
A Comparative Study for the Effects of Laboratory and Commercially Prepared Plum Extracts on Colon-26 Adenocarcinoma Cells
*Corresponding Author(s):
Rafat A SdidiquiFood Chemistry And Nutrition Science Laboratory, Agriculture Research Station, College Of Agriculture, Virginia State University, Virginia, United States
Tel:+1 8045245957,
Email:rsiddiqui@vsu.edu
Abstract
Methods
Results
Conclusion
Keywords
INTRODUCTION
The digestive tract is made up of the esophagus, stomach, and the small and large intestines. The colon is part of the body’s digestive tract which processes nutrients from foods and helps pass waste material out of the body. The colon is the first part of the large intestine and is about 5 feet long. Colorectal cancer (CRC) begins when the process of normal replacement of the lining cells of the colon is deregulated. Errors in normal checks and balances that control growth during cell division transform a normal cell causing it to divide and grow uncontrollably and contribute to growth within the colon known as polyps. Polyps are precancerous lumps that grow slowly over a long period; however, the subsequent genetic mutations in these cells make then invasive and malignant [1]. Colorectal cancers are the third most common causes of cancers in the United States in both women and men. For 2018, the American Cancer Society’s estimates 97,220 new cases of colon cancer and 43,030 new cases of rectal cancer which is expected to cause about 50,630 deaths. (https://cancerstatisticscenter.cancer.org). Approximately 95% of all CRCs occur in people age 45 and above. According to the National Cancer Institute, the median age of individuals diagnosed with the disease is 69 years [2]. The mortality and incidence rates are 40% and 30% in men than in women, respectively even though the lifetime risks of CRC is similar in both cases (4.7% vs. 4.3%) [2].
There are several risk factors for developing CRC. It is believed that environmental factors or inherited genes or the combination of both may increase the chances of an individual to develop CRC [3]. Eating food that is high in processed or red meat (e.g., hot dogs, lamb, and beef) increases an individual’s chances of initiating CRC. Broiling, grilling, frying or other techniques of cooking meat at extreme temperatures create chemicals that could also increase the risk for developing CRC [4]. Free radicals are produced during metabolism, which are highly reactive to cellular proteins, lipids, and nucleic acid and cause cellular damage. The most sensitive of all is nucleic acids whose reaction with free radicals causes changes in genetic structures and that can progress to cancer. People who embrace a sedentary lifestyle have high chances of develop CRC whereas people involve in physical activities have a reduced risk [5]. Smoking is generally associated with lung cancer; however, is also linked with other types of cancer including CRC [6]. The prevention of CRC is generally based on the modifiable risk factors which include maintenance of reasonable degree of physical exercises, avoiding weight gain, reducing alcohol intake, avoidance of smoking, and overall healthy dietary patterns. A number of dietary agents such as consumption of fruits and vegetables can help prevent the initiation and/or progression of CRC [7].
Various dietary micronutrients such as Vitamins A, C, E, beta carotene and selenium have been proposed to have anti-carcinogenic effects, based on their anti-inflammatory or anti-oxidant properties. Anti-oxidants neutralize these free radicals and, therefore, minimize the cellular damage and thus reduce the risk of developing cancer. A plum is a delicious and juicy fruit that belongs to the genus Prunes and family Rosacea. The fruit contains assortments of healthy minerals and vitamins. They are excellent source of vitamins A, K, C, and folate. They are also good source if thiamine, riboflavin, niacin and alpha-tocopherol. The available minerals in this fruit include zinc, calcium, iron, magnesium, phosphorus, fluoride and potassium. Plums also help in supplying dietary fiber as well as other low calories to the body without any risky fats. Plums are also a rich source of bioactive polyphenol including chlorogenic acids, anthocyanins, zeaxanthin and kryptoxanthin, catechins, and quercetin which contribute to their anti-oxidation and anti-inflammatory activities and also induce powerful anti-cancer effects [8,9]. The above observations suggest that plums can be very effective in preventing cancer from different origins, including CRC. The aim of present investigation was to study the effect of plum’s extract on colon cancer cells. We Hypothesize that plums because of their high content of bioactive polyphenols with anti-oxidation activity can effectively inhibit the proliferation of colon cancer cells. We used commercially available standardized preparation of plum extract named “PE60”, which contained approximately 60% polyphenols to test its anti-oxidation and anti-cancer properties and compared to that of laboratory prepared extracts from plum’s skin and pulp. Our data suggest that amounts of polyphenols in plum extracts are important for their anti-cancer activity in colon cancer.
