatry Clin Neurosci 259: 352-361.
- Montag C, Brockmann EM, Lehmann A, Muller DJ, Rujescu D, et al. (2012) Association between oxytocin receptor gene polymorphisms and self-rated 'empathic concern' in schizophrenia. PLoS One 7: 51882.
- Pijnenborg GH, Spikman JM, Jeronimus BF, Aleman A (2013) Insight in schizophrenia: Associations with empathy. Eur Arch Psychiatry Clin Neurosci 26: 299-307.
- Chiang SK, Hua MS, Tam WCC, Chao JK, Shiah YJ (2014) Developing an alternative chinese version of the interpersonal reactivity index for normal population and patients with schizophrenia in taiwan. Brian Impairment 15: 10-131.
- Lee J, Zaki J, Harvey PO, Ochsner K, Green MF (2011) Schizophrenia patients are impaired in empathic accuracy. Psychol Med 41: 2297-2304.
- Derntl B, Seidel EM, Schneider F, Habel U (2012) How specific are emotional deficits? A comparison of empathic abilities in schizophrenia, bipolar and depressed patients. Schizophr Res 142: 58-64.
- Miller EK, Wallis JD (2009) Executive Function and Higher-Order Cognition: Definition and Neural Substrates. Enc NeoroSci 4: 99-104.
- Kern RS, Nuechterlein KH, Green MF, Baade LE, Fenton WS, et al. (2008) The MATRICS consensus cognitive battery, part 2: Co-norming and standardization. Am J Psychiatry 165: 214-220.
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, et al. (2008) The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. Am J Psychiatry 165: 203-213.
- McGuire J, Brüne M, Langdon R (2017) Judgement of moral and social transgression in schizophrenia. Compr Psychiatry 76: 160-168.
- Didehbani N, Shad MU, Tamminga CA, Kandalaft MR, Krawczyk DC, et al. (2012) Insight and empathy in schizophrenia. Schizoph Res 142: 246-247
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009) The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med 6: 1000097.
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC (2010) Antipsychotic dose equivalents and dose-years: A standardized method for comparing exposure to different drugs. Biol Psychiatry 67: 255-262.
- Decety J, Jackson PL (2006) A social-neuroscience perspective on empathy. Current Directions In Psychological Science 15: 54-58
- Hedges LV (1981) Distribution theory for glass's estimator of effect size and related estimators. Journal of Educ 6: 107-128.
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to meta-analysis. John Wiley & Sons, Wiltshire, UK.
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2009) Introduction to Meta-Analysis. John Wiley & Sons, Ltd, Chippenham, Wiltshire, UK.
- Duval S, Tweedie R (2000) Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta?analysis. Biometrics 56: 455-463
- Card NA (2012) Applied Meta-Analysis for Social Science Research. Guilford Press, New York, USA.
- Lipsey MW, Wilson DB (2001) Practical Meta-Analysis. SAGE Publications, California, USA.
- Cohen J (1992) A power primer. Psychol Bull 112: 155-159.
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR (2011) Comprehensive Meta-Analysis (Version 2). Biostat, Englewood, USA.
- Andrews SC, Enticott PG, Hoy KE, Fitzgerald PB (2013) Mirror systems and social cognition in schizophrenia. Schizophr Bull 39: 218.
- Berrada-Baby Z, Oker A, Courgeon M, Urbach M, Bazin N, et al. (2016) Patients with schizophrenia are less prone to interpret virtual others’ empathetic questioning as helpful. Psychiatry Research 242: 67-74.
- Mehrabian A (2000) Manual for the Behavioural Emotional Empathy Scale (BEES).
- Mehrabian A, Epstein N (1972) A measure of emotional empathy. J Pers 40: 525-543.
- Andreasen NC, (1989) Scale for the Assessment of Negative Symptoms (SANS). The British Journal of Psychiatry 155: 53-58.
- Andreasen NC (1984) Scale for the assessment of positive symptoms: SANS/SAPS. Dept. of Psychiatry, College of Medicine, the University of Iowa, Iowa City, Iowa, USA.
- Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychological Reports 10: 799-812.
- Nuechterlein KH, Green MF (2006) MATRICS Consensus Cognitive Battery Manual. MATRICS Assessment Inc, Los Angeles, USA.
- Lehrl S, Triebig G, Fischer B (1995) Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand 91: 335-345.
- Nelson HE (1982) National Adult Reading Test (NART) test manual (Part 1). NFER-Nelson, Windsor, UK.
- Wechsler D (1997) Wechsler Adult Intelligence Scale (3rd edn). Psychological Corporation, San Antonio, USA.
- Luteijn F, Van der Ploeg FAE (1983) Groninger Intelligentie Test. Swets and Zeitlinger, Lisse, Netherlands.
- Watkins J (1996) Living with Schizophrenia. Hill of Content Publishing Co Pty Ltd, Victoria, Australia.
- Horan WP, Green MF, Kring AM, Nuechterlein KH (2006) Does anhedonia in schizophrenia reflect faulty memory for subjectively experienced emotions? J Abnorm Psychol 115: 496-508.
