
A Study of OCT Imaging in Acute Angle-Closure Glaucoma Eyes Treated with Phacoemulsification
*Corresponding Author(s):
Bakunina NADepartment Of Ophthalmology, N I Pirogov City Clinical Hospital No1, Leninskiy Prospect 8, Moscow, Russian Federation
Tel:+7 9057575186,
Email:nata-oko@mail.ru
Abstract
Purpose: To analyze the main pathogenic factors of closing and opening of the anterior chamber angle in patients with acute primary angle closure glaucoma before and after phacoemulsification based on Optical Coherence Tomography (OCT) data.
Material and Methods: The study included 35 patients with acute primary angle closure glaucoma. The control group was composed of 25 healthy individuals (50 eyes) with transparent lens and normal IOP and 22 patients (44 eyes) with Primary Open-Angle Glaucoma (POAG). All the patients underwent phacoemulsification.
Results and Conclusion: The clinical studies show increased choroidal thickness especially in the parafovea (254±0.18 microns) compared to control groups without glaucoma. The clinical studies shows increase in the difference between central corneal thickness and peripheral corneal thickness in patients with primary angle closure glaucoma (620±32.25 microns and 750±75.12 microns) compared to control groups without glaucoma (610±30.15 microns and 620±31.11 microns). Phacoemulsification of the lens in acute primary angle closure glaucoma leads to the reduction of choroidal thickness by 64±1.5 microns in the fovea and by 30±1.4 microns in the parafovea; decreases IOP, improves visual functions, extends the anterior chamber angle, and increases the volume and depth of the anterior chamber of the eye. OCT data may be useful in the diagnosis and prognosis of the acute primary angle closure glaucoma treatment outcome.
Keywords
INTRODUCTION
At emergency hospitals, in most of the cases laser management is practically impossible for the patients with Acute Angle Closure Glaucoma (AACG) due to the frank corneal edema.
Hypotensive surgeries in anatomically short eyes practically cause cataract progression. While the removal of the lens in AACG eyes influences the hydrodynamics of the eye [5,6]. However, under high Intraocular Pressure (IOP) the removal of the lens is often problematic due to the high probability of intraoperative complications. Phacoemulsification of the lens in ACG eyes is characterized by pathogenicity, minimum surgical injury and high efficiency which leads to IOP decrease and the improvement of visual functions due to the changes in the anatomical topographic correlations in the eyes of PACG patients [6]. The closed surgery significantly decreases the threat of expulsive hemorrhage and in this case it enables to stop the development of the complication at the stage of transchoroidal exudation.
The introduction of Optical Coherent Tomography (OCT) in clinical practice made it possible to conclude that thicker choroid occurs in the eyes with smaller Anterior-Posterior Axis (APA), thicker lens, and flatter cornea i.e., in hypermetropic patients predisposed to ACG [7]. And in hypermetropia higher then 1.0 diopters the decrease of the Anterior-Posterior Axis (APA) by 1 mm corresponds to the increase of Choroidal Thickness (CT) by 30 microns on average [8].
According to H. Quigley, the cause of the anterior lens dislocation is due to the increase of CT especially in the fovea and peripapillary zone both in ACG and the primary closure of the Anterior Chamber Angle (ACA) [9-12]. According to H. Quigley data, the increase of the choroidal volume by 20% in the eyes predisposed to ACG leads to slit-like anterior chamber and the increase of IOP by 60 mm of mercury, which induces further anterior dislocation of the lens and the development of the pupillary block [13].
The biggest CT is registered in AACG patients. This makes the authors [14] conclude that CT is significant for the ACG pathogenesis and the development of the AACG. It is proved by positive water-drinking test of ACG patients showing increase of CT, swallowing of the anterior chamber and the decrease of the perfusion pressure [15].
There are many publications on the study of the anterior segment spatial structures of PACG eyes by Ultrasound Biomicroscopy (UBM) [16-18] and few publications on the study of Optical Coherence Tomography (OCT) [19]. OCT is a noninvasive contactless investigation technique of big practical interest that offers a quick and simple analysis of the anterior chamber structures.