METHODS AND MATERIALS
Isolation and extraction of plum fractions
A known quantity (5g) of dried skin or pulp was mixed with 200ml of water or 80% methanol and placed on a shaker at room temperature overnight. The next day, the mixture was centrifuged at 2000g for 20mins using a Thermos Scientific Centrifuge (Waltham, MA) and the supernatant was collected. The methanol extract was dried in a nitrogen evaporator (Organomation Associates, Inc, Berlin, MA) and then placed in freeze dryer under vacuum over night to ensure the complete removal of residual methanol. The water extract was freeze dried. The dried extract was stored in a -20°C freezer. The dried powder of plums was dissolved in DMSO and the stock solution was prepared at 250mg/ml.
Characterization of plum extracts
Nuclear staining
DATA ANALYSIS
The data is expressed as mean ± SD for at least 3 individual experiments. All comparisons were made by one-way ANOVA with Tukey’s -HSD-post-hoc test using SPSS 20 Statistical software. Significant differences were reported at P<0.05 and indicated by “*” where as non-significant differences were not marked.
RESULTS
Characterization of plum extracts
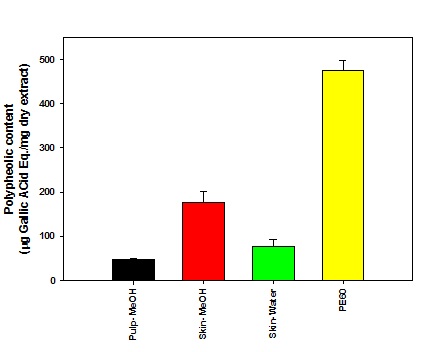 Figure 1: Determination of total phenolic content in plum extracts.
Figure 1: Determination of total phenolic content in plum extracts.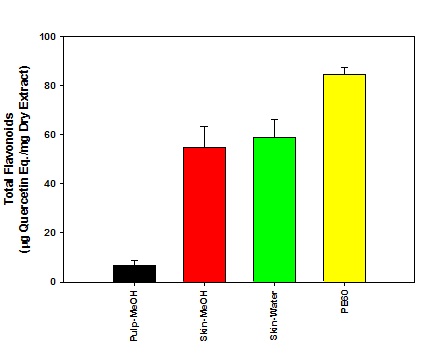 Figure 2: Determination of total flavonoid content in plum extracts.
Figure 2: Determination of total flavonoid content in plum extracts.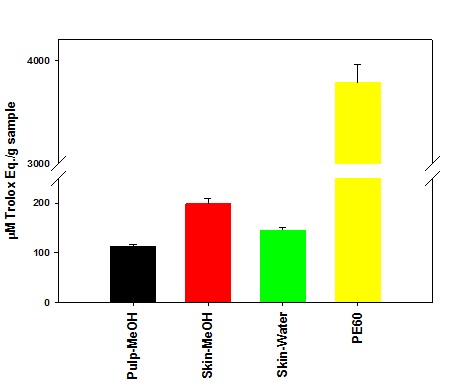 Figure 3: Anti-oxidation activity in plum extracts.
Figure 3: Anti-oxidation activity in plum extracts.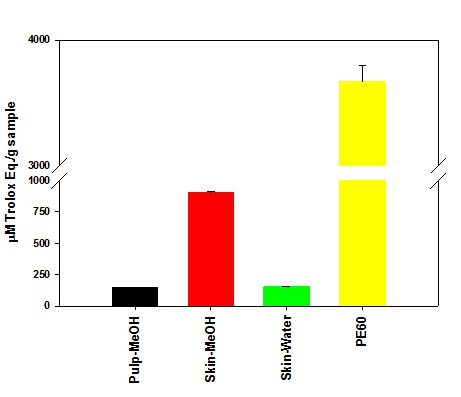 Figure 4: Oxygen scavenging activity in plum extracts.
