
A Comparative Study on Nutritional Composition, Mineral Content and Amino Acid Profile of the Skin of Four Different Animals
*Corresponding Author(s):
Akomolafe SFDepartment Of Biochemistry, Ekiti State University, Ado-Ekiti, Nigeria
Tel:+234 8030460149,
Email:purposefulseun@yahoo.co.uk
Abstract
The increase in human population comes with it an increase in the demand for protein (and amino acids) from different sources. This study seeks to compare the proximate, mineral, amino acid compositions as well as Mineral Safety Index (MSI) of the skin of four different animals (dog, goat, pig and cane rat). The proximate analysis revealed the values of carbohydrate, fat, crude protein, crude fibre, ash and moisture contents. The protein level in cane rat skin was the highest (40.30%), followed by dog (32.17%) and goat (27.36%) skins respectively, while pig skin (25.36%) had the least protein value. The mineral analysis indicated high Ca, Mg and K contents in pig and cane rat skins, while P content was more concentrated in dog and goat skins. Also, Na, Mn and Zn contents were almost similar in all the skin samples. Leucine was the most concentrated essential amino acid in all the skin samples, with ca skin having the highest content followed by pig, dog and goat skins respectively, while the most concentrated amino acid was glutamic acid, with cane rat and pig skins having the highest value followed by goat and dog skins respectively. The content of total essential amino acids in the animal’s skins are 3.56, 5.22, 5.12 and 5.43mg/g for dog, goat, pig and cane rat respectively. All the skin samples were adequate only in Leu, Met + Cys and Phy + Tyr based on FAO/WHO provisional pattern, while they recorded low activities in some essential amino acids (Val, Thre and Lys). The total amino acid levels being 9.82, 8.81, 9.36 and 11.15mg/g crude protein in dog, goat, pig and cane rat skins respectively. Overall, the findings indicate that all these skins which are usually avoided by some people can contribute useful amount of nutrients, mineral elements and amino acids to human diet. Although, cane rat skin had the highest qualities of them all, followed by dog, pig and goat respectively. There is need, however, to determine the vitamins and fatty acids present in these samples. This will help to adequately establish their importance in human nutrition and provide basis for their maximum utilization.
Keywords
INTRODUCTION
Animals are among the most important species of livestock [1] in Nigeria. The major products from animals are meat, milk, and skins [2]. Demand for food of animal origin in developing countries is expected to double by the year 2020 [3].Animal products contribute significantly to the total nutrients in our food supply [4]. They provide a nearly ideal pattern of amino acids and account for over 60% of the total protein intake in the United States. The goal of increasing the efficiency of animal production has been, and continues to be an important consideration in producing animal-derived food products. There is also increasing recognition that foods can be contributing factors in the prevention and development of some disease conditions. As a result, additional focus has been given to designing foods with enhanced components that have beneficial effects on human health [4].The composition of amino acids is the factor determining the quality of protein in food. In general, high protein food is also high in the contents of amino acids including essential amino acids [5]. It is generally accepted that the nutritional quality of proteins depends on the content and availability of their Essential Amino Acids (EAA) [6]. It is well accepted that the nutritional value of proteins may differ substantially depending on their (essential) amino acid composition and digestibility [7].Food products derived from animals are also known to contain micro components that have positive effects on human health and disease prevention beyond those associated with traditional nutritive values [8-11]. In addition, they are primary sources of many vitamins and minerals, including vitamin B12, vitamin B6, riboflavin, niacin, zinc, phosphorus, and calcium. Animals fulfil a variety of functions within the agricultural economy. They provide human beings with food, industrial raw materials (particularly wool and hides), energy for traction and carriage of goods, and also manure. Animal skins have a greater nutritional value compared to plant products. It has been reported by many researchers that animal skins played a significant role in satisfying the dietary and nutritional requirements of human [12-15]. The study reported in this article is an attempt to assess and compare the nutritional quality of the skin of four different animals namely: Dog (Capra aegagrus hircus), goat (Capra aegagrus hircus), pig (Sus scrofa domesticus) and cane rat (Thryonomys swinderianus) by evaluating their proximate, minerals, amino acids, mineral ratios and mineral safety index. It is hoped that this will contribute information to food composition tables.
MATERIALS AND METHODS
Sample collection and preparation
Proximate analysis
Mineral analysis
The detection limits for the metals in aqueous solution had been determined just before the mineral analyses using the methods of Varian Techtron, giving the following values in μg/ml: Fe (0.01), Cu (0.002), Na (0.002), K (0.005), Ca (0.04), Mg (0.002), Zn (0.005) and Mn (0.01). The optimal analytical range was 0.1 to 0.5 absorbance units with coefficients of variation from 0.9-2.2%.