- Jablensky A, Sartorius N (2008) What did the WHO studies really find? Schizophr Bull 34: 253-255.
- Leff J (2001) The unbalanced mind. Columbia University Press, New York, USA.
- Gulati G, Lynall ME, Saunders K (2014) Schizophrenia. In Psychiatry Lecture Notes (11th Edn). Wiley Blackwell, Oxford, UK.
- Heilbronner U, Samara M, Leucht S, Falkai P, Schulze TG (2016) The Longitudinal Course of Schizophrenia Across the Lifespan: Clinical, Cognitive, and Neurobiological Aspects. Harv Rev Psychiatry 24: 118-128.
- Derntl B, Finkelmeyer A, Toygar TK, Hülsmann A, Schneider F, et al. (2009) Generalized deficit in all core components of empathy in schizophrenia. Schizophr Res 108: 197-206.
- Bora E (2017) A comparative meta-analysis of neurocognition in first-degree relatives of patients with schizophrenia and bipolar disorder. Eur Psychiatry 45: 121-128.
SUPPLEMENTARY FILE
|
Measures of Empathy |
Original Article |
Studies in Meta-Analysis |
Description of Tasks and Scores Produced |
|
Interpersonal Reactivity Index (IRI) |
[1] |
[2-35] |
A 28-item self-report scale including four sub-scales: Empathic Concern, Perspective-Taking, Personal Distress and Fantasy. The Empathic Concern sub-scale taps into ‘other-orientated’ feelings of sympathy and concern for unfortunate other. The Perspective-Taking sub-scale assesses the ability to see things from the others perspective or how the other person thinks. The Personal Distress sub-scale measures levels of anxiety, sorrow or emotional distress in emergency situations. The Fantasy sub-scale measures the ability to relate to fictional characters (e.g. books or movies). Items on these sub-scales are measured on a 5-point Likert-scale, with responses ranging between does not describe me well, to describes me very well |
|
Empathy Quotient (EQ) |
[36] |
[37] |
A 60-item self-report scale. 40 items measure empathy on the cognitive and affective dimension and the remaining are included as control items. Each response is measured on a 4-point Likert scale, with responses ranging between strongly agree-to-strongly disagree |
|
Questionnaire for Cognitive and Affective Empathy (QCAE) |
[38] |
[17,39] |
A 31-item self-report scale consisting of five sub-scales: Perspective-Taking and Online Stimulation, measuring cognitive empathy. Emotion Contagion, Proximal Responsivity and Peripheral Responsivitymeasuring affective empathy |
|
Balanced Emotional Empathy Scale (BEES) |
[40] |
[41] |
A 30-item self-report scale measuring spontaneous, or vicarious emotional reactions in response to another’s emotional distress (i.e. affective/emotional empathy). Each itemis rated on a 9-point extent to which you agree-disagree spectrum |
|
Questionnaire Measure of Emotional Empathy (QMEE) (also referred to as the emotional empathic tendency scale) |
[42] |
[43] |
A 33-item self-report scale assessing affective role-taking empathy. In other words, this scale measures the extent to which the respondent agrees with the self-orientated emotional responses someone would typically experience in response to another’s emotional distress. Items on this scale are measured on a 4-point Likert scale, ranging between strongly agree to strongly disagree |
|
Social context emotional recognition task |
[44] |
[44] |
In this task, participants watched short films representing a happy, sad, angry and fearful context. Participants rated their emotional reaction (affective empathy) to each film and the intensity of the emotion they felt using a rating scale. The rating scale consisted of a continuous 10 cm line on which participants had to make a mark: Scores to the extreme left corresponded to the lowest intensity (0 cm) and scores to the extreme right corresponded to the highest intensity |
Table S1: List of empathy measures used by studies included in the meta-analysis.
|
Symptom Assessment Measure |
Studies in meta-analysis |
Description of Measure |
|
Positive and Negative Syndrome Scale (PANSS) [45] |
[2-44] |
A 30-item semi-structured measure completed by clinicians in an interview or observation format. 7 items measure positive symptoms of schizophrenia, 7 items measure negative symptoms and 16 items measuresgeneral psychopathology |
|
Schedule for the ASSESSMENT of Positive Symptoms(SAPS) [46] |
[23-25,29,30,32,41] |
A 34-item clinician rated scale which is used to measure the followingpositive symptoms: Bizarre behaviour, formal thought disorder, hallucinations and delusions |
|
Schedule for the Assessment of Negative Symptoms (SANS) [47] |
[17,23-25,29,30,32,41] |
The originally published scale consisted of 25 items. Currently, SANS comprises of 19-items, representing 5 scales: Blunted/flattened affect, alogia, avolition-apathy, anhedonia/associability and inattention. Items on this scale are rated by clinicians |
|
Brief Psychiatric Rating Scale (BPRS)-positive symptom sub-scale [48] |
[16,17,20] |
A 24-item scale assessing positive symptoms of schizophrenia via self-report and clinical observations. Each item onthis scale is measured on a 2 (very mild) to 7 (extremely severe) anchor points |
|
BPRS-Negative symptom subs-cale [48] |
[16,20] |
This sub-scale consists of items assessing negative symptoms of schizophrenia and is measured in the same way as the BPRS-positive symptom sub-scale |
Table S2: List of symptom assessments used by studies included in the meta-analysis.