Figure 4: Oxygen scavenging activity in plum extracts.Effect of plum extract on colon-26 cancer cells viability
 Figure 5: The effect of plum extract on colon-26 cancer cells viability after 24 hrs incubation.
Figure 5: The effect of plum extract on colon-26 cancer cells viability after 24 hrs incubation.The Effect of PE60 and plum extract from skin, pulp on colon 26 cells was also tested after 48hrs of treatment. It is evident from data shown in figure 6 that the cells viability was also not affected on longer incubation at 48hr and data very much mirrors the effects observed after 24hrs of incubation. The PE60 showed a dose-dependent effect whereas the water extract of plum’s skin shown no significant effect on all the concentration and the methanol extract of plum’s skin caused a significant reduction by 30% (P<0.05) at 300μg/ml. The methanol extract of pulp has no significant effect at any tested concentration.
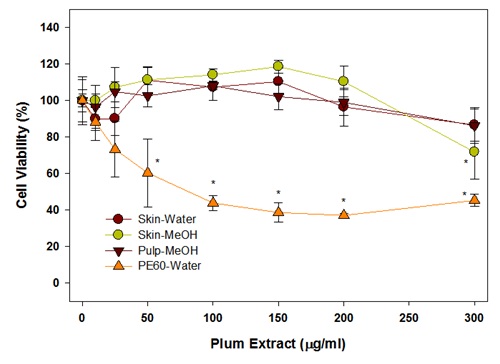 Figure 6: Effect of plum extract on colon-26 cancer cells after 48hrs of incubation.
Figure 6: Effect of plum extract on colon-26 cancer cells after 48hrs of incubation.From these initial experiments, it was evident that the laboratory preparation of water or methanol extract of plum’s skin and methanol extract of plum’s pulp have no or very little effect on colon cell viability. However, the commercial preparation of Plum Extract (PE60) significantly inhibited colon-26 cell proliferation. This difference in effect between laboratory plum extracts and PE60 was due to probably because of their higher content of polyphenols in the PE60 preparation. Based on data in figure 1, further experiments were, therefore, performed using plum skin extract adjusted to their polyphenol in range that was present in PE60. However, we did not further test pulp extracts because it contained low amounts of polyphenols and adjustment of polyphenols to an equivalent levels to PE60 required a large amount of extract that was difficult to dissolve in the test volumes for desired concentrations. The effects of methanol or water extracts of plum’s skin adjusted for polyphenolic content after 24hr treatment are shown in figure 7. The data show that a concentration of polyphenols in methanol extract of plum’s skin at 25μg/ml inhibited colon cell viability significantly by 55% (P<0.05). The cell viability was further decreased by 70% at 75μg/ml reaching a plateau of a maximum inhibition by 80% at 300μg/ml. The polyphenols in water extract also inhibited colon-26 cell viability but to a lesser extent than that of methanol extract. The polyphenols at 25μg/ml inhibited cell viability significantly by 45% (P<0.05); however, the cell variability remained at 45%-50% inhibition on further increasing the concentration of polyphenols up to 300μg/ml.
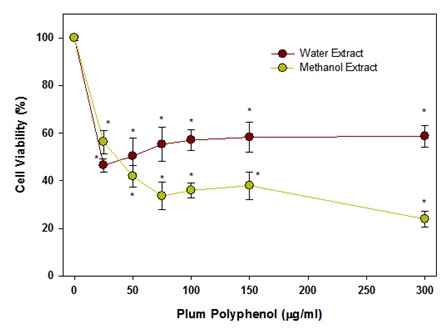 Figure 7: Effect of polyphenols in plum skin extracts on colon-26 cell viability after 24hr of incubation.