The coefficients of variation per cent were calculated [18]. The percentage contribution to Energy due to Protein (PEP), due to total fat (PEF) and due to carbohydrate (PEC) as PEP %, PEF % and PEC % respectively were calculated. The percentage Utilizable Energy Due to Protein (UEDP %) was also calculated. Ca/P, Na/K, Ca/Mg and the millequivalent ratio of [K/(Ca + Mg)]; the Mineral Safety Index (MSI) of Na, Mg, Mn, P, Ca, Fe and Zn were also calculated [19]. To calculate MSI, we have: RAI is recommended adult intake; CV in the table will represent Calculated Value (CV) of calculated MSI from research results. The differences between the standard MSI and the MSI of the samples were also calculated.
Amino acid analysis
Determination of quality parameters
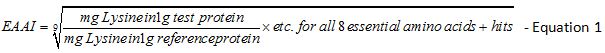
Amino acid score = (amount of amino acid per test protein (mg/g)) / (amount of amino acid per protein in reference pattern (mg/g)). In this method, Met + Cys and Phe + Tyr were each taken as a unit. Also, only essential amino acids determined were scored. Amino acid score was also calculated based on the composition of the amino acids obtained in the samples compared with the suggested pattern of requirements for pre- school children (2-5 years). Here, Met + Cys and Phe + Tyr were each taken as a unit. Also, only essential amino acids with (His) were scored.
Determination of the essential amino acid index: The Essential Amino Acid Index (EAAI) was calculated by using the ratio of test protein to the reference protein for each of the eight essential amino acid plus histidine in the equation that follows [23]. Met and Cys are counted as a single amino acid value in the equation, as well as Phe + Tyr.
Determination of the predicted protein efficiency ratio: The predicted protein efficiency ratio (P - PER) was determined using one of the equations derived by Alsmeyer et al. [24].
P - PER = -0.468 + 0.454 (Leu) - 0.105 (Tyr) - Equation 2
Other determinations: Determination of the Total Essential Amino Acid (TEAA) to the Total Amino Acid (TAA), (TEAA/TAA); Total Sulphur Amino Acid (TSAA); percentage cystine in TSAA; the Leu/Ile ratios were calculated using the equation of the form [25].
Statistical analysis
RESULTS AND DISCUSSION
Table 1 contains the proximate composition of the skin of four different mammals (dog, goat, pig and cane rat). As indicated by the results, the protein level in cane rat skin was the highest, followed by dog and goat skins respectively, while pig skin had the least protein value. The crude protein in all the animal skins were higher than that found in camel [26] and beef [27]. Protein is the most important muscle constituents and is made up of myofibrilla, sarcoplasmic and connective tissues. Also, the crude fibre content in pig skin was of higher value compared to the rest of the skins of other animals, followed by the cane rat skin. The crude fibre level in cane rat skin doubled the level in dog and goat skins while that of pig skin is four-fold times the level in dog and goat skins and one-half fold times the level in cane rat skin. Results compiled in table 1 also showed that the total ash in cane rat skin was much more higher than in skins of other animals, meaning that the cane rat skin would likely contain higher levels of minerals than the skins of other animals (dog, goat and pig). Also, the fat constituent of the pig and cane rat skins were higher when compared with other two animal skins (dog and goat ) on a dry-matter basis, which makes the skins of the two animals a good source of oil, which can be a supplement to fat foods. The fat content was 28.96% and 20.36% for pig and cane rat skins respectively, which was higher from the value of raw and cooked camel meat reported by Muhammad and Abubakar [26]. This increment may be due to the fact that meat fat is usually deposited in connective tissues, muscles and under the skin. Fat is a source of concentrated energy in meat, and are often avoided by consumers. However, fat serves as a transport compound that is very essential for the development of eating flavor in foods. Table 1 still contains other parameters calculated from the proximate values. It shows the various energy values as contributed by protein, fat and carbohydrate. The daily energy requirement for an adult is between 2500 - 3000kcal (10455 - 12548KJ) depending on his physiological state while that of infants is 740kcal (3094.68KJ) [28]. This implies that while an adult man would require between 590 - 709g (taking the calculated energy of 1501KJ/100g) of his energy requirement, infants would require 174.9g (taking the calculated energy of 1501KJ/100g). On the whole this meant that samples with higher energy value would require lower quantity of sample to satisfy the energy needs of man and infants. The Utilizable Energy Due to Protein (UEDP%) for the samples (assuming 60% utilization) are 21.23, 17.45, 12.39 and 27.58% for dog, goat, pig and cane rat skins respectively. The UEDP % of dog, goat and cane rat were higher while that of pig compared favourably with the recommended safe level of 8% for an adult man who requires about 55g protein per day with 60% utilization. The PEF% values were generally high in all the skin samples and far above the recommended level of 30% [4] and 35% [4] for total fat intake but nevertheless pig skin had the highest PEF%; this is useful for people wishing to adopt the guidelines for a healthy diet.
