
Factors Related with Frontal Dysfunction in Early Stages of Parkinson Disease
*Corresponding Author(s):
Julio López ArgüellesDepartament Of Neurology, University Hospital Gustavo Aldereguía Lima, Cuba
Tel:+53 54218333,
Email:juliola@jagua.cfg.sld.cu, julito.arguelles@gmail.com
Abstract
Introduction
Parkinson Disease (PD) although described initially by James Parkinson as a disease with motor disorder it has been demonstrated that the cognitive disorders in the form of disejecutive syndrome are frequent worsening with the evolution. Objective: To characterize the Frontal Dysfunction (FD) in the patients with PD and to determine the factors related with frontal dysfunction in early stages.
Method
We studied 125 patients with diagnosis of idiopathic PD and Hoehn and Yahr Stages <2, to those which it was carried to them out survey with demographic, clinical and neuropsychological data studies included the Frontal Assessment Battery (FAB).
Results
The mean age was of 68.1±8.6, the onset age was of 62.6±10.5, the diestrums predominated and those which initiated with tremor. Of the 125 patients 71.4% presented FD, with an average of the FAB of 11.82±3.7. The age (R=-0.45; p<0.001) and onset age (R=-0.33; p=0.02) showed inversely proportional correlation with the FD. Other related variables were the schooling up to second level (p=0.003) and the rural origin with significance <0.001.
Conclusion
The age and onset age higher than 60 years, the lower schooling than second level of teaching, rural origin and the presence of cognitive dysfunction are related to FD in early stages of PD.
Keywords
INTRODUCTION
Parkinson Disease (PD) was described for first time in 1817 by James Parkinson in their monograph of 66 pages An Essay on the shaking palsy [1]. PD kept as the second degenerative disorder of the central nervous system after Alzheimer disease [2-4]. Although was initially described as a disease with motor disorder has been demonstrated that the cognitive disorders in the form of disejecutive syndrome are frequent in PD worsening with the evolution [5-13]. The patients have difficulty in maintaining adaptive responses with visuospatials and visuoperceptuals deficits, who leads to alteration of the memory of work and attentional deficits [14]. Has been demonstrated that the signs of frontal disease are well represented in subcortical pattern dementias, whose prototype can be PD [15-20]. With the present article we intended to characterize the frontal dysfunction in patients with PD and to determine the factors related with frontal dysfunction in early stages of PD.
METHODS
Procedure the study was carried out in two phases
In this phase an interview structured with clinical, sociodemographic data and risk factors for frontal dysfunction, where it will be included age, sex, race and other demographic data as well as it will be carried out the scale UPDRS motor and scale of Hoehn and Yahr in order to determine the stage of its disease.
Second phase (phase of neuropsychological study)
In this phase worked with the 125 patients, sample obtained then to be classified the patients according to the Stages of Hoehn and Yahr to those which were carried them out several neuropsychological tests: The Mini Mental State Test (MMSE), Montreal Cognitive Assessment (MoCA), depression scale of Hamilton or Ysavage for patients older than 60 years. For the assessment of the frontal dysfunction was in addition used the Frontal Assessment Battery (FAB).
Frontal Assessment Battery (FAB)
Cognitive deterioration
Statistical analysis
Consequently, during the planning of this research we will respect the ethical principles of research on human beings [12,13].
RESULTS
In table 1 we found the onset age 62.6±10.5 years old and the current age 68.1±8.6 years old, remain as well as the world average in the sixth decade of life, prevailing the form of beginning tremoric, in addition the time of evolution mean was of 5.6±4.3 years in spite of being in the first motor stages of the DP, with a mean of the motor UPDRS in On and Off that does not arrive at the 20 points.
The majority of the patients (71.4%) presented frontal lobe dysfunction (Graph 1).
|
Variables |
Mean±SD |
|
Age |
68.1±8.6 |
|
Onset age |
62.6±10.5 |
|
Time of evolution (years) |
5.6±4.3 |
|
UPDRS motor On |
14.7±6.0 |
|
UPDRS motor Off |
18.6±6.4 |
|
Clinical Variables of the Sample Given in Percentage |
|
|
Variables |
Percentage |
|
Clinical onset tremoric/Rigid-acinetic |
77.6/27.7 |
|
Diestrums/Left-handed |
81.6/2.6 |
|
Predominant symptom tremor/Rigidity |
53.7/46.3 |
|
Source: Conducted survey |
|
Table 1: Relation of the clinical variables of the sample.
