
A Modern Approach to the Problem of Cognitive and Motor Impairment in Dementia
*Corresponding Author(s):
Ivanova NEPolenov Neurosurgical Institute Branch Of The Almazov National Medical Research Centre, Saint Petersburg, Russian Federation
Tel:+78-911-218-71-49,
Email:ivamel@yandex.ru
Abstract
Background: The combination of Alzheimer's disease (AD) in vascular dementia (VaD) leads to an increase of cognitive impairment and ataxia and worsens prognosis. Physical exercise is a crucial key to avoid falls. Translingual neurostimulation (TLNS) has been used to improve dynamic and static balance and gait of several years. A non-pharmacological intervention programme for symptoms of dementia can help improve neuropsychiatric symptoms.
Objectives: To study the dynamics of cognitive impairment and the quality of life of patients with mixed dementia, depending on the methods of patient care.
Methods: Cohort study of 80 cases, from March 2019 to January 2020, moderate and severe cognitive impairment and previously established diagnoses of chronic cerebrovascular ischemia (without any notes about dementia). Inclusion criteria: MMSE<26 points, FAB<11 points. The average age of the patients was 77 ± 6.3 years.
Results: The quality of life (EQ-5D VAS) increased in the group 1 in 100% of cases 23 ± 4.7 points. In the group 2, changes in the quality of life were not statically significant, the average value decreased by 2 ± 0.7 points. The improvement in the stabilometry was confirmed by a statically significant improvement in the mean values of BBS and DGI. The use of TLNS (PoNS ™) with physical exercises under the control of stabilometry with biofeedback system (H. Motion Lab ™) in patients with high risk of falling can improve gait and balance, including patient with dementia.
Conclusion: A differentiated approach to the problem of cognitive impairment in the early stages of dementia (taking into account the etiology of dementia) increases the compliance of the patient and relatives and the effectiveness of drugs therapy.
Keywords
Dementia; Mixed dementia; Risk of falls; Non-pharmacological program; Stabilometry; Translingual neurostimulation
BACKGROUND
There were 46.8 million people with dementia in the world in 2015. Every 20 years the number of patients should double and their number should reach 75 million by 2030, and 131.5 million-by 2050. Growth the number of patients with dementia is related to the quality of the life and it is especially pronounced in low and middle income countries. A in developed countries positive trend towards the decrease the number of patients with dementia is associated with improved living conditions and the quality of medical care [1].
The majority of authors included mixed cases vascular dementia (VaD) and Alzheimer's disease (AD) in vascular dementia rates. In our country, cognitive impairment is more often diagnosed as vascular. In this group of patients, the results of a neuropsychological examination and brain MRI/CT often indicate a mixed etiology of cognitive impairment - a combination of vascular and degenerative deceases. Post-stroke cognitive impairment can be reversible or partially reversible [1]. Mistakes in the diagnosis of cognitive impairment in cases of chronic cerebrovascular ischemia delay the start of specific treatment and lead to a decrease in the quality of life.
Recent epidemiological data from clinical and neuropathological series identify mixed dementia as one of the most common causes of dementia. Zekry D. et al. [2] Observed in a group of patients with AD revealed 50% vascular disorders of different severity. At the same time, 80% (!) of patients diagnosed with VaD had typical AD changes. The pure VaD was observed in 8-15% cases. The combination of AD and VaD leads to an increase of cognitive impairment and ataxia and worsens prognosis [3].
The falling remains one of the most acute problem among the elderly. Physical exercise is a critical key to avoid falls. Using biofeedback systems while exercises facilitate understanding of the task for patients with cognitive impairment [4-6].
Translingual neurostimulation (TLNS) has been used to improve dynamic and static balance and gait of several years. Non-invasive brain stimulation techniques have shown similarly promising potential to modulate brain plasticity in humans. In stroke specifically, both sensorimotor and higher-order cognitive impairments, such as aphasia and neglect, have demonstrated varying degrees of recoverability [7,8]. This fact allowed to start studies TLNS for cognitive impairments of the other etiology.
More than 90% of people with dementia experience neuropsychiatric symptoms which are often distressing and can result in early diminished quality of life, increased frequency of emergency department visits along with stress and ill-health in caregivers. A non-pharmacological intervention programme for symptoms of dementia can help improve neuropsychiatric symptoms. The programme has a multicomponent, structured approach which includes initial person-centred assessment on needs, environment and potential behaviour triggers; education of the family, caregivers and community on dementia care and management; and a personalised, one-on-one programme of selected non-pharmacological intervention sessions [9].
Researchers recognize the need for such further studies and future trials to document the efficacy of non-pharmacological interventions for dementia.
Goals. To study the dynamics of cognitive impairment and the quality of life of patients with mixed (vascular and degenerative) dementia, depending on the methods of patient care (drug treatment or a combination of drug treatment and social involvement of the patient).
The objectives of the study. To determine the relationship between the presence of signs of a mixed (vascular and degenerative) nature of brain damage, the severity of cognitive, motor impairment and the dynamics of quality of life.