THE AIM OF THE WORK
MATERIALS AND METHODS
Initial glaucoma was detected in 10 eyes, developed glaucoma was detected in 13 eyes and advanced glaucoma was detected in 12 eyes. Before the surgery, IOP was decreased to 25-28 mm of mercury by the prescription of Carbonic Anhydrase (CA) blockers, diuretics and glycerol per os. In this case, the CA blockers were taken as a choice of therapy because in ACG timololum, elevated IOP should be used in combination with myotics and not as a monotherapy. The administration of myotics before the phacoemulsification of the lens makes the procedure more complicated. After the IOP decrease, 40% glucose solution is administered which subsides corneal edema that helps to do OCT of the cornea and the uvea.
The preoperational refraction of all the eyes was established as the hypermetropia of various degrees. In 25 eyes (71.4%) there was phacosclerosis, in other eyes there was complicated initial or immature cataract with various levels of manifestation, visual acuity before the surgery was 0.01- 0.2 without correction. After 2-3 days the reduction of the corneal edema visual acuity with hypermetropic correction increased up to 0.5- 0.7.
The control groups were composed of 25 healthy individuals (50 eyes) with transparent lens without any changes in IOP and 22 patients (44 eyes) with POAG. The patient’s age was from 55 to 80 years old. The gonioscopic visualized open ACA with a possibility to examine identification zones and the absence of goniosynechiae. The visual acuity in control groups amounted 0.5-0.8 with a small myopic correction. The lens opacity was conditioned by the myopization. The degree of the lens opacity in the control group decreased the social adaptation of able-bodied patients; however, it allowed the unobstructed OCT of the cornea and uvea.
The groups of the study were homogeneous by their age and sex composition. Patients with eye comorbidity (diabetic retinopathy, vitreomacular traction syndrome, etc.) were excluded from the study.
All the patients had standard preoperational and post operational ophthalmological examination and the OCT of the anterior and posterior segments.
The OCT of the anterior and posterior segments is provided for the identification of central and peripheral corneal thickness, level of ACA opening and the CT. The level of ACA opening was evaluated by the anterior chamber opening distance and depth. IOLmaster provided for the identification of the depth of the anterior chamber. The ACA opening distance is the distance between the posterior surface of the cornea and the iris in 500 microns from the scleral spur.
The CT was measured by Cirrus HDOCT (Carl Zeiss Meditec Inc., Dublin, CA) tomographer with the possibility to use the EDIOCT (Enhanced Depth Imaging) program. The investigation was performed in HD 5 line raster mode with the flag on EDI sign. The thickness was measured on the cross-section going through the central fovea and in the parafovea (1000 microns from the fovea towards the temporal side) from the outward border of the pigment epithelium to the inner border of the sclera (lamina fusca). The quantitative evaluation of the main biomechanical components of ACA closing in the experimental and control groups is given in table 1.
|
Parameter |
Group withAACG |
Group with POAG |
Group without glaucoma |
|
Distance of ACA opening in 500 microns from the scleral spur, mm |
0.05±0.05 |
0.27± 0.05 |
0.25 ± 0.05 |
|
Choroidal thickness in the fovea, microns |
334±0.23 |
240.5±0.11 |
311.5±0.12 |
|
Choroidal thickness in the parafovea, microns |
254±0.18 |
121.2±0.12 |
145.1±0.14 |
|
APA, mm |
21.48±0.62 |
23.1±0.65 |
23.2±0.57 |
|
The depth of the anterior chamber, mm |
2.11±0.19 |
3.19±0.29 |
3.29±0.28 |
86% patients admitted to the hospital with AACG had the anterior chamber angle closed (Figure 1). 5 (14.3%) patients of the main group had the slit-like angle of the anterior chamber before the surgery.
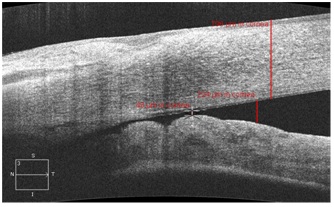
Figure 1: Anterior chamber angle in the OCT image in a patient with acute angle closure glaucoma. Peripheral corneal thickness.
According to a-scanning APS was within 19.4-21.6 mm, the average value 20±1.6 mm.
The ultrasound phacoemulsification of the lens was performed on INFINITI Vision System, «Alkon». All the patients had soft IOL implanted intraoperatively. Its size was 4-5 times smaller than the size of the genuine lens.