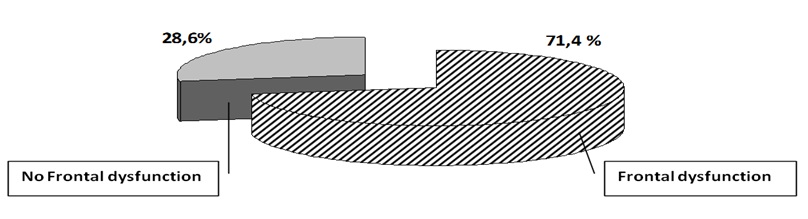 Graph 1: Distribution of the patients according to the frontal activity.
Graph 1: Distribution of the patients according to the frontal activity.
With regard to the variables related to the frontal dysfunction (Table 2) we found that the patients who presented frontal dysfunction had an average of age higher than those which remained with the adequate frontal functions (p=0.02), occurring the same with the variables that measure global cognitive alteration basically the MoCA where its values differ between groups presenting a mean of 16.9±4.3 (p<0.0001).The MMSE also showed a relevant difference between groups with an statistical significance (p=0.01).
|
|
Frontal Dysfunction |
Without Frontal Dysfunction |
p |
|
Age |
69.91±7.89 |
64.39±9.8 |
0.02 |
|
UPDRS motor On |
14.74±5.9 |
14.38±5.35 |
0.82 |
|
UPDRS motor Off |
18.41±6.23 |
18.50±5.9 |
0.96 |
|
MoCA |
16.9±4.3 |
21.56±2.77 |
<0.0001 |
|
MMSE |
22.17±3.4 |
24.13±2.09 |
0.01 |
|
Source: Conducted survey |
|||
Table 2: Relationship between the mean±SD of clinical/demographic variables and the presence of frontal dysfunction.
In table 3 we found that the average of the FAB was lower in the patients with rural origin (p<0.001), those which attended only up to the second level of teaching (10.9±3.9; p=0.003), onset age and current age older than 60 years with a significance of 0.02 and 0.025 respectively. Not occurring statistical significance among the stages I and II of Hoehn and Yahr although showed mean of the FAB lower in the stage I.
|
Variables |
Mean±SD |
p |
|
|
Age |
≤60 años |
14.30±2.7 |
0.025 |
|
> 60 años |
11.43±3.8 |
||
|
Onset age |
≤60 años |
13.22±2.8 |
0.02 |
|
> 60 años |
10.78±3.9 |
||
|
Origin |
Rural |
10.13±3.6 |
<0.001 |
|
Urban |
14.22±2.9 |
||
|
Schooling |
Up to second level |
10.9±3.9 |
0.003 |
|
After the second level |
13.95±2.7 |
||
|
Stage of Hoehn and Yahr |
STAGE I |
11.11±2.6 |
0.43 |
|
STAGE II |
12.21±3.9 |
||
|
Source: Conducted survey |
|||
Table 3: Relation of the mean±SD of the FAB and clinical/demographic variables according to t student.
Upon establishing the correlation among the clinical and demographic variables with the values of the FAB (Table 4) we find that the age (R=-0.45; p<0.001) and onset age (R=-0.33; p=0.002) showed an inversely proportional correlation in relation with regard to the values of the FAB. The schooling showed gave directly proportional values (R=0.43; p=0.001), that is to lower values of the schooling lower values of the FAB. The rest of the clinical variables did not show statistical significance including the evolution time.
|
m=11.82±3.7(a) |
R |
p |
|
Age |
-0.45** |
<0.001 |
|
Onset age |
-0.33 |
0.02 |
|
Schooling |
0.43 |
0.001 |
|
UPDRS motor On |
-0.08 |
0.6 |
|
UPDRS motor Off |
-0.1 |
0.9 |
|
Time of evolution (years) |
0.14 |
0.3 |
Table 4: Correlation of demographic and motor variables with the FAB’s scoring.
** The correlation is significant at level 0.01 (bilateral).
(a) Mean of the FAB ± Standard deviation.
In the graph 2 can see as the means items of the FAB, in all study’s pacients, that presented lower values were the fluidity and motor series, occurring the opposite with the behavior of compenzación with an almost equal average value to 3.00, that is maximum value.