MATERIALS AND METHODS
Cohort study of 80 cases, from March 2019 to January 2020, moderate and severe cognitive impairment and previously established diagnoses of chronic cerebrovascular ischemia or discirculatory encephalopathy degree II and III (without any notes about dementia).
Inclusion criteria: MMSE<26 points, FAB<11 points. Exclusion criteria: previously verified dementia of any type. The average age of the patients was 77 ± 6.3 years.
The group 1 included 63 patients residing in a long-term care (LTC) clinic from month to year. On the basis of clinical data and brain MRI (atrophic changes in the temporal and parietal lobes, atrophy of the hippocampi), 30 cases were verified-AD, 2 cases of Lewy Body Dementia (LBD), 13 cases of VaD, 1 case of progressive supranuclear palsy (PSP), 1 case of primary progressive aphasia (PPA), 16 cases of mixed (degenerative and vascular) dementia with a history of hemorrhagic stroke (4 cases) or ischemic stroke (5 cases), including after not neurosurgical intervention (4 cases).
The group 2 included of 17 patients because of the refusal of relatives from the proposed placement of the patient for a long stay in our clinic. Patients in this group were examined by a neurologist and neuropsychologist, and drugs were prescribed them by a neurologist. Their relatives were interviewed and we taught them the specifics of contact with a patient with dementia. Based of clinical and MRI data were diognosed 1 case frontotemporal dementia (FTD), 1 case LBD, 1 case VaD and 14 cases of AD.
The most common MRI-sign in both group was a combination of atrophy of the temporal and parietal lobes with severe subcortical and periventricular white matter lesions.
Due to the presence of ataxia in the group 1, a subgroup of 10 patients was identified that had a high risk of falling according to the Berg balance score (BBS)<43 points and dynamic gait index (DGI)<19 points. Patients in this subgroup did physical exercises on training static and dynamic balance using the TLNS method (PoNS ™, Helius, USA) under the control of stabilometry with biofeedback system (H. Motion Lab ™, Habilect, Russia).
Clinical and instrumental complex: neurological examination, MRI, CT, MMSE, FAB, GDS, EQ-5D VAS. Patients from the group 1 had individual cognitive trainings, classes with an occupational therapist and a specialist in social adaptation, classes in adaptive physical education and increased stability. For both groups were prescribed standart drugs like memantine and acethylcholinesterase inhibitors (AChEI) in therapeutics doses.
Non-pharmacological methods: therapeutic conversation (to reduce the manifestation of anxiety and depression), orientation in place, orientation in time and self (to improve the patient’s cognitive status and social involvement), cognitive stimulation therapy, therapy by memories (restoring the patient’s life history, recognizing relatives), in spring and summer classes (planting and caring for plants, taking into account ergonomics for the elderly - the location of boxes with plants at the patient’s waist level), art, music (visiting concerts, performing favorite songs by patients themselves), aromatherapy (using aromatic oils in the room, when taking a general or local bath), sensory integration (vary in intensity of stimulation from the least sensitive to the most sensitive areas).
RESULTS
Most often problem for patient with cognitive impairment were the orientation in time and place and the decreasing in non-specific modal memory.
In the group 1, the MMSE and FAB scales obtained average values of 17.2 ± 6.4 and 8.7 ± 3.1. In the group 2, the average values of MMSE and FAB were 18.6 ± 5.4 and 9.2 ± 2.3. The quality of life on the visually analogous EQ-5D VAS in the group 1 averaged 74 ± 8.2 points and group 2 averaged 77 ± 5.1 points.
Differences in values were statistically unsignificant, which allowed the groups to be compared.
Average during the stay of patients in the LTC clinic was 58 ± 17,6 days (the minimum time was 26 days, the maximum was 311 days, this patient stay in the clinic now). After 6 months changes of MMSE and FAB data were not statistically significant that means no futher cognitive impairment.
Six months later the quality of life (EQ-5D VAS) increased in the group 1 in 100% of cases 23 ± 4.7 points. In the group 2, changes in the quality of life were not statically significant, the average value decreased by 2 ± 0.7 points. Further exams are planned after 12 and 24 months to assess the quality of life of the patient and his family and the dynamics of the severity of cognitive impairment.
In the subgroup of all 10 patients with the high risk of falling, before to the TLNS, the support of 1 caregiver was requiring.
After 2 weeks of training, as a result of improving the static and dynamic balance, 5 out of 10 patients were able to move on a flat surface independently and needed supporting only when climbing stairs and when walking in the park, 3 patients needed only visual or verbal control, 2 patients were able to move independently on any surface [10-13] (Figure 1).
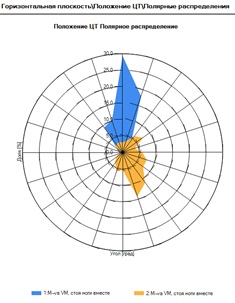 Figure 1: Patient M-va, 74y., the reduced angle of deviation of the center of gravity in the Romberg test (with open eyes) before (blue color) and after (yellow color) the TLNS course according to stabilometry.