Figure 7: Effect of polyphenols in plum skin extracts on colon-26 cell viability after 24hr of incubation.The effects of the methanol or water extracts of plum’s skin after 48hr of treatment were also assayed and are shown in figure 8. The data show that a concentration of polyphenols in methanol extract of plum’s skin at 25μg/ml inhibited colon cell viability significantly by 25% (P<0.05). The cell viability was further decreased by 80% in a dose-dependent manner up to 150μg/ml and then reached a plateau. Similar to 24hr incubation, the polyphenols in water extract also inhibited colon-26 cell viability but to a lesser extent than that of methanol extract. The polyphenols at 25μg/ml inhibited cell viability significantly by 40% (P<0.05), and then caused a gradual reduction in cell viability by 50% at 300μg/ml. However, the cell viability reduced by almost 80% at 500μg/ml, which was similar to the methanolic extract.
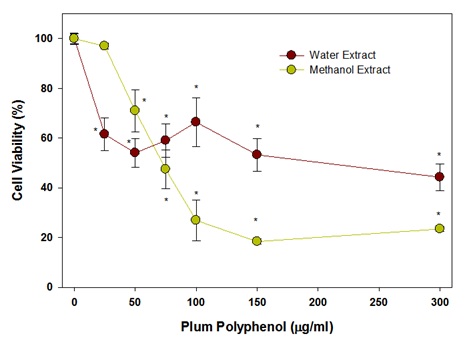 Figure 8: Effect of polyphenols in plum’s skin extract on colon-26 cancer cells after 48hrs of incubation.
Figure 8: Effect of polyphenols in plum’s skin extract on colon-26 cancer cells after 48hrs of incubation.The effect of PE60 adjusted for its polyphenols contents on colon-26 cancer cells after 24hrs incubation is shown in figure 9. It is evident from the data that a significant inhibition of colon cell growth by 20% (P<0.05) was observed at 25μg/ml of polyphenols in PE60. On further increasing the polyphenol concentration in PE60, the cell viability decreased in a dose-dependent manner reaching a plateau of almost a significant 50% (P<0.05) inhibition at 75μg/ml. No further decrease in cell viability was observed on further increasing the concentration of polyphenols up to 150μg/ml.
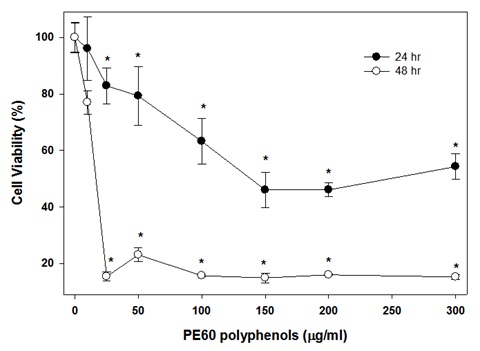 Figure 9: Effect of polyphenols in PE60 on colon-26 cancer cells.
Figure 9: Effect of polyphenols in PE60 on colon-26 cancer cells.The effect polyphenols in PE60 on colon-26 cancer cells after 48hrs incubation is shown in figure 9. The viability of colon-26 cells was effectively inhibited by polyphenols in PE60 extract on 48 incubation. The cell viability inhibited significantly by 85% (P<0.05) at 12.5μg/ml. There was only a slight improvement in inhibition and a maximum inhibition of 90% (P<0.05) was observed on further increasing the concentration over 12.5μg/ml.
Effect of plum extracts on colon-26 cellular structure
The methanol extract of plum skin caused bubble formation at a lower concentration of 25μg/ml (data not shown), and formed optimal circular bubbles at 100μg/ml before the cells started to die (Figure 10). In contrast, the water extract from plum skin started inducing the formation of circular bubbles at 100μg/ml (data not shown). Pulp extract was not used in this experiment because of lack of their effects on colon-26 proliferation. The PE60 extracts, which were more cytotoxic to colon-26 cells, also caused bubble formation but to a lesser extent than that of plum skin extract, prior to undergoing cell death (Figure 11).