|
Neurocognitive Domain |
Neurocognitive Measures |
Description of Measure |
Studies in Meta-Analysis |
|
Attention/ Vigilance (reported by three studies) |
The test of everyday attention [49] |
This test included three tasks: |
[41] |
|
Continuous performance task-identical pairs [50] |
A computerised test assessing sustained attention. A button must be pressed each time the participant sees two numbers matching onscreen |
[6,29] |
|
|
2. Verbal learning (reported by six studies) |
Hopkins verbal learning test-revised [51] |
The task administrator presents 12 words from three categories (e.g. animal, colours and numbers). Participant is assessed on how many words they can recall after each of three learning trials |
[6,7,15] |
|
|
Rey auditory verbal learning test (english version) [52] |
Participants are presentedwith 15 words over five trials. Participants must say the words immediately. An interference trial is then presented which involved presenting new words. Participants are asked to recall words from the initial list presented |
[26,43] |
|
|
California verbal learning test-second edition [53]
|
Participants are presented with a list of 16 words which they recall immediately over five trials. This is followed by an interference list, in which 16 words are presented in a single trial which must be recalled immediately. 20 minutes later a recognition trial is administered. Recall can be free or category-cued |
[29] |
|
3a. Working Memory (verbal) (Reported by Five Studies) |
Wechsler memory scale-third edition (WMS-III): Letter-number span [54] |
Participants are instructed to mentally re-order strings of numbers and letters and repeat them orally to the test administrator |
[6,15,24,29] |
|
WAIS Digit Span Forward/Backwards Subtest [55] |
Participants are instructed to repeat the numbers presented to them either in the same or reverse order. Over the course of the task, the number sequence increases |
[29] |
|
|
Repeatable battery for the assessment of neuropsychological status-story memory sub-test [56] |
A 12-item short story is presented visually in three sperate parts over two trials. Each story is read aloud with a low reading speed. Participants recall as much of the story as they can after each presentation. A verbatim criterionis used to score participant response |
[25] |
|
|
3b. Working memory |
WAIS-Revised (R)-working memory subtest [57] |
This test uses an arithmetic and digit span test. For the digit span test, participant recalls a series of number in a specific order (i.e., ascending, backward or same order). For the arithmetic test, participants work within a specified time limit to mentally resolve a series of mental arithmetic problems |
[28] |
|
WMS-III: Spatial span [54] |
Participants are presented with 12 blocks on which a sequence is tapped by the administrator. Participants must tap the blocks in the order requested by the administer (either reverse or same order) |
[7,15,29] |
|
|
Short recognition memory test for faces [58] |
Participants are presented with 25 grey scale faces of male actors at a rate of 1 face every 3 seconds. Participants decide (using a forced choice option) whether the image presented is pleasant or unpleasant immediately post stimulus onset. Each stimulus item is paired with a distractor item |
[41] |
|
|
4. Speed of processing (reported by eight studies) |
Trail Making Test A (TMT A) [59] |
In this test, numbers a placed irregularly on a sheet of paper, which participants are instructed to join correctly? This is a timed pencil and paper test |
[6,15,28,29,43] |
|
|
Trail Making Test B (TMT B) [59] |
In part B, participants are presented with numbers and letters in random order, which they connect in alternating order |
[28,43] |
|
|
Brief Assessment of Cognition in Schizophrenia (BACS): Symbol coding [60] |
This is a timed test in which participants are required to write down the digit corresponding to nonsense symbols within 90 seconds |
[6,15] |
|
|
WAIS-III Digit symbol substitution sub-test [55] |
Participants are presented with a series of numbers and symbols in a grid. Participants reproduce symbolscorresponding to the numbers in the grid within a 120 second time limit |
[29] |
|
|
Category fluency-animal subtest [61] |
In this test, participants are instructed to generate exemplars of animals within 60 seconds. The total number of true animal exemplars within the time frame is measured |
[6,15,29] |
|
|
Five-point test [62] |
There are two parts to this test: Verbal and non-verbal. In the verbal test participants must make words that begin with a specific letter (e.g., “A”) within three minutes. Participants are instructed not to produce nouns or repeat words |
[14] |
|
|
Repeatable battery for the assessment of neuropsychological status-coding sub-set [63] |
A page filled with symbols is presented to participants. Each symbol corresponds to a number on top of the page. Participant must match the symbol to its corresponding number within 90 seconds |
[25] |
|
5. Cognitive flexibility (reported by Three studies) |
Stroop test [64] |