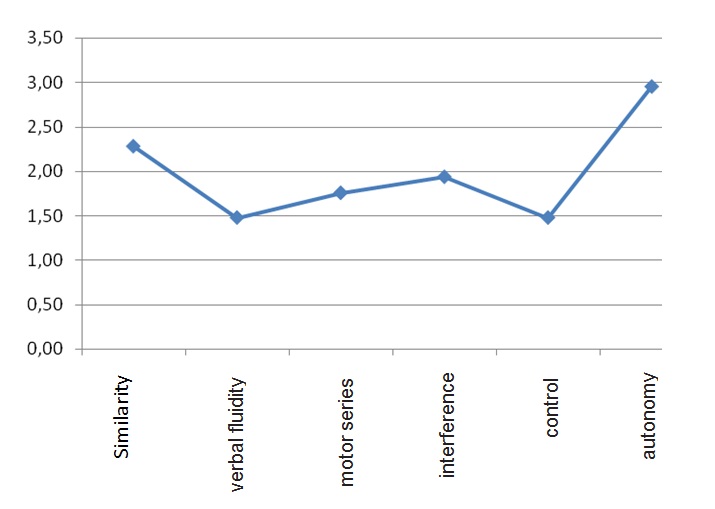 Graph 2: More affected frontal functions in early stage of Parkinson's disease (according to FAB).
Graph 2: More affected frontal functions in early stage of Parkinson's disease (according to FAB).
DISCUSSION
The present study emphasizes us that the frontal affectations are presented at onset of the PD.
Analyzing the demographic variables these do not differ from the reviewed studies that have dealt with widely this subject, being noteworthy that the majority of the patients present a schooling that does not surpass the secondary teaching bearing no relation this with the origin that presents both similar percentages for those which are of rural origin and urban [24].
The age is kept as one of the principal variables related to the frontal dysfunction, being able to be in relation this with which as advances the age occur cognitive alterations, although the average for this of our study was of 68.1 years, being noteworthy that the motor scales of the PD did not show a statistically significant relation, occurring the opposite with the neuropsychological scales that demonstrate the presence of global cognitive alteration, represented by the association of frontal dysfunction with the MoCA and MMSE what translates that in the beginning of the EP not only are affected prefrontal cortical areas, but also parietal areas and rising subcorticales systems, that they utilize other neurotransmitters; in addition to parts of the striate one as putamen, caudate or nucleus accumbens that is associated with the implicit learning of habits or of incentive response and sensoriomotor coordination, most adequate planifcación of each incentive [25,26].
Furthermore find that both the age and the onset age present an inversely proportional correlation, which is to greater age and greater onset age smaller scoring of the FAB and greater frontal dysfunction, with a greater significance for a point of cut off greater than 60 year [27,28].
With the results of this study is shown that the affectation in the frontal lobes comes together with the onset of the disease, then seem to be affected different cortico-subcortical circuits that act parallels and similar with regard to their structure and organization, affecting in this form the closed circuit that is originated in a private area of the frontal córtex that transmits the information through the basal nodes and returns to the place of departure in the lobe frontal.
Furthermore occurs specific disorder: In the motor circuits that leads to the classical acinesia or bradycinesia, the dorsolateral frontal circuit that is translated into a disejecutive syndrome with disability for the mental flexibility and the change of criteria, in planning and generation of strategy, in the organization of the actions, in the utilization of the experience and in the production of a spontaneous activity, in addition to the previous cingular circuit by the presence of the reduction of the initiative and the maintenance of the attention.
CONCLUSION
The present study demonstrates that already in early stages of PD occurs degeneration in different frontal cortical regions as the motor cortex, premotor, dorsolateral and cingular area. With variables that are related to the frontal dysfunction as the age and onset age above 60 years, the lower schooling than second level of teaching as well as the presence of generalized cognitive affectation.
ACKNOWLEDGEMENT
None.
CONFLICT OF INTEREST
The author declares there is no conflict of interest.
REFERENCES
- Parkinson J (1817) An Essay on the Shaking Palsy Londr, Sherwood, Neely and Jones.
- López-Argüelles J, Carbajal AB, García S, Sosa LM, et al. (2014) Cognitive deterioration in initial stage of Parkinson Disease. Rev Neuropsic neuropsiq neurociencias 14: 77-79.
- Kitayama M, Wada-Isoe K, Nakaso K, Irizawa Y, Nakashima K (2007) Clinical evaluation of Parkinson’s disease dementia: association with aging and visual hallucination. Acta Neurol Scand 116: 190-195.
- Ransmayr G (2007) Clinical criteria of Parkinson’s disease. Ther Umsch 64: 5-8.
- Kandiah N, Narasimhalu K, Lau PN, Seah SH, Au WL, et al. (2009) Cognitive decline in early Parkinson’s disease. Mov Disord 24: 605-608.
- Muslimovi? D, Post B, Speelman JD, De Haan RJ, Schmand B (2009) Cognitive decline in Parkinson’s disease: a prospective longitudinal study. J Int Neuropsychol Soc 15: 426-437.