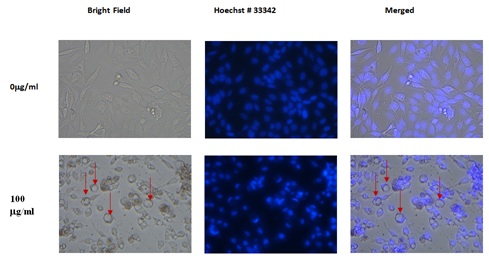 Figure 10: Effect of skin-methanol extract of plums on colon-26 Cells.
Figure 10: Effect of skin-methanol extract of plums on colon-26 Cells.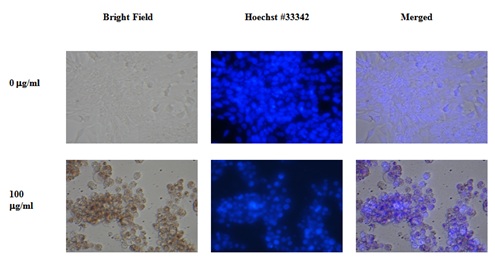 Figure 11: Effect of PE60 Extract on colon-26 Cell.
Figure 11: Effect of PE60 Extract on colon-26 Cell.DISCUSSION
In this study, we investigated the effect of plum extract prepared in the laboratory and compared that with PE60, a commercially standard plum extract on colon cancer cell growth. We initially characterized the phenolic and flavonoids contents and also assayed their anti-oxidation activity and compared the data of laboratory prepared plum extracts to commercially obtain standard preparation. The commercial preparation, PE60 should contain approximately 600mg of TPC in gram of the PE extracts. The TPC amount in PE60 from our analysis came out in range of 490-500mg/g of dry extract. Our data, therefore, validated the high phenolic contents in the PE60 plum extract. In our laboratory preparation of plum extracts, methanol extraction from plum skin contained about 200mg/g dry weight of TPC while the pulp only contained about 50mg/g of flavonoids dry weight. Our data show that the phenolic compounds are concentrated in skin rather than in the pulp. Our data is consistent with other studies that have also shown that phenolic compounds are mostly localized in skin of fruits [14].
Out of about 500mg of phenolic compounds, PE60 contained about 200mg of flavonoids whereas the laboratory preparation of skin contained about 50mg/g dry weight of flavonoids out of about 200mg/g of total phenolic compounds. This data suggests that plum extracts in addition to flavonoids also contained phenolic compounds of other classes. The PE60 is known to have procyanidins and chlorogenic acid classes of polyphenols. During present investigation, we did not determine the phenolic classes in the laboratory prepared extracts of plum skin and pulp.
The anti-oxidation data show that PE60 possessed a very high activity for inhibiting DPPH oxidation whereas our laboratory preparation of plum extracts, compared to that of PE60, only contained about only 24% of the anti-oxidation activity. The low anti-oxidation activity in our laboratory preparation of plum extracts may have been due to their low content of phenolic compounds. Future experiments are planned to further validate the anti-oxidation activity of these extract in a cellular model.
In the initial experiments, PE60 inhibited the growth of colon cancer cells at about 150μg/ml whereas laboratory preparation of plum extract was not effective. It was clear that due to very low contents of polyphenols in the laboratory preparation of plum extracts, we did not find their effects on colon cancer cells. We, therefore, further tested the laboratory preparation of plum extract by adjusting their amount so that their polyphenolic contents were in the range of PE60 phenolic contents. It is evident from our results that the methanolic extract of plum skin was more effective than that of water extract. Our data showed that laboratory preparation of plum methanolic-extracts at 75μg/ml phenolic content inhibited about 70%-75% growth of colon cancer cells after 24hr. incubation. In comparison, PE60 at 75μg/ml only inhibited 40-50% colon cancer growth. This data, therefore, suggest that our laboratory preparation of plum’s skin extract was more effective than PE60 when adjusted to their phenolic contents. However, there are limitations with MTT assay because drugs can interfere mitochondrial activity and, therefore, the effect of drugs may be over or under estimated [15]. During the present investigation, the effects of MTT were validated by microscopic examination and no discrepancies between two assays were noticed.