| Parameters | Dog | Goat | Pig | Cane Rat |
| Proximate Composition (mg/g) | ||||
| Crude protein | 32.17 ± 0.01a | 27.36 ± 0.05b | 25.36 ± 0.04c | 40.30 ± 1.77d |
| Crude fat | 07.07 ± 0.02a | 07.32 ± 0.01a | 28.96 ± 0.07b | 20.36 ± 0.12c |
| Total ash | 01.39 ± 0.03a | 01.96 ± 0.01a | 01.45 ± 0.03a | 20.80 ± 0.25b |
| Crude fibre | 02.20 ± 0.12a,b | 03.26 ± 0.05b | 03.22 ± 0.01b | 01.10 ± 0.03a |
| Moisture content | 13.83 ± 0.71a | 09.31 ± 0.02b | 06.55 ± 0.01c | 12.44 ± 0.05d |
| Carbohydrate % | 43.34 ± 0.10a | 50.79 ± 0.25b | 34.46 ± 0.10c | 27.00 ± 0.02d |
| PEC % | 736.78 | 863.43 | 585.82 | 459 |
| PEF % | 261.59 | 270.84 | 1071.52 | 346.12 |
| PEP % | 546.89 | 465.12 | 431.12 | 685.1 |
| UEDP % | 21.23 | 17.45 | 12.39 | 27.58 |
| Energy (KJ/100g) | 1545 | 1600 | 2088 | 1897 |
| Mineral Composition (mg/g) | ||||
| Phosphorus (P) | 26.11 ± 0.17a | 25.18 ± 0.25a | 21.52 ± 0.02b | 18.47 ± 0.01c |
| Calcium (Ca) | 13.39 ± 0.10a | 16.01 ± 1.77b | 25.49 ± 0.12c | 24.56 ± 0.01c |
| Magnesium (Mg) | 05.31 ± 0.03a | 06.22 ± 0.03a | 09.80 ± 0.02b | 11.32 ± 0.05c |
| Sodium (Na) | 12.81 ± 0.05a | 09.31 ± 0.17b | 13.17 ± 0.25a | 12.31 ± 0.02a |
| Potassium (K) | 16.32 ± 0.01a | 17.02 ± 0.10a | 23.14 ± 0.03b | 19.32 ± 0.02c |
| Zinc (Zn) | 02.51 ± 0.05a | 02.27 ± 0.25a | 01.19 ± 0.02a | 01.98 ± 1.77a |
| Manganese (Mn) | 0.61 ± 0.25a | 0.81 ± 0.04b | 0.86 ± 0.01b | 0.63 ± 0.05a |
| Iron (Fe) | 02.49 ± 0.01a | 02.38 ± 0.17a | 02.62 ± 0.05a | 02.47 ± 0.02a |
| Copper (Cu) | 0.02 ± 0.00a | 0.02 ± 0.00a | 0.04 ± 0.01a | 0.02 ± 0.01a |
| Mineral Ratio Parameters | ||||
| K/Na | 1.27 | 1.83 | 1.76 | 1.57 |
| Na/K | 0.78 | 0.55 | 0.57 | 0.64 |
| Ca/P | 0.51 | 0.64 | 1.18 | 1.33 |
| Ca/Mg | 2.52 | 2.57 | 2.60 | 2.17 |
| [K/(Ca+Mg)]a | 1.74 | 1.54 | 1.32 | 1.08 |
PEP = Proportion of Total Energy Due to Protein
PEF = Proportion of Total Energy Due to Fat
PEC = Proportion of Total Energy Due to Carbohydrate
UEDP = Utilizable Energy Due to Protein
Table 1 also depicts the mineral composition of the skin of four different animals (dog, goat, pig and cane rat). In the table, for the pig and cane rat skins, elements Ca, Mg and K were more concentrated than dog and goat skins, whereas element P was more concentrated in dog and goat skins than pig and cane rat skins. Elements Na, Mn and Zn were almost similar in all the skin samples.