The additional increase of corneal transparency in patients with high IOP and corneal edema was performed intra operationally by diepitalization (mechanical removal of the epithelium in the diameter of 5.0-6.0 mm) and the discharge of the Intraocular Fluid (IOF) through paracentesis was sufficient for the visualization of all the stages of the surgery. In high intraocular pressure and shallow anterior chamber, the staged decompression of the eyeball including posterior sclerectomy and active draining of the vitreous body by Tsur Nedden needle was performed. The fluid was evacuated along the needle independently due to the impact of the increased IOP. Simultaneously, in the anterior chamber viscoelastic was introduced which opens the anterior chamber angle partially removing the pretrabecular retention. The nucleus was chopped by the branches of capsulorhexis forceps that were placed into the received grooves of the nucleus and moved apart (RF patent #2528633) [20]. This technique does not allow excessive rocking of Zinn’s zonules that are weak in ACG due to the sparing rotation of the nucleus of lens. The latter is explained by the fact that the first two grooves are made from one point and the nucleus can already be chopped by capsulorhexis forceps under the decrease of depth of the nucleus of lens by half. After the IOL implantation, patients had goniosynechiae removed (if there were any) along the full circumference by micro spatula in the anterior chamber filled by viscoelastic.
RESULTS AND DISCUSSION
The investigation of anatomical biometric parameters by OCT and IOL master was carried out 1 month after cataract phacoemulsification with IOL implantation. The changes of the pathogenic components of ACA closing after cataract phacoemulsification + IOL implantation are given in tables 2-4.
|
Parameter |
AACG group (35 eyes) |
POAG group (44 eyes) |
Group without glaucoma (50 eyes) |
|
Anterior chamber before the surgery |
2.11±0.19 |
3.19±0.29 |
3.29±0.28 |
|
Anterior chamber after the surgery (in 3-7 days) |
3.28±0.25 |
3.48±0.24 |
3.74±0.24 |
Table 2: Dynamics of the changes in the depth of the anterior chamber (mm) 1 month after phacoemulsification with IOL implantation according to IOL master data.
|
Parameter |
AACG group (35 eyes) |
POAG group(44 eyes) |
Group without glaucoma (50 eyes) |
|
ACA opening distance before phacoemulsification + IOL, mm |
0.05±0.05 |
0.37±0.05 |
0.42±0.05 |
|
ACA opening distanceafter phacoemulsification + IOL, mm |
0.31±0.1 |
0.38±0.05 |
0.45±0.05 |
Table 3: Dynamics of the width of the iridocorneal angle (mm) after phacoemulsification with IOL implantation according to OCT data.
|
Parameter |
AGA group (35 eyes) |
POAG group(44 eyes) |
Group without glaucoma (50 eyes) |
|
CT before phacoemulsification+IOL in the fovea |
334±1.23 |
240.5±0.11 |
311.5±0.12 |
|
CT before phacoemulsification+IOL in the theparafovea |
254±1.18 |
121.2±0.12 |
145.1±0.14 |
|
CT after phacoemulsification+IOL in the fovea |
270±1.13 |
235±0.12 |
310±0.12 |
|
CT after phacoemulsification+IOL in the parafovea |
224±1.14 |
120±0.11 |
142±0.09 |
Table 4: Dynamics of CT changes (microns) in the fovea and in the peripapillary zone in 1 month after phacoemulsification with IOL implantation according to OCT.
After the surgery TC significantly decreased in AACG group (p≤0.05) - by 64±1.5 microns in the fovea and by 30±1.4 microns in the parafovea, in our opinion this played a role in IOP decrease (Figure 2 and 3).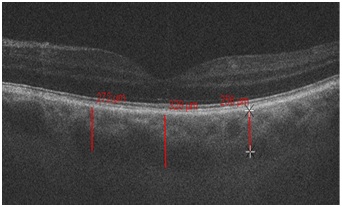
Figure 2: Choroidal thickness in the fovea and parafovea in a patient with acute angle closure glaucoma before phacoemulsification.
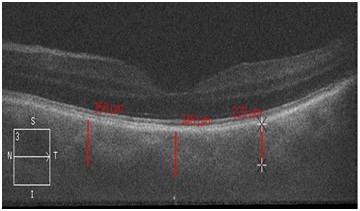
Figure 3: Choroidal thickness in the fovea and parafovea in a patient with the acute angle closure glaucoma after the phacoemulsification of the lens.