In addition to the cytotoxic effects of plum extracts, we also observed that the laboratory prepared plum extract induced a big bubble formation in colon-26 cells which was also evident when cells were treated with PE60, but to a lesser extent. Initially it was thought that the bubble structures are probably swollen nuclei. To test this possibility, cells were stained with Hoechst No. 33342 dye to stain nuclei. To our surprise, the round structures were not nuclei but appear to be vacuoles in each cell. The identity of the vacuoles is not known, but it may be water vacuoles as colon cells accumulate water in adverse situations and cause diarrhea. The data suggest that the laboratory prepared extracts may have some phenolic compounds that have caused the vacuoles formation and these compounds may be present in a lesser quantity in the PE60 extract. Further experiments are planned to isolate polyphenols from laboratory prepared extracts and PE60 and to test them head-to-head for further validation of polyphenols role in plums in preventing colon cancer. In addition, future experiments are also required to investigate if the plum extracts are active on other colon cancer cell lines as well as in in vivo animal models, and also to determine their effects on normal colon cells both under in vivo and in vitro conditions.
Plums have various shades of purple color. The purple pigment also has various substances such as chlorogenic acid, flavonoid and anthocyanin which work simultaneously through different signaling pathways to induce death in colon cancer cells [9]. It is interesting to note that colon cancer is also associated with high blood pressure and purple potatoes help in regulating and lowering blood pressure due to their effects on blood vessels and capillaries. Similarly, purple potatoes also have the high concentration of chlorogenic acid, which is shown to reduce colon cancer in an animal model [16]. Similar to purple potatoes, plums also have variable shades of purple pigments which are rich in chlorogenic acid [17] and chlorogenic acid has been shown to inhibit growth of human cancer cells lines [18]. During the present investigation, no attempt was made to identify the phenolic compounds in plum extracts. Since, methanolic extract was more effective than water extract; it suggests that high molecular weight polyphenolic compounds may be responsible for the anticancer activity for colon cancer because they dissolve to a much greater extent in methanol. We found low concentration of phenolic compounds in pulp; however, the mass of pulp is far more than that of skin. It is, therefore, possible that the total amount of phenolic compounds present in the whole pulp may have considerable effect on inhibition of colon cancer growth despite the lower concentration of polyphenols. Furthermore, plum pulp is also a good source of dietary fibers. Fibers are known to produce butyric acid from digestion by gut bacteria [19]. Butyric acid has been shown to contain potent anticancer activity for colon cancer [20]. We did not test the effect of whole pulp fibers on colon cancer during present investigation.
Our study concludes that the effects of plums were dependent on their phenolic contents. Plum skin contained more concentrated phenolic compounds and greater anti-oxidation activity than pulp. Methanol extracts of plum skin was better than water extract of plum skin and effectively inhibited proliferation of colon-26 cells. The amount of plum skin is very small compare to that of pulp. In order to meet the high polyphenol intake, perhaps one need to eat a large quantity of plums, which may be a difficult task. Alternatively, the skin of plums can be removed and the active compounds can be extracted in the methanolic extract. After removing the methanol, the dried powder can be used in a tablet or capsule form to meet the high intake of plum-derived bioactive compounds for preventing colon cancer. In addition, plum extract can be used as an adjuvant supplement with current chemotherapies to better treat colon cancer patients.
In addition, future studies are required to isolate and identify the chemical nature of the active compounds. This information will help to understand the mechanism of its effect on colon cancer cells. The active compound (s) has a potential to be used as a pharmaceutical drug in the future, if found to be potent in pre-clinical or clinical studies, In conclusion, regular consumption of whole plum fruits or in the form of concentrated “whole fruit juice” may be a potential strategy for preventing colon cancer.
AUTHORS’ CONTRIBUTION
Concept, design, manuscript preparation, manuscript editing, and manuscript review: Rafat A Siddiqui, Paul Kaseloo and Sarah Witiak.
Experimental studies, data acquisition, data analysis, statistical analysis: Haiwen Li and Noura Alzahrani.
FINANCIAL SUPPORT AND SPONSORSHIP
The work was supported by funds from Evans-Allen Research program FY2017, USDA, Agricultural Research Station, Virginia State University, Petersburg, Virginia. The financial support to Noura Alzahrani’s Master studies was provided by Saudi Arabian Culture Mission, Kingdom of Saudi Arabia.