The list of the nutritionally important minerals as well as the computed mineral ratios present in the skin samples were also presented in table 1 and table 2. Minerals are important in human nutrition. It is well known that enzymatic activities as well as electrolytic balance of the blood fluid are related to the adequacy of Na, K, Mg and Zn. Potassium is very important in maintaining the blood fluid volume and osmotic equilibrium. Metal deficiency syndrome like rickets and calcification of bone is caused deficiency. Appreciable levels of all the essential minerals were present in the skin samples. In the tables, for the pig and cane rat skins, elements Ca, Mg and K were each more concentrated than dog and goat skins, whereas element P was more concentrated in dog and goat skins than pig and cane rat skins. Elements Na, Mn and Zn were almost similar in all the skin samples. The Ca/P of all the skins were comparably higher than 0.5 which is the minimum ratio required for favourable calcium absorption in the intestine for bone formation [29] although the level of Ca/P has been reported to have some effects on calcium in the blood of many animals [30]. The value of ratio of Na/K of goat and pig skins (0.55, 0.57 respectively) were lower than 0.6, the value that favours non-enhancement of high blood pressure disease in man while that of dog and cane rat were higher. Although for normal retention of protein during growth and for balancing fluid a K/Na ratio of 1.0 is recommended [31], the high value of K/Na ratio (1.27, 1.83, 1.76 and 1.57 for dog, goat, pig and cane rat respectively) obtained in the present report suggests that bringing the ratio down would require the consumption of food sources rich in Na. The Ca/Mg value obtained for the present skin samples (2.52, 2.57, 2.60 and 2.17 for dog, goat, pig and cane rat respectively) were fairly higher than the 1.0 recommended. It means that both Ca and Mg would need adjustment for normal health.
| Parameters | Dog | Goat | Pig | Cane Rat | |||||
| TV | CV | D | CV | D | CV | D | CV | D | |
| Phosphorus (P) | 10 | 2.17 | +7.83 | 0.21 | +9.79 | 0.18 | +9.82 | 0.15 | +9.85 |
| Calcium (Ca) | 10 | 0.11 | +9.89 | 0.13 | +9.87 | 2.12 | +7.88 | 0.20 | +9.80 |
| Magnesium (Mg) | 15 | 0.20 | +14.80 | 0.23 | +14.77 | 0.37 | +14.63 | 0.42 | +14.58 |
| Sodium (Na) | 48 | 0.12 | +47.88 | 0.09 | +47.91 | 0.13 | +47.87 | 0.12 | +47.88 |
| Potassium (K) | 48 | 0.16 | +47.84 | 0.16 | +47.84 | 0.22 | +47.87 | 0.19 | +47.81 |
| Zinc (Zn) | 33 | 5.52 | +27.48 | 4.99 | +28.01 | 2.62 | +30.38 | 4.36 | +28.64 |
| Manganese (Mn) | 15 | 0.02 | +14.98 | 0.03 | +14.97 | 0.03 | +14.97 | 0.02 | +14.98 |
| Iron (Fe) | 6.7 | 1.11 | +5.59 | 1.06 | +5.64 | 1.17 | +5.53 | 1.10 | +5.60 |
The milliequivalent ratio [K/(Ca+Mg)] of all the skin samples were comparably lower than 2.2 recommended, which means that the samples would not promote hypomagnesaemia in man [4,32]. Iron and Zinc are among the required elements for humans and their daily requirements for adults are 0.1 and 0.15mg/g respectively. Levels obtained in the present report are ((Zn) 2.51 ± 0.05, 2.27 ± 0.25, 1.19 ± 0.02, 1.98 ± 1.77mg/g and (Fe) 2.49 ± 0.01, 2.38 ± 0.17, 2.62 ± 0.05, 2.47 ± 0.02mg/g) for dog, goat, pig and cane rat respectively. However zinc requirements can easily be met by consuming these samples. Generally from the recommendation set out by NRC/NAS, the daily requirement of Zn, Mn and Cu can easily be met while the diets may be supplemented with foods high in K, Na, Ca and P.
The Mineral Safety Index (MSI) of the skin samples as presented in table 2 revealed that all the minerals tested in all the skin samples have their TV>CV, giving positive difference with Calculated Value (CV) ranging between 0.02 to 5.52. This meant that the MSI of all the skin samples are relatively low and might not be overloading the body in these minerals. It has been reported that abnormal high levels of Na, Mg and P in any food could cause the reduction of zinc in the small intestine [33].