There was a significant increase in the anterior chamber depth (p≤0.05) - by 0.19±0.05 mm (Table 2) and a significant increase in ACA opening distance (p≤0.05) after the phacoemulsification of the lens in AACG - by 0.26±0.05 microns (Table 3). This was confirmed by gonioscopy data.
The gonioscopy shows that, after surgery in all the AACG eyes, the anterior chamber angle profile widened to the average and wide level of opening (Van Beuningen classification) (Figure 4). The quantitative analysis of the anterior segment parameters identified by OCT confirms high reliability which is comparable to the subjective gonioscopy data.
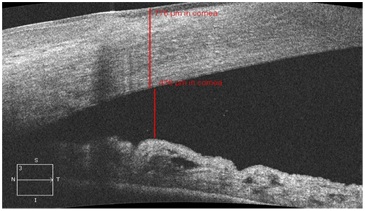
Figure 4: Opening of the anterior chamber angle in the OCT image in a patient with acute angle closure glaucoma after the cataract phacoemulsification.
During the surgeries, clinically significant complications were not noticed. In the postop period 8 (22.8%) patients of the main group had residual corneal edema, 3 (8.57%) patients with the developed and advanced ACG had postop inflammation reaction. During discharge from the hospital the visual acuity of patients with the initial stage of ACG was 0.85±0.05; with the developed stage – 0.62±0.1, with the advanced stage -0.59±0.12. Increase of visual acuity was linked to the reduction of the corneal edema and IOP normalization. All the patients had the stabilization of visual functions during 1 year after the surgery. IOP average level was 12.5±0.87 mm of mercury.
Patients with initial glaucoma had IOP normalized without hypotensive drops. Patients with the developed stage had tonometric IOP within normal values. 3 patients (8.57%) from this group needed hypotensive therapy for the normalization of hydrodynamic indicators. In 3 eyes (8.57%) with advanced glaucoma we failed to normalize the pressure by medications. They had Hypertensive Non penetrating Deep Sclerotomy (HDNS) in the period of 2 to 4 months after cataract phacoemulsification. HDNS gave a positive effect since it was performed on the ACA opened after the phacoemulsification of the lens.
After the lens phacoemulsification in AACG patients, the anterior chamber was deepened on an average by 1.55 times, while in the control groups by 1.09 and 1.13 respectively. The biggest angle opening took place in the group of AACG patients where the opening distance increased by 6.2 times in the postop period (Figure 4). In the two control groups these indicators increased by 1.02 and 1.07 times respectively. The difference in ACA widening between the main and the control groups was statistically significant (p<0.05). The changes of the topographic correlations of the anterior segment structures detected in the early postop period remained in the next periods of the observation.
We noticed the decrease in TC in the main group especially in the fovea (by 1.23 times), while in the control groups; this indicator practically did not change (Figure 3). In our opinion, this is one of the hypotensive factors in AACG.
In the postoperative period, 14 days-1 month after the reduction of the postoperative edema the corneal thickness was evaluated. According to the anterior segment OCT data, central corneal thickness amounted 620 ±32.25 mm. Besides, we identified increase in the peripheral corneal thickness comparing to the eyes of the control groups (Figure 1). The difference between central and peripheral corneal thickness in ACG was more than 130 microns (Table 5) which was more significant than in the control groups where the difference did not exceed 100 microns (p<0.05). That made us concludes that together with other specifics of a hypermetropic eye, the thicker cornea in the periphery can play its role in closing of ACA in the acute glaucoma too.
|
Parameter |
AACG group |
POAG group |
Group without glaucoma |
|
Central corneal thickness, microns |
620±32.25 |
590±22.27 |
610±30.15 |
|
Peripheral corneal thickness, microns |
750±75.12 |
610±20.15 |
620±31.11 |
After the lens removal, in such eyes significant deepening of the anterior chamber, ACA widening and TC decrease take place, thus, PACG pathogenic mechanisms are neutralized improving the intraocular fluid circulation that is very important for IOP regulation in PACG eyes. Based on the results which are received allows suggesting phacoemulsification of the lens for acute angle closure glaucoma management.
CONCLUSION
2) Significant difference between the central and peripheral corneal thickness after the acute angle closure glaucoma relief in comparison with the control groups was established that could be considered one of the pathogenic factors of ACG
3) The data of the OCT of the cornea, ACA and choroid allowed the extension of indications to the phacoemulsification of the lens in ACG and can be useful in the diagnosis and prognosis of acute glaucoma management
REFERENCES
- Libman ES (2009) Epidemiological characteristics of glaucoma. Glaucoma 1: 2-3.