There are three sub-tests to this task: The colour word sub-test in which participants must read the colour of the word presented in black ink. The colour name sub-test, in which participants must name the colour of the triangle, and in the interference sub-test, participants ignore the word they see and say the colour of the word (e.g. if the word black is written in the colour red, then the correct answer would be red) |
[14,16] |
|
|
Delis-kaplan executive function scale-colour word interference sub-test [65] |
In this test, a participant must inhibit a dominant and automatic verbal response of a word presented, and instead, name the colour of the ink for the word presented |
[24] |
|
6. Reasoning and problem solving (reported by six studies) |
Tower of London Test [66] |
Participants are presented with coloured beads arranged vertically on pegs of different heights. How they must be arranged,andthe number of moves allowed is determined by the experimenter, which they (the participant) must follow in order to achieve a specific arrangement |
[21] |
|
|
Neuropsychological assessment battery: Mazes sub-test [67] |
Participants are presented with seven mazes, each increasing in difficulty. Participants complete each maze within a 300-second time limit |
[6,15] |
|
|
WAIS-III-Matrix reasoning sub-test [55] |
Participants are presented with different figures. Each figure must be analysed in order to determine whichfigurebest fits theorder of the sequence presented |
[29] |
|
|
Wisconsin card sorting test [68] |
Participants sort a series of cards by a specific rule (e.g. by colour, shape or number of shapes). Feedback on performance is provided. After ten correct sorts, the rules for sorting are changed to a new rule without warning |
[18,19] |
|
7. Visual learning (reported by three studies) |
Brief visuospatial memory test-revised [69] |
The instructor presents six geometric figures which participants reproduce from memory |
[6,15] |
|
|
The Judgement of Line Orientation test [70] |
Participants are presented with two angle lines. They are instructed to matchtheseto a set of 11 lines by re-arranging them so that all the lines are 18 degrees apart and form a semi-circle |
[21] |
Table S3: List of studies included in meta-analysis measuring neurocognition in schizophrenia and healthy controls.
|
Noncognitive Domain |
Neuro-cognitive Measures |
Description of Task |
Studies in Meta-Analysis |
|
Pre-morbid verbal IQ (reported by 13 studies) |
Multiple-choice vocabulary test (german version) [71] |
This measure presents 37 rows of five words. From each row participants pick the actual word and rule out the pseudo-words. The number of correctly identified words provides the test result |
[8,9,13,18,26-28] |
|
National adult reading test [72] |
This test comprises of 50 words with irregular spellings (e.g. aisle). Participants are assessed on their vocabulary comprehension rather than their ability to apply regular pronunciation rules |
[11,22,24,25,32,39] |
|
Verbal IQ (reported by two studies) |
WAIS-III- verbal subset [55] |
In this test, participants name the object in the picture or define the words presented to them |
[12,19] |
Table S4: List of the studies in the meta-analysis measuring verbal comprehension in schizophrenia and healthy controls.
|
Neuro-cognitive Domain |
Neurocognitive Measures |
Studies in Meta-Analysis |
Description of General IQ Tests |
|
General IQ (reported by 10 studies) |
Wechsler abbreviated adult intelligence scale [73] |
[12,15,37] |
The many versions of the Wechsler’s Adult Intelligence Scales measure a person’s ability to act purposefully, reason and deal effectively with his/her surrounding/environment [74]. This aim is fulfilled using several verbal ability and cognitive reasoning/style sub-tests (for a detailed description of each sub-test refer to Wechsler’s administration manual and scales [55,75,73,54,57] |
|
|
Wechsler Adult Intelligence Scale III [55] |
[2,5,7,35] |
|
|
Wechsler adult intelligence scale-IV [75] |
[34] |
|
|
|
Raven’s progressive matrices test [76] |
[21] |
This is a non-verbal group test designed to measure abstract reasoning |
|
|
Groninger Intelligence Test [77] |
[43] |
This test is used in the Netherlands as a reliable alternative to the Wechsler Adult Intelligence Tests. As such, this test includes examining the same cognitive and verbal abilities as the WAIS sub-tests [55] |
Table S5: List of studies in the meta-analysis that examined general IQ.
|
k |
B |
SE |
95% CI |
Z |
P |
I2 |
|
|
Age at Diagnosis |
20 |
-0.06 |
0.018 |
[-0.09, -0.02] |
-3.35 |
0.0008 |
28.70 |
|
Duration of Illness |
29 |
0.012 |
0.008 |
[0.001,0.03] |
2.11 |
0.03 |
30.55 |
|
Positive symptoms |
33 |
0.008 |
0.008 |
[-0.007,0.024] |
1.004 |
0.31 |
30.73 |
|
Negative Symptom severity |
33 |
0.004 |
0.007 |
[-0.009,0.01] |
0.67 |
0.50 |
30.04 |
|
General Symptom Severity |
15 |
0.01 |
0.01 |
[-0.01,0.03] |
1.06 |
0.28 |
5.04 |
|
CPZ-Equivalent -mg/day |
16 |
-0.0005 |
0.0006 |
[-0.001,0.0008] |
-0.77 |
0.43 |
13.50 |
Table S6: Moderating effect of clinical characteristics on the difference in performance between schizophrenia patients and healthy controls on cognitive empathy.