The total amino acids in the current report were 9.82, 8.81, 9.36 and 11.15mg/g crude protein in dog, goat, pig and cane rat skins respectively (Table 3). Leucine was the most concentrated essential amino acid in all the skin samples, leucine content of cane rat skin was the highest followed by pig, dog and goat skins respectively (Table 3). Glutamic acid was the most concentrated amino acid in all the skin samples, also the value was higher in cane rat and pig skins followed by goat and dog skins respectively (Table 3). Tryptophan (Try) concentrations could not be determined. It has been reported that most animal protein are low in Cys [34]. The case is the same in the present study; the Cys in the present study were 0.71, 0.47, 0.78 and 0.22 for dog, goat, pig and cane rat respectively. Information on the advantages of increasing the concentration of sulphur-containing amino acid in staple foods shows that Cys had positive effects on mineral absorption, particularly zinc [35].
| Amino Acids | Dog | Goat | Pig | Cane Rat |
| Lysinea (Lys) | 0.58 | 0.50 | 0.70 | 0.45 |
| Histidine (His) | 0.40 | 0.50 | 0.72 | 0.25 |
| Argininea (Arg) | 0.40 | 0.58 | 0.33 | 0.55 |
| Aspartic acid (Asp) | 0.40 | 0.58 | 0.41 | 0.75 |
| Threoninea (Thre) | 0.56 | 0.50 | 0.48 | 0.30 |
| Serine (Ser) | 0.28 | 0.39 | 0.40 | 0.52 |
| Glutamic acid (Glu) | 1.10 | 1.25 | 1.60 | 1.60 |
| Proline (Pro) | 0.40 | 0.52 | 0.82 | 0.32 |
| Glycine (Gly) | 0.50 | 0.90 | 1.20 | 1.20 |
| Alanine (Ala) | 0.48 | 0.53 | 0.35 | 0.49 |
| Cystine (Cys) | 0.71 | 0.47 | 0.78 | 0.22 |
| Valinea (Val) | 0.40 | 0.44 | 0.66 | 0.48 |
| Methioninea (Met) | 0.41 | 0.42 | 0.56 | 0.26 |
| Isoleucinea (Ile) | 0.50 | 0.60 | 0.40 | 0.40 |
| Leucinea (Leu) | 0.68 | 0.71 | 0.85 | 0.87 |
| Tyrosine (Tyr) | 0.40 | 0.48 | 0.30 | 0.24 |
| Phenylalaninea (Phe) | 0.61 | 0.45 | 0.59 | 0.48 |
| Total | 9.82 | 8.81 | 9.38 | 11.15 |
Table 4 shows the concentrations of Total AA (TAA), Total Essential AA (TEAA), Total Acidic AA (TAAA), Total Neutral AA (TNAA), Total Sulphur AA (TSAA), Essential Aromatic AA (EArAA) and their percentage values. The Leu/Ile ratios, their differences and percentage differences are contained in table 4. Non- essential amino acids have the highest % concentration (61.60, 53.50) in dog and pig skins respectively while TEAA total essential amino acids had the highest % concentration (51.20, 58.80) in goat and cane rat skins respectively. The content of TEAA of dog, goat and pig skins (38.40, 51.20, and 46.50mg/g cp) were close to the value for egg reference protein (56.6mg/g cp) [21] while that of cane rat (58.80mg/g cp) was higher. Also, these values were comparably higher to the values reported for soya bean (44.4mg/g cp) [36] except that of dog which was lower. Table 4 also depicts the percent of Total Acidic Amino Acids (TAAA) (9.71%, 14.50%, 18.30% and 25.40%) for dog, goat, pig and cane rat skins respectively. The percent of Total Acidic Amino Acids (TAAA) of pig and cane rat skins were found to be greater than the percent of Total Basic Amino Acids (TBAA) (15.90% and 13.60%) respectively, indicating that the protein in the two animal skins is probably acidic in nature while the percent of Total Acidic Amino Acids (TAAA) of dog and goat skins were found to be lower than the percent of Total Basic Amino Acids (TBAA) (18.00% and 15.80%) respectively, indicating that the protein in the two animal skins (dog and goat) is probably basic in nature. The content of TSAA of all the samples (1.13, 0.89, 1.34, 0.48mg/g for dog, goat, pig and cane rat skins respectively) were lower than the 58mg/g cp recommended for infants [37].