- Nesterov A P Glaucoma (1995) ?.: Medicine (In Russian).
- Ereskin NN, Zuev VK, Sokolovskaya TV (1997) Integrated approach to the treatment of primary narrow-angle glaucoma. In: Proceedings of Russian conference "Eroshevsky reading" Samara 1995: 106-107 (In Russian).
- Egorova EV, Faizieva US (2009) Phacoemulsification of the lens with residual closure of the anterior chamber angle after laser iridectomy in patients with primary angle-closure glaucoma in Uzbekistan. Bulletin of SO RAMS 4: 16-21 (In Russian).
- Malov IV, Gabdrahmanov LM, Galeev RS, Malov VM, Eroshevskaya VB, et al. (2009) ?hacoemulsification of lens in patients with primary angle-closure glaucoma. Vestnik Orenburgskogo gosudarstvennogo universiteta 12: 88-90 (In Russian).
- Balashevich L, Pravosudova ? (2006) Phaco therapy for angle-closure glaucoma. Ophthalmology Times Europe 8: 40-43.
- Wei WB, Xu L, Jonas JB, Shao L, Du KF, et al. (2013) Subfoveal choroidal thickness: the Beijing eye study. Ophthalmology 120: 175-180.
- Elagouz M, Stanescu-Segall D, Jackson TL (2010) Uveal effusion syndrome. Surv Ophthalmol 55: 134-145.
- Quigley HA, Friedman S, Congdon NG (2003) Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma 12: 167-180.
- Wang W, Zhou M, Huang W, Chen S, Ding X, et al. (2013) Does acute primary angle closure cause an increased choroidal thickness? Invest Ophthalmol Vis Sci 54: 3538-3545.
- Zhou M, Wang W, Ding X, Huang W, Chen S, et al. (2013) Choroidal thickness in fellow eyes of patients with acute primary angle closure measured by enhanced depth imaging spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 54: 1971-1978.
- Kurysheva NI, Ardzhevnishvili TD, Kiseleva TN, Fomin AV (2013) Choroid at glaucoma: results of study by optical coherent tomography. Glaucoma 3: 73- 83 (In Russian).
- Quigley HA (2009) What’s the choroid got to do with angle closure? Arch Ophthalmol 127: 693- 694.
- Kurysheva NI, Boyarinceva MA, Fomin AV (2013) Choroidal thickness in primary angle-closure glaucoma: the results study by Optical Coherence Tomography. Ophthalmology 10: 26-31. (In Russian).
- Arora KS, Jefferys JL, Maul EA, Quigley HA (2012) Choroidal thickness change after water drinking is greater in angle closure than in open angle eyes. Invest Ophthalmol Vis Sci 53: 6393-6402.
- Dolzhich GI, Nesterova EE (2009) A clinicoanatomic classification of the iridociliary area in healthy individuals. Vestn Oftalmol 5: 18-21.
- Takhchidi K P, Egorova EV, Shermuhamedov AA (2009) The Informative value of ultrasound biomicroscopy in the diagnosis of intraocular blocks in patients with primary angle-closure glaucoma. Ophthalmokhirurgija 3: 39-44 (In Russian).
- Egorova EV, Malyugin BE, Morozova TA (2010) Anatomo-topographic features of the anterior segments of the eye artifacing the results of the study by means of ultrasound biomicroscopy. Ophthalmokhirurgija 4: 4-9 (In Russian).
- Kucumen RB, Yenerel NM, Gorgun E, Kulacoglu DN, Dinc UA, et al. (2008) Anterior segment optical coherence tomography measurement of anterior chamber depth and angle changes after phacoemulsification and intraocular lens implantation. J Cataract Refract Surg 34: 1694-1698.
- Bakunina NA (2014) The method of phacoemulsification. Patent RF N2528633 (in Russian).
Citation: Bakunina NA, Kolesnikova LN (2017) A Study of OCT Imaging in Acute Angle-Closure Glaucoma Eyes Treated with Phacoemulsification. J Ophthalmic Clin Res 4: 036
Copyright: © 2017 Bakunina NA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