Note: K = Number of studies. B = regression coefficient. SE = standard error. 95% CI = 95% confidence interval. Z = indicates the extent of uncertainty in the regression coefficient. p = statistical significance, 2-tailed. I2 indicates the amount of between-study heterogeneity. CPZ-equivalent-mg/day = Chlorpromazine Equivalent, milligram per day.
|
Affective Empathy |
k |
B |
SE |
95% CI |
Z |
P |
I2 |
|
General IQ, Schizophrenia Spectrum Disorders |
9 |
0.003 |
0.003 |
[-0.002,0.010] |
1.12 |
0.25 |
4.98 |
|
Verbal IQ, Schizophrenia Spectrum Disorders |
15 |
0.0001 |
0.002 |
[-0.005,0.005] |
0.04 |
0.96 |
12.51 |
|
Global Neuro-cognition, Schizophrenia Spectrum Disorder |
11 |
-0.004 |
0.008 |
[-0.020,0.012] |
-0.52 |
0.60 |
6.54 |
Note: K = Number of studies. B = regression coefficient. SE = standard error. 95% CI = 95% confidence interval. Z = indicates the extent of uncertainty in the regression coefficient. p = statistical significance, 2-tailed. I2 indicates the amount of between-study heterogeneity. CPZ-equivalent-mg/day = Chlorpromazine Equivalent in milligram per day
|
Affective Empathy |
k |
B |
SE |
95% CI |
Z |
P |
I2 |
|
Age, Schizophrenia spectrum |
35 |
0.007 |
0.009 |
[-0.01,0.02] |
0.79 |
0.42 |
32.38 |
|
Lower Education in Schizophrenia compared to Healthy Controls |
21 |
-0.04 |
0.05 |
[-0.15,0.06] |
-0.75 |
0.45 |
18.47 |
|
Higher Proportion of Male Schizophreniathen Female Schizophrenia |
28 |
0.003 |
0.005 |
[-0.006, 0.01] |
0.71 |
0.47 |
26.14 |
|
Higher Proportion of Non-Caucasian Schizophrenia then Caucasian Schizophrenia |
7 |
-0.0004 |
0.006 |
[-0.01,0.01] |
0-0.06 |
0.94 |
5.05 |
|
Year of Study Publication |
36 |
-0.02 |
0.02 |
[-0.06,0.02] |
-1.009 |
0.31 |
33.49 |
Note: K = Number of studies. B = regression coefficient. SE = standard error. 95% CI = 95% confidence interval. Z = indicates the extent of uncertainty in the regression coefficient. p = statistical significance, 2-tailed. I2 indicates the amount of between-study heterogeneity. CPZ-equivalent-mg/day = Chlorpromazine Equivalent in milligram per day
|
Affective Empathy |
k |
B |
SE |
95% CI |
Z |
P |
I2 |
|
Age at Diagnosis |
21 |
-0.02 |
0.02 |
[-0.08, 0.02] |
-1.01 |
0.31 |
23.26 |
|
Duration of Illness |
32 |
0.007 |
0.01 |
[-0.01,0.02] |
0.68 |
0.49 |
33.15 |
|
Severity of Positive Symptom |
36 |
0.012 |
0.007 |
[-0.001,0.02] |
1.68 |
0.09 |
39.85 |
|
Severity of Negative Symptoms |
36 |
0.007 |
0.005 |
[-0.002,0.01] |
1.52 |
0.12 |
39.60 |
|
Severity of General symptom |
17 |
-0.004 |
0.01 |
[-0.03,0.02] |
-0.30 |
0.76 |
16.32 |
|
CPZ-Equivalent Mg/Day |
18 |
-0.00015 |
0.0006 |
[-0.001,0.001] |
-0.24 |
0.80 |
4.97 |
Note: K = Number of studies. B = regression coefficient. SE = standard error. 95% CI = 95% confidence interval. Z = indicates the extent of uncertainty in the regression coefficient. p = statistical significance, 2-tailed. I2 indicates the amount of between-study heterogeneity. CPZ-equivalent-mg/day =Chlorpromazine Equivalent in milligram per day.