|
Amino Acids |
Dog |
Goat |
Pig |
Cane Rat |
|
Total Amino Acid (TAA) |
9.27 |
10.19 |
10.98 |
9.22 |
|
Total Non Essential Amino Acid (TNEAA) |
5.71 |
4.97 |
5.86 |
3.79 |
|
% TNEAA |
61.60 |
48.80 |
53.50 |
41.10 |
|
Total Essential Amino Acid (TEAA) |
3.56 |
5.22 |
5.12 |
5.43 |
|
% TEAA |
38.40 |
51.20 |
46.50 |
58.80 |
|
Essential Aliphatic Amino Acid (EAAA) |
1.87 |
2.40 |
2.30 |
1.85 |
|
Essential Aromatic Amino Acid (EArAA) |
1.01 |
0.93 |
0.89 |
0.72 |
|
Total Neutral Amino Acids (TNAA) |
1.58 |
1.78 |
1.55 |
1.69 |
|
Total Acidic Amino Acid (TAAA) |
0.90 |
1.48 |
2.01 |
2.35 |
|
% TAAA |
9.71 |
14.50 |
18.30 |
25.40 |
|
Total Basic Amino Acid (TBAA) |
1.67 |
1.61 |
1.75 |
1.25 |
|
% TBAA |
18.00 |
15.80 |
15.90 |
13.60 |
|
Total Sulphur Amino Acid (TSAA) |
1.13 |
0.89 |
1.34 |
0.48 |
|
% Cysteine in TSAA |
63.40 |
62.80 |
58.20 |
45.80 |
|
Leu/IIe ratio |
1.36 |
1.18 |
2.13 |
2.16 |
|
Leu - IIe (Difference) |
0.18 |
0.11 |
0.45 |
0.47 |
|
% Leu - IIe (Difference) |
26.47 |
15.49 |
52.94 |
54.02 |
|
Predicted Protein Efficiency Ratio (P-PER) |
-0.20 |
-0.19 |
-0.11 |
-0.09 |
|
Essential Amino Acid Index (EAAI) |
1.79 |
1.82 |
1.98 |
1.31 |
Table 4: Summary of some essential parameters of amino acid of the skin of the animals (mg/g crude protein).
The Aromatic Amino Acids (ArAA) are the precursors of epinephrine and thyroxin [38]. The Essential Aromatic Amino Acid (EArAA) in the study are 1.01, 0.93, 0.89, and 0.72 for dog, goat, pig and cane rat skins respectively. The % ratios of TEAA/TAA in all the samples (dog, goat, pig and cane rat respectively) were 38.4, 51.2, 46.6 and 58.9% which were well above 39% (except that of dog skin) considered to be adequate for ideal protein food for infants; 26% for children and 11% for adults [37]. The TEAA/TAA were strongly comparable to that of egg (50%) [39].
The P-PER values of dog, goat, pig and cane rat skins (-0.20, -0.19, -0.11 and -0.09 respectively) were low. Clinical, biochemical and pathological observations in experiments conducted in humans and laboratory animals showed that high leucine in the diet impairs the metabolism of tryptophan and niacin and is responsible for niacin deficiency in sorghum eaters [40]. These studies suggested that the leucine/isoleucine balance is more important than dietary excess of leucine alone in regulating the metabolism of tryptophan and niacin and hence the disease process. The present Leu/Ile ratios of all the samples were moderate in value but nevertheless cane rat skin had the highest value. The EAAI can be useful as a rapid tool to evaluate food formulations for protein quality. The EAAI for soy flour is 1.26 [41] which is lower for the current result of 1.79, 1.82, 1.98 and 1.31 of dog, goat, pig and cane rat skins respectively.
According to the amino acid scores (Table 5), the contents of essential amino acid in all the skin samples were almost the same. All the skin samples were adequate only in Leu, Met + Cys and Phy + Tyr based on FAO/WHO provisional essential amino acid scoring pattern, while they recorded low activities in some essential amino acids (Val, Thre and Lys), the skin samples may require supplementation with other rich sources in order to be used as confectionaries/or as a food supplement for any food material that is not adequate in essential amino acid. It has been reported that the essential amino acids most often acting in a limiting capacity are Met (and Cys), Lys, and Try [42]. The first two limiting amino acids in this study were dog skin (Lys and Val), goat skin (Lys and Val), pig skin (Lys and Val) and cane rat skin (Lys and Val) (Table 5). Try could not be determined. Also, table 5 showed that Val had the lowest score with a value of 0.008 and 0.009 in dog and goat skins respectively. For dog skin, the correction value is 100/0.8 (or 125) × protein of sample while for goat skin, the correction value is 100/0.9 (or 111) × protein of sample. This implies that this quantity of protein has to be taken (eaten) when they are the sole source of protein in the diet [43]. Ile had the lowest score with a value of 0.010 in pig skin while Lys and Thr had the lowest score with a value of 0.008 in cane rat skin.