- D'Amelio M, Ragonese P, Morgante L, Reggio A, Callari G, et al. (2006) Long-term survival of Parkinson’s disease: a population-based study. J Neurol 253: 33-37.
- Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV (1991) Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain 114: 2095-2122.
- Capriotti T, Terzakis K (2006) Parkinson Disease. Home Healthc Now 34: 300-307.
- Oliveira GN, Souza CP, Foss MP, Tumas V, (2015) An analysis of the cognitive items of the movement disorders society checklist for the diagnosis of dementia in patients with Parkinson’s disease. Parkinsonism Relat Disord 21: 1260-1263.
- Vera-Cuesta H, Vera-Acosta H, Varez-Gonzalez L, Fernandez-Maderos I, Casabona-Fernandez E (2006) Disfunción frontal en la enfermedad de Parkinson idiopática. Rev Neurol 42: 76-84.
- Manzini JL (2000) Declaración de Helsinki: Principios éticos para la investigación médica sobre sujetos humanos. Acta bioeth 6: 321-334.
- Asociacin Americana de Psiquitría. Manual diagnóstico y estadístico de los trastornos mentales. Barcelona: Masson; 2002.
- Toribio-Diaz ME, Carod-Artal FJ (2015) Subtipos de deterioro cognitivo leve en la enfermedad de Parkinson y factores predictores de conversión a demencia. Rev Neurol 61: 14-24.
- Lannuzel A, Hoglinger GU, Verhaeghe S, Gire L, Belson S, et al. (2007) Atypical parkinsonism in Guadeloupe: a common risk factor for two closely related phenotypes? Brain 130: 816-827.
- Moorhouse P, Gorman M, Rockwood K (2009) Comparison of EXIT-25 and the Frontal Assessment Battery for evaluation of executive dysfunction in patients attending a memory clinic. Dement Geriatr Cogn Disord 27: 424-428.
- Nagata T, Ishii K, Ito T, Aoki K, Ehara Y, et al. (2009) Correlation between a reduction in Frontal Assessment Battery scores and delusional thoughts in patients with Alzheimer's disease. Psychiatry Clin Neurosci 63: 449-454.
- Thabit H, Kennelly SM, Bhagarva A, Ogunlewe M, McCormack PM, et al. (2009) Utilization of Frontal Assessment Battery and Executive Interview 25 in assessing for dysexecutive syndrome and its association with diabetes self-care in elderly patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 86: 208-212.
- Lima CF, Meireles LP, Fonseca R, Castro SL, Garrett C (2004) The Frontal Assessment Battery (FAB) in Parkinson's disease and correlations with formal measures of executive functioning. J Neurol 255: 1756-1761.
- Jódar-Vicente M (2004) Funciones cognitivas del lóbulo frontal. Rev Neurol 39: 179-182.
- Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinsin’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: 181-184.
- Dubois B, Slachevsky A, Litvan I, Pillon B (2000) The FAB: a frontal assessment battery at bedside. Neurology 55: 1621-1626.
- Rodriguez del AA, Catalan Alonso MJ, Carrasco ML (2003) [FAB: a preliminar Spanish application of the frontal assessment battery to 11 groups of patients]. Rev Neurol 36: 605-608.
- Kummer A, Harsányi E, Dias FM, Cardoso F, Caramelli P, et al. (2009) Depression impairs executive functioning in Parkinson disease patients with low educational level. Cogn Behav Neurol 22: 167-172.
- Braak H, Rüb U, Jansen Steur EN, Del TK, de Vos RA (2005) Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology 64: 1404-1410.
- Peng D, Shi Z, Xu J, Shen L, Xiao S, et al. (2016) Demographic and clinical characteristics related to cognitive decline in Alzheimer disease in China: A multicenter survey from 2011 to 2014. Medicine (Baltimore) 95: 3727.
- Zhang S, Ou R, Chen X, Yang J, Zhao B, et al. Correlative factors of cognitive dysfunction in PD patients: a cross-sectional study from Southwest China. Neurol Res 38: 434-440.
- Bocanegra Y, Trujillo-Orrego N, Pineda D (2014) Dementia and mild cognitive impairment in Parkinson's disease: A review. Rev Neurol 59: 555-569.
Citation: Argüelles JL, Carvajal ABR, Alba GG, Aguila LMS, Manso LM (2019) Factors Related with Frontal Dysfunction in Early Stages of Parkinson Disease. J Alzheimers Neurodegener Dis 5: 018.
Copyright: © 2019 Julio López Argüelles, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