|
Affective Empathy |
k |
B |
SE |
95% CI |
Z |
P |
I2 |
|
General IQ, Schizophrenia |
10 |
-0.0005 |
0.003 |
[-0.007,0.005] |
-0.17 |
0.85 |
54.02 |
|
Pre-morbid/Verbal IQ, Schizophrenia |
15 |
-0.00009 |
0.002 |
[-0.005,0.005] |
-0.03 |
0.97 |
14.22 |
|
Global Neuro-cognition, Schizophrenia |
13 |
-0.005 |
0.005 |
[-0.016,0.005] |
-1.03 |
0.29 |
7.01 |
Note: K = Number of studies. B = regression coefficient. SE = standard error. 95% CI = 95% confidence interval. Z = indicates the extent of uncertainty in the regression coefficient. p = statistical significance, 2-tailed. I2 indicates the amount of between-study heterogeneity. CPZ-Equivalent-mg/day = Chlorpromazine Equivalent in milligram per day
|
Affective Empathy |
k |
B |
SE |
95% CI |
Z |
P |
I2 |
|
Age, Schizophrenia |
36 |
0.01 |
0.01 |
[-0.005,0.03] |
1.43 |
0.15 |
39.60 |
|
Fewer Years in Education in Schizophrenia compared to Healthy Controls |
23 |
-0.04 |
0.07 |
[-0.19,0.10] |
-0.55 |
0.57 |
25.46 |
|
Higher Proportion of Male Schizophrenia then Female Schizophrenia |
29 |
-0.001 |
0.006 |
[-0.01,0.01] |
-0.30 |
0.76 |
31.43 |
|
Higher Proportion of Non-Caucasian Schizophrenia compared to Caucasian Patients |
7 |
0.005 |
0.007 |
[-0.009,0.021 |
0.74 |
0.45 |
5.28 |
|
Year of Study Publication |
39 |
-0.04 |
0.02 |
[-0.09,0.008] |
-1.65 |
0.09 |
41.16 |
Note: K = Number of studies. B = regression coefficient. SE = standard error. 95% CI = 95% confidence interval. Z= indicates the extent of uncertainty in the regression coefficient. p = statistical significance, 2-tailed. I2 indicates the amount of between-study heterogeneity. CPZ-equivalent-mg/day =Chlorpromazine Equivalent in milligram per day
REFERENCES
- Davis MH (1980) A multidimensional approach to individual differences in empathy. Diss Abstr Int 40: 3480.
- Achim AM, Ouellet R, Roy MA, Jackson PL (2011) Assessment of empathy in first-episode psychosis and meta-analytic comparison with previous studies in schizophrenia. Psychiatry Res 190: 3-8.
- Andrews SC, Enticott PG, Hoy KE, Fitzgerald PB (2013) Mirror systems and social cognition in schizophrenia. Schizophr Bull 39: 218.
- Brown, EC, Gonzalez Liencres, C, Tas, C, Brune M (2016) Reward modulates the mirror neuron system in schizophrenia: A study into the mu rhythm suppression, empathy, and mental state attribution. Soc Neurosci 11: 175-186.
- Chiang SK, Hua MS, Tam WCC, Chao JK, Shiah YJ (2014) Developing an alternative Chinese version of the interpersonal reactivity index for normal population and patients with schizophrenia in Taiwan. Brain Impair 15: 120-131.
- Corbera S, Wexler BE, Ikezawa S, Bell MD (2013) Factor structure of social cognition in schizophrenia: is empathy preserved? Schizophrenia Research and Treatment 1-13.
- Corbera S, Cook K, Brocke S, Dunn S, Wexler BE, et al. (2014) The relationship between functional deficits and empathy for emotional pain in schizophrenia. Biol Psychiatry 75: 200.
- Derntl B, Finkelmeyer A, Voss B, Eickhoff SB, Kellermann T, et al. (2012) Neural correlates of the core facets of empathy in schizophrenia. Schizophr Res 136: 70-81.
- Derntl B, Seidel EM, Schneider F, Habel U (2012) How specific are emotional deficits? A comparison of empathic abilities in schizophrenia, bipolar and depressed patients. Schizophr Res 142: 58-64.
- Fischer Shofty M, Brüne M, Ebert A, Shefet D, Levkovitz Y, et al. (2013) Improving social perception in schizophrenia: The role of oxytocin. Schizophr Res 146: 357-362.
- Fujino J, Takahashi H, Miyata J, Sugihara G, Kubota M, et al. (2014) Impaired empathic abilities and reduced white matter integrity in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 48: 117-123.
- Fujiwara H, Shimizu M, Hirao K, Miyata J, Namiki C, et al. (2008) Female specific anterior cingulate abnormality and its association with empathic disability in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 32: 1728-1734.
- Gizewski ER, Müller BW, Scherbaum N, Lieb B, Forsting M, et al. (2013) The impact of alcohol dependence on social brain function. Addict Biol 18: 109-120.
- Haker H, Rössler W (2009) Empathy in schizophrenia: Impaired resonance. Eur Arch Psychiatry Clin Neurosci 259: 352-361.
- Hooker CI, Bruce L, Lincoln SH, Fisher M, Vinogradov S (2011) Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biol Psychiatry 70: 1169-1178
- Horan WP, Pineda JA, Wynn JK, Iacoboni M, Green MF (2014) Some markers of mirroring appear intact in schizophrenia: Evidence from mu suppression. Cogn Affect Behav Neurosci 14: 1049-1060.
- Horan WP, Reise SP, Kern RS, Lee J, Penn DL, et al. (2015) Structure and correlates of self-reported empathy in schizophrenia. J Psychiatr Res 66-67: 60-66.
- Lehmann A, Bahcesular K, Brockmann EM, Biederbick, SE, Dziobek I, et al. (2014) Subjective experience of emotions and emotional empathy in paranoid schizophrenia. Psychiatry Res 220: 825-833.