|
Amino acids |
PAAESPa |
Dog |
Goat |
Pig |
Cane Rat |
|
Ile |
40 |
0.013 |
0.015 |
0.010 |
0.010 |
|
Leu |
70 |
0.014 |
0.010 |
0.015 |
0.012 |
|
Met + Cys |
35 |
0.032 |
0.025 |
0.038 |
0.014 |
|
Lys |
55 |
0.011 |
0.009 |
0.013 |
0.008 |
|
Phy + Tyr |
60 |
0.017 |
0.016 |
0.015 |
0.012 |
|
Thr |
40 |
0.021 |
0.013 |
0.012 |
0.008 |
|
Try |
10 |
ND |
ND |
ND |
ND |
|
Val |
50 |
0.008 |
0.009 |
0.013 |
0.010 |
|
Total |
360 |
0.112 |
0.097 |
0.113 |
0.074 |
CONCLUSION
This present study has provided some biochemical information on the proximate composition, mineral element and amino acid profile of four different animal’s skins. There are indications that all these skins which are usually avoided by some people can contribute useful amount of nutrients, mineral elements and amino acids to human diet. Although cane rat skin had the highest qualities of them all, followed by dog, pig and goat respectively. There is need, however, to determine the vitamins and fatty acids present in these samples. This will help to adequately establish their importance in human nutrition and provide basis for their maximum utilization.
REFERENCES
- Otunaiya AO, Ologbon OAC, Adigun GT (2015) Rural Households Willingness to Pay for Small Ruminants Meat in South-Western Nigeria. Agriculture, Forestry and Fisheries 4: 117-122.
- Ehui SK, Ahmed MM, Berhanu G, Benin SE, Nin Pratt A et al. (2003) 10 years of Livestock Policy Analysis. Policies for improving productivity, competitiveness and sustainable livelihoods of smallholder livestock producers. ILRI (International Livestock Research Institute), Nairobi, Kenya.
- Delgado C, Rosegrant M, Steinfeld H, Ehui S, Courboi C (1999) Livestock to 2020: The next food revolution. Food, Agriculture, and the Environment Discussion Paper 28. IFPRI, Washington D.C, USA.
- National Research Council (1989) Recommended Dietary Allowances, (10th edn) Food and Nutrition Board National Academy Press, Washington D.C., USA.
- Kim BH, Lee HS, Jang YA, Lee JY, Cho YJ et al. (2009) Development of amino acid composition database for Korean foods. Journal of Food Composition and Analysis.
- Tuan YH, Phillips RD, Dove CR (1999) Predicting integrated protein nutritional quality part 2: integrated protein nutritional quality predicted from amino acid availability corrected amino acid score (AACAAS). Nutrition Research 19: 1807-1816.
- Schaafsma G (2000) The protein digestibility-corrected amino acid score. J Nutr 130: 1865-1867.
- Allen LH (1993) The nutrition CRSP: what is marginal malnutrition, and does it affect human function? Nutr Rev 51: 255-267.
- Knekt P, Järvinen R, Seppänen R, Pukkala E , Aromaa A (1996) Intake of dairy products and the risk of breast cancer. Br J Cancer 73: 687-691.
- Parodi PW (1997) Cow’s milk fat components as potential anticarcinogenic agents. J Nutr 127: 1055-1060.
- Molkentin J (1999) Bioactive lipids naturally occurring in bovine milk. Nahrung 43: 185-189.
- Sales J, Marias D, Kruger M (1996) Fat content, caloric value, cholesterol content and fatty acids composition of raw and cooked ostrich meat. J. Food Compos Anal.
- Adeyeye EI, Faleye FJ (2004) Mineral components for health from animal sources. Pak J Sci Indus Res.
- Assogbadjo AE, Codjia JTC, Sinsin B, Ekue HRM, Mensah GA (2005) Importance of rodents as a human food source in Benin. Belg J Zool 135: 11-15.
- Oyarekua MA, Ketiku AO (2010) The Nutrient Composition of the African Rat. Advance Journal of Food Science and Technology 2: 318-324.
- AOAC (2002) International Official Methods of Analysis, (17th edn), Association of Analytical Chemists, Washington D.C., USA.
- Pearson D (1976) The chemical analysis of foods, (7th edn), Churchill Livingstone, London.
- Steel RGD (1960) Principles and Procedures of Statistics: A Biometrical Approach. McGraw-Hill, New York, USA.
- Hathcock JN (1985) Quantitative evaluation of vitamin safety. Pharmacy Times 104-113.