Figure 1: Patient M-va, 74y., the reduced angle of deviation of the center of gravity in the Romberg test (with open eyes) before (blue color) and after (yellow color) the TLNS course according to stabilometry.
The improvement in the stabilometry was confirmed by a statically significant improvement in the mean values of BBS and DGI (Figure 2). Before the start of classes, the BBS median value was M=42 (41; 43, min=31, max=47). After classes, the BBS median value was M=50 (47; 55, min=38, max=56). DGI results also had positive dynamics: from first values of the median DGI M=18 (41; 43, min=31, max=47), to the values after classes DGI M=(41; 43, min=31, max=47). All of the described changes helped to reduce the risk of falls in patients with ataxia, despite of the cognitive impairment in this group of patients.
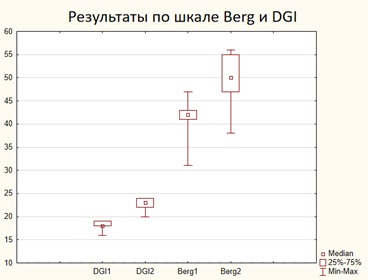 Figure 2: Dynamics of values on the Berg and DGI scales for 2 weeks of TLNS classes and motor rehabilitation aimed at improving the static and dianmic balance.
Figure 2: Dynamics of values on the Berg and DGI scales for 2 weeks of TLNS classes and motor rehabilitation aimed at improving the static and dianmic balance.
The described model of an individual approach is recommended for working with patients with dementia all over the world. But even in developed countries there are difficulties with its full implementation in clinics. Feature of this study is the possibility of long-term (from a month to several years) follow-up of patients in professional nursing care at the same time by neurologists, neuropsychologists, rehabilitation therapists, occupational therapists, and social workers.
CONCLUSION
Cognitive training, occupational therapy and social adaptation, physical exercises should be focused on individual problems of the patient and take into account the etiology of dementia, the severity of cognitive impairment, other diseases, and only then can improve quality of life of the patient and his family.
The use of TLNS (PoNS ™) with physical exercises under the control of stabilometry with biofeedback system (H. Motion Lab ™) in patients with high risk of falling can improve gait and balance, including patients with dementia. The question about the possibility of the cumulative effect of TLNS in this category of patients remains open.
DISCUSSION
The group of patients with mixed dementia (AD and VaD) should be considered separately as the group with the highest risk of developing early mental symptoms and motor disorders that increase the risk of falling.
A differentiated approach to the problem of cognitive impairment in the early stages of dementia (taking into account the etiology of dementia) increases the compliance of the patient and relatives. That can help to improve their quality of life and the effectiveness of drugs therapy.
No potential conflict of interest was reported by the authors.
REFERENCES
- Vasenina EE, Levin OS, Sonin AG (2017) Modern trends in epidemiology of dementia and management of patients with cognitive impairment. Journal of Neurology and Psychiatry 117: 87-95.
- Zekry D, Hauw JJ, Gold G (2002) Mixed dementia: Epidemiology, diagnosis, and treatment. J Am Geriatr Soc 50: 1431-1458.
- Katunina EA (2015) Heterogeneity of vascular cognitive impairment and therapy issues. Neurology, Neuropsychiatry, Psychosomatics 7: 62-69.
- Barabas J, Bednar T, Vychlopen M (2019) Kinect-Based Platform for Movement Monitoring and Fall-Detection of Elderly People.12th International Conference on Measurement IEEE 2019: 199-202.
- https://www.theseus.fi/handle/10024/161281
- https://soar.wichita.edu/handle/10057/16234
- Paltin D, Tyler M, Danilov Y (2017) Cognitive enhancement exciting discovery using trans-lingual neuro-stimulation. J Neurol Neurorehabil Res 2: 39-45.
- http://openict4d.wikidot.com/open-development:edited-volume-and-conference-2010
- Carter MMcL, Wei A, Li X (2019) An individualised, non-pharmacological treatment strategy associated with an improvement in neuropsychiatric symptoms in a man with dementia living at home. BMJ Case Rep 12:
- Avdi? D, Skrbo A (2003) Co-relation between risk factors of falls down and the Berg Balance Scale in elderly people (third age). Bosn J Basic Med Sci 3: 49-55.
- Barry E, Galvin R, Keogh C, Horgan F, Fahey T (2014) Is the Timed Up and Go test a useful predictor of risk of falls in community dwelling older adults: A systematic review and meta-analysis. BMC Geriatr 14: 14.
- Kikkert LHJ, de Groot MH, van Campen JP, Beijnen JH, Hortobágyi T, et al. (2017) Gait dynamics to optimize fall risk assessment in geriatric patients admitted to an outpatient diagnostic clinic. PloS One 12: e0178615.
- Tan ECK, Johnell K, Bell JS, Garcia-Ptacek S, Fastbom J, et al. (2020) Do Acetylcholinesterase Inhibitors Prevent or Delay Psychotropic Prescribing in People With Dementia? Analyses of the Swedish Dementia Registry. Am J Geriatr Psychiatry 28: 108-117.
Citation: © 2020 Borisov AV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Copyright: © 2020 Borisov AV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