- Lee SJ, Kang DH, Kim CW, Gu BM, Park JY, et al. (2010) Multi-level comparison of empathy in schizophrenia: An fMRI study of a cartoon task. Psychiatry Res 181: 121-129.
- Lee J, Zaki J, Harvey PO, Ochsner K, Green MF (2011) Schizophrenia patients are impaired in empathic accuracy. Psychol Med 41: 2297-2304.
- Lam BY, Raine A, Lee TM (2014) The relationship between neurocognition and symptomatology in people with schizophrenia: Social cognition as the mediator. BMC Psychiatry 14: 138.
- Matsumoto Y, Takahashi H, Murai T, Takahashi H (2015) Visual processing and social cognition in schizophrenia: Relationships among eye movements, biological motion perception, and empathy. Neurosci Res 90: 95-100.
- McCormick LM, Brumm MC, Beadle JN, Paradiso S, Yamada T, et al. (2012) Mirror neuron function, psychosis, and empathy in schizophrenia. Psychiatry Res 201: 233-239.
- McGuire J, Barbanel L, Brüne M, Langdon R (2015) Re-examining Kohlberg,s conception of morality in schizophrenia. Cogn Neuropsychiatry 20: 377-381.
- McGuire J, Brüne M, Langdon R (2017) Judgment of moral and social transgression in schizophrenia. Compr Psychiatry 76: 160-168.
- Montag C, Heinz A, Kunz D, Gallinat J (2007) Self-reported empathic abilities in schizophrenia. Schizophr Res 92: 85-89.
- Montag C, Brockmann EM, Lehmann A, Muller DJ, Rujescu D, et al. (2012) Association between oxytocin receptor gene polymorphisms and self-rated ‘empathic concern’ in schizophrenia. PLoS One 7: 51882.
- Regenbogen C, Kellermann T, Seubert J, Schneider DA, Gur RE, et al. (2015) Neural responses to dynamic multimodal stimuli and pathology-specific impairments of social cognition in schizophrenia and depression. Br J Psychiatry 206: 198-205.
- Smith MJ, Horan WP, Cobia DJ, Karpouzian TM, Fox JM, et al. (2014) Performance-based empathy mediates the influence of working memory on social competence in schizophrenia. Schizophr Bull 40: 824-834.
- Singh S, Modi S, Goyal S, Kaur P, Singh N, et al. (2015) Functional and structural abnormalities associated with empathy in patients with schizophrenia: An fMRI and VBM study. J Biosci 40: 355-364.
- Shamay-Tsoory SG, Shur S, Harari H, Levkovitz Y (2007) Neurocognitive basis of impaired empathy in schizophrenia. Neuropsychology 21: 431-438.
- Sparks A, McDonald S, Lino B, O'Donnell M, Green MJ (2010) Social cognition, empathy and functional outcome in schizophrenia. Schizophr Res 122: 172-178.
- Thirioux B, Tandonnet L, Jaafari N, Berthoz A (2014) Disturbances of spontaneous empathic processing relate with the severity of the negative symptoms in patients with schizophrenia: A behavioural pilot-study using virtual reality technology. Brain Cogn 90: 87-99.
- Vistoli D, Lovoie, MA, Sutliff S, Jackson PL, Achim AM (2017) Functional MRI examination of empathy for pain in people with schizophrenia reveals abnormal activation related to cognitive perspective-taking but typical activation linked to affective sharing. J Psychiatry Neurosci 42: 262-272.
- Wojakiewicz A, Januel D, Braha S, Prkachin K, Danziger N, et al. (2013) Alteration of pain recognition in schizophrenia. Eur J Pain 17: 1385-1392.
- Baron-Cohen S, Wheelwright S (2004) The empathy quotient: An investigation of adults with asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord 34: 163-175.
- Didehbani N, Shad MU, Tamminga CA, Kandalaft MR, Allen TT, et al. (2012) Insight and empathy in schizophrenia. Schizophr Res 142: 246-247.
- Reniers RL, Corcoran R, Drake R, Shryane NM, Völlm BA (2011) The QCAE: A questionnaire of cognitive and affective empathy. J Pers Assess 93: 84-95.
- Berrada-Baby Z, Oker A, Courgeon M, Urbach M, Bazin N, et al. (2016) Patients with schizophrenia are less prone to interpret virtual others’ empathetic questioning as helpful. Psychiatry Res 242: 67-74.
- Mehrabian A (2000) Manual for the Behavioural Emotional Empathy Scale (BEES). Monterey, CA, USA.
- Kucharska-Pietura K, Tylec A, Czernikiewicz A, Mortimer A (2012) Attentional and emotional functioning in schizophrenia patients treated with conventional atypical antipsychotic drugs. Med Sci Monit: 18: 44-49.
- Mehrabian A, Epstein N (1972) A measure of emotional empathy. J Pers 40: 525-543.
- Pijnenborg GHM, Spikman JM, Jeronimus BF, Aleman A (2013) Insight in schizophrenia: Associations with empathy. Eur Arch Psychiatry Clin Neurosci 26: 299-307.