- Sparkman DH, Stein WH, Moore S (1958) Automatic recoding apparatus for use in chromatography of amino acids. Analytical Chemistry 30: 1190-1206.
- Paul AA, Southgate DAT, Russel J (1976) First supplement to McCance and Widdowsons’s the composition of foods. Her Majesty’s Stationery Office, London, UK.
- World Health Organization (1973) Energy and protein requirements. WHO Technical Report Series 522, World Health Organization, Geneva, Switzerland.
- Steinke FH, Prescher EE, Hopkins DT (1980) Nutritional evaluation (PER) of isolated soybean protein and combinations of foods proteins. Journal of Food Science 45: 323-327.
- Alsmeyer RH, Cunningham AE, Happich ML (1974) Equations to predict PER from amino acid analysis. Food Technology.
- Olaofe O, Akintayo ET (2000) Prediction of isoelectric points of legume and oilseed proteins from their amino acid compounds. Journal of Food Science and Technology 4: 48-53.
- Muhammad BF, Abubakar FM (2011) Chemical Composition of Raw and Cooked Camel (Camelus dromedarius) Meat Cuts. Savannah Journal of Agriculture 6: 31-36.
- Ezekwe AG, Okonwo TM, Ukaegbu UG and Sangode AA (1997) Preliminary study of meat quality characteristics of young N’dama and Muturu bulls. Nigerian Journal of Animal Production 24.
- Bingham S (1978) Nutrition: A consumer’s guide to good eating, Transworld, London, UK. Pg no: 123-127.
- Nieman DC, Butterworth DE, Nieman CN (1992) Nutrition. William C Brown Publishers. Dubuque, USA.
- Adeyeye EI, Orisakeye OT, Oyarekua MA (2012) Composition, mineral safety index, calcium, zinc and phytate interrelationships in four fast- foods consumed in Nigeria. Bulletin of Chemical Society of Ethiopia 26: 43-54.
- Helsper PFG, Hoogendijk MJ, Van Norel A, Burger-Meyer K (1993) Antinutritional factors in faba beans (Vicia faba L.) as affected by breeding toward the absence of condensed tannin. Journal of Agriculture and Food Chemistry 41: 1058-1061.
- Adeyeye EI, Adesina JA (2012) Nutritional Composition of the Flour of African Breadfruit (Treculia africana) Seeds Testa. Journal of Agricultural Research and Development 11.
- Adeyeye EI, Oyarekua MA, Adesina AJ (2014) Proximate, mineral, amino acid composition and mineral safety index of callinectes latimanus. International Journal of Development Research 4: 2641-2649.
- Adeyeye EI, Adamu AS (2005) Chemical composition and food properties of Gymnarchus niloticus (Trunk fish). Biosciences Biotechnology Research Asia 3: 265-272.
- Mendoza C (2002) Effect of genetically modified low phytic acid plants on mineral absorption. International Journal of Food Sciences and Technology 37: 759-767.
- Altschul AM (1958) Processed plant protein foodstuff. New York: Academic Press.
- World Health Organization (1985) Energy and protein requirements. WHO Technical Report Series No. 724. World Health Organization, Geneva, Switzerland.
- Robinson DS (1987) Food: Biochemistry and Nutritional value. Longman Science and Technical. London, UK.
- FAO/WHO (1991) Protein Quality Evaluation. Food and Nutrient, FAO Rome, Report of Joint FAO/WHO Expert Consultation FAO, Italy.
- Ghafoorunissa, Rao BS (1973) Effect of leucine on enzymes of the tryptophan-niacin metabolic pathway in rat liver and kidney. Biochem J 134: 425-430.
- Nelson SS (1994) Introduction to the chemical analysis of foods. Jones and Bartletes Publishers, Burlington, USA.
- Oleszek W, Price KR, Colquhoun IJ, Jurzysta M, Ploszynski M et al. (1990) Isolation and identification of alfalfa (Medicago sativa L.) root saponins: their activity in relation to a fungal bioassay. J Agric Food Chem 38: 1810-1817.
- Bingham S (1977) Proximate Analysis, Mineral Contents, Amino Acid Composition, Anti-Nutrients and Phytochemical Screening of Brachystegia Eurycoma Harms and Pipper Guineense Schum and Thonn. American Journal of Food and Nutrition 2: 76-281.
Citation: Ajayi OB, Akomolafe SF (2016) A Comparative Study on Nutritional Composition, Mineral Content and Amino Acid Profile of the Skin of Four Different Animals. J Food Sci Nutr 2: 012.
Copyright: © 2016 Ajayi OB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