CONFLICTS OF INTEREST
There are no conflicts of interest.
REFERENCES
- Tanaka T (2009) Colorectal carcinogenesis: Review of human and experimental animal studies. J Carcinog 8: 5.
- Grivennikov SI (2013) Inflammation and colorectal cancer: Colitis-associated neoplasia. Semin Immunopathol 35: 229-244.
- Thélin C, Sikka S (2015) Epidemiology of Colorectal Cancer-Incidence, Lifetime Risk Factors Statistics and Temporal Trends. In: Ettarh R (ed.). Screening for Colorectal Cancer with Colonoscopy. InTech, London, UK.
- Oft M, Heider KH, Beug H (1998) TGFbeta signaling is necessary for carcinoma cell invasiveness and metastasis. Curr Biol 8: 1243-1252.
- Segditsas S, Tomlinson I (2006) Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 25: 7531-7537.
- Charames GS (2010) Molecular mechanisms of the cooperation between Rac1/1b GTPases and the canonical Wnt signaling pathway in colorectal cancer. University of Toronto, Ontario, Canada.
- Willett WC (2000) Diet and cancer. The Oncologist 5: 393-404.
- Byrne DH, Noratto GD, Cisneros-Zevallos L, Porter W, Vizzotto M (2009) Health benefits of peach, nectarine and plums. Acta Horticulturae 841: 267-274.
- Watson RR, Preedy VR (2009) Bioactive foods in promoting health: Fruits and vegetables. Academic Press, Massachusetts, USA.
- Yu L, Haley S, Perret J, Harris M (2002) Antioxidant properties of extracts from hard winter wheat. Food Chemistry 78: 457-461.
- Parry J, Su L, Moore J, Cheng Z, Luther M, et al. (2006) Chemical compositions, antioxidative capacities, and antiproliferative activities of selected fruit seed flours. J Agric Food Chem 54: 3773-3778.
- Cheng Z, Moore J, Yu L (2006) High-throughput relative DPPH radical scavenging capacity assay. J Agric Food Chem 54: 7429-7436.
- Llobera A, Cañellas J (2008) Antioxidant cctivity and dietary fibre of prensal blanc white grape (Vitis vinifera ) by-broducts. International Journal of Food Science and Technology 43: 1953-1959.
- 14. Ribera AE, Reyes-Díaz M, Alberdi M, Zuñiga GE, Mora ML (2010) Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in highbush blueberry (Caccinium corymbosum L .) grown in Southern Chile. J Soil Sci Plant Nutr 4: 509-536.
- Stepanenko AA, Dmitrenko VV (2015) Pitfalls of the MTT assay: Direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 574: 193-203.
- Charepalli V, Reddivari L, Radhakrishnan S, Vadde R, Agarwal R, et al. (2015) Anthocyanin-containing purple-fleshed potatoes suppress colon tumorigenesis via elimination of colon cancer stem cells. J Nutr Biochem 26: 1641-1649.
- Bouayed J, Rammal H, Dicko A, Younos C, Soulimani R (2007) Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J Neurol Sci 262: 77-84.
- Thurow T (2012) Effect of chlorogenic acid and neochlorogenic acid on human colon cancer cells. University of Arkansas, Fayetteville, USA.
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, et al. (2008) Review article: The role of butyrate on colonic function. Aliment Pharmacol Ther 27: 104-119.
- Nepelska M, Cultrone A, Béguet-Crespel F, Le Roux K, Doré J, et al. (2012) Butyrate produced by commensal bacteria potentiates phorbol esters induced AP-1 response in human intestinal epithelial cells. PLoS One 7: 52869.
Citation: Alzahrani NM, Li H, Kaseloo P, Witiak SM, Siddiqui RA (2018) A Comparative Study for the Effects of Laboratory and Commercially Prepared Plum Extracts on Colon-26 Adenocarcinoma Cells. J Food Sci Nut 4: 031.
Copyright: © 2018 Noura M Alzahrani, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