- Ramos-Loyo J, Mora-Reynoso L, Sanchez-Loyo LM, Medina-Hernandez V (2012) Sex differences in facial, prosodic and social context emotional recognition in early-onset schizophrenia. Schizophr Res Treatment: 1-12.
- Kay SR, Fisbein A, Opler LA (1987) The positive and negative symptom scale (PANSS) for schizophrenia. Schizophr Bull 13: 261-276.
- Andreasen NC (1984) Scale For The Assessment Of Positive Symptoms (SAPS). University of Iowa, Iowa, USA.
- Andreasen NC (1983) Scale for the assessment of negative symptoms (SANS). Iowa City, Iowa, USA.
- Overall JE, Gorham DR (1962) The brief psychiatric rating scale. Psychological Reports 10: 799-812.
- Robertson IH (1994) The test of everyday attention. Thames Valley Test Company, England, UK.
- Cornblatt B, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L (1988) The Continuous Performance Test, Identical Pairs Version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res 26: 223-238.
- Brandt J (1991) The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clinical Neuropsychologist 5: 125-142.
- Heubrock D (1994) Auditiv-verbales Lernenunter standard-isierten Bedingungen: Erste deutsche Normenfür 16- bis26-jährige Männer und Frauen zum Auditiv-VerbalenLerntest (AVLT) [Standardized auditory-verbal learning: Preliminary German Auditory-Verbal Learning Test (AVLT) norms for amale and female population aged 18 to 26]. J Individ Diff 15: 65-76.
- Delis DC, Kramer JH, Kaplan E, Ober BA (2000) California Verbal Learning Test Manual: Second edition, adult version. Pearson, London, UK.
- Wechsler D (1997) WMS-III: Wechsler Memory Scale Administration and Scoring Manual. Psychological Corporation, San Antonio, USA.
- Wechsler D (1997) Wechsler Adult Intelligence Scale - 3rd Edition (WAIS-III). The Psychological Corporation, San Antonio, USA.
- Randolph C (2012). RBANS Update : Repeatable Battery for the Assessment of Neuropsychological Status. NSC Pearson, Bloomington, Indiana.
- Wechsler D (1955) Manual for the Wechsler Adult Intelligence Scale. Psychological Corporation, New York, USA.
- Warrington E (1996) The Camden Memory Tests: Short Recognition Memory Test for Faces (Volume 5). Psychology Press, New York, USA.
- Reitan RM (1955) The relation of the Trail Making Test to organic brain damage. J Consult Psycho 19: 393-394.
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, et al. (2004) The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 68: 283-297.
- Benton AL, Hamsher deS, Sivan AB (1994) Multilingual Aphasia Examination, Third Edition. Psychological Assessment Resources, Inc, Iowa, USA.
- Regard M, Strauss E, Knapp P (1982) Children’s production on verbal and non-verbal fluency tasks. Percept Mot Skills 55: 839-844.
- Randolph C (1999). Repeatable Battery for the Assessment of Neuropsychological Status. Psychological Corporation, Bloomington, Indiana.
- Stroop JR (1935) Studies of interference in serial verbal reactions. Journal of Experimental Psychology 18: 643-662.
- Delis DC, Kaplan E, Kramer J (2001) Delis–Kaplan executive function scale. The Psychological Corporation, San Antonio, USA.
- Shallice T (1982) Specific impairments of planning. In: Broadbent DE, Weiskrantz L, (eds.). The neuropsychology of cognitive function. The Royal Society, London, UK.
- Stern RA, White T (2003) Neuropsychological Assessment Battery. Psychological Assessment Resources Inc, Florida, USA.
- Grant DA, Berg EA (1948) A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J Exp Psychol, 38: 404-411.
- Benedict RHB (1997) Brief visuospatial memory test-revised: Professional manual. Psychological Assessment Resources Inc, Florida, USA.
- Benton AL, Sivan AB, deS Hamsher K, Varney NR, Spreen O (1994) Contributions to neuropsychological assessment. Oxford University Press Inc, New York, USA.
- Lehrl S, Triebig G, Fischer B (1995) Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand 91: 335-345.
- Nelson HE (1991) National Adult Reading Test. NFER-Nelson, Windsor, UK.
- Psychological Corporation Harcourt Brac (1999) WASI Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation Harcourt Brace, New York, USA.
- Wechsler D (1958) The measurement of adult intelligence. Baltimore: William & Wilkins, Literary Licensing, Maryland, USA.
- Wechsler D (2008) WAIS-IV administration and scoring manual. PsychCorp, San Antonio, USA.
- Raven J, Raven JC, Court JH (1998) Manual for Raven's progressive matrices and vocabulary scales. Oxford Psychologists Press, Oxford, UK.
- Luteijn F (1983) GIT Groninger Intelligentie Test. Swets & Zeitlinger bv, California, US
Citation: Varachhia S, Ferguson E, Doody GA (2018) A Meta-Analysis Taxonomizing Empathy in Schizophrenia. J Psychiatry Depress Anxiety 4: 016.
Copyright: © 2018 Sumayyah Varachhia, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

