
AI-Driven Personalized Nutrition for Metabolic Care: A Perspective on Equitable Digital Health Solutions
*Corresponding Author(s):
Anand K GavaiIndustrial Engineering & Business Information Systems, University Of Twente, Enschede, Netherlands
Email:anand.gavai@gmail.com
Abstract
Metabolic diseases, including obesity and type 2 diabetes, pose a growing public health and economic burden worldwide. Conventional dietary guidelines often fail to address individual nutritional needs or to achieve adherence in resource-constrained settings. This Perspective highlights an integrative framework that leverages Large Language Models (LLMs), Retrieval-Augmented Generation (RAG), and federated learning to enable culturally sensitive, privacy-preserving, and scalable dietary interventions. Building on prior simulation studies demonstrating over 80% compliance with clinical nutrition targets such as DASH and EASD guidelines, this approach supports real-time, explainable nutrition guidance through virtual nutritionist systems. The framework aligns with clinical nutrition practices, emphasizes sustainable and localized dietary strategies, and has the potential to enhance digital health equity by extending evidence-based nutritional support to underserved populations. Future real-world validation and long-term studies are needed to assess the public health impact of such AI-driven nutrition systems.
Keywords
Clinical nutrition; DASH diet; Digital health; Federated learning; Large language models; Obesity; Personalized dietary interventions; Type 2 diabetes
Introduction
Metabolic diseases such as obesity and type 2 diabetes affect over 537 million adults globally, with economic costs exceeding US$1.3 trillion annually [1]. In the Netherlands, more than half of adults are overweight and approximately 1.2 million live with type 2 diabetes [2]. Clinical nutrition guidelines-though essential for public health-often lack the granularity required to address individual variation in dietary needs and cultural eating patterns. This gap is particularly evident in underserved or low-resource settings, where traditional nutrition counseling remains difficult to scale.
The expanding availability of digital platforms presents an opportunity to deliver personalized nutrition interventions that are both adaptive and clinically aligned. Advances in Artificial Intelligence (AI), Especially Large Language Models (LLMs), have opened pathways for translating dietary guidelines into actionable, context-aware recommendations [3]. This Perspective explores the role of AI-powered nutrition frameworks that integrate personalized dietary guidance with clinical and public health goals. Building on prior simulation studies of AI-driven recipe generation for obesity and type 2 diabetes [4], the proposed framework aligns with the broader objective of improving dietary adherence and managing metabolic risk factors at scale.
Potential of LLMs in Metabolic Nutrition Care
LLMs can analyze unstructured dietary data, such as food diaries and lifestyle metrics, to provide tailored recommendations that align with clinical nutrition targets. When combined with Retrieval-Augmented Generation (RAG), these models integrate evidence from structured dietary databases, such as USDA guidelines and Dutch RIVM data, to produce balanced meal plans that meet caloric, fiber, and glycemic criteria [4]. Recent experiments have shown that LLM-generated recipes can achieve compliance rates exceeding 80% for DASH-aligned dietary targets, including ≤ 350 kcal per meal and ≥8g of dietary fiber [4].
Federated learning extends this capability by enabling localized model training on region-specific dietary data while preserving data privacy [5]. This ensures that dietary recommendations are not only nutritionally sound but also culturally relevant-for example, substituting local lentils for quinoa or adapting seasoning patterns to regional preferences. Moreover, the interpretability of outputs (e.g., “chia seeds help stabilize postprandial glucose levels”) improves trust and usability across diverse literacy levels [5].
Challenges and Limitations
Despite these advantages, LLM-driven nutrition systems face notable limitations. Unvalidated outputs may include unsafe or nutritionally imbalanced suggestions-such as recommending high-sugar foods for diabetic patients-if robust safety layers are not in place [6]. This highlights the need for virtual nutritionist layers that cross-check model outputs against validated dietary guidelines before presentation to users.
Biases in training datasets, which are often Western-centric, may lead to culturally inappropriate recommendations or exclusion of local foods [7]. Additionally, federated learning requires standardized data formats across institutions, and LLMs currently lack intrinsic accountability for clinical decision-making [8]. These challenges underscore the importance of rigorous human oversight, robust testing in clinical nutrition settings, and adherence to regulatory standards before implementation.
Misinformation and Trustworthiness
Trust in AI-driven nutrition tools depends on their transparency, clinical alignment, and reproducibility. Human-in-the-loop systems involving registered dietitians or primary care providers can ensure that AI-generated outputs support rather than replace professional judgment. Auditing mechanisms-such as prompt logging and reproducibility tracking-further strengthen reliability. Regulatory frameworks like the FDA’s Digital Health Software Precertification Program provide a potential pathway for validating such tools in clinical nutrition care.
Related Work and Research Gaps
Current digital health platforms often lack real-time personalization or culturally adaptive meal planning. Existing tools such as NGQA or MOPI-HFRS offer limited integration of federated learning or retrieval-based personalization [9,10]. Previous studies using Dutch cohorts demonstrate feasibility in generating personalized nutrition plans [11,12], but large-scale clinical deployment remains underexplored.
A critical gap lies in combining local food systems, patient biometrics, and clinical dietary guidelines into a unified platform. Few systems successfully merge DASH or ADA guidelines with dynamic personalization and cultural adaptation. Addressing these gaps requires cross-disciplinary efforts spanning nutrition science, data ethics, and AI.
Key Opportunities For Translation
The integration of AI in clinical nutrition offers multiple avenues for impact:
- Global Clinical-Nutritional Repositories: By linking biomarkers, diet logs, and regional food databases, multimodal datasets can enable fine-tuned LLM personalization aligned with DASH and Mediterranean diets [13].
- Ethical Deployment Ecosystems: Federated models adhering to GDPR and HIPAA principles ensure data security while reducing the risks of misinformation [14].
- Explainable AI in Nutrition Counseling: Substituting high-carb foods with low-glycemic alternatives through transparent explanations supports adherence and patient understanding [15].
- Bias Mitigation: Leveraging multilingual and inclusive datasets can reduce failure rates in recipe generation and improve cultural alignment [16].
- Community Engagement: The use of “digital navigator” roles-community health workers trained to bridge usability gaps-can increase digital literacy in low-resource settings [17,18].
- Sustainable Business Models: Virtual nutritionist systems can generate employment opportunities while scaling dietary counseling in rural or underserved communities [19].
Figure 1 illustrates this sociotechnical framework, highlighting the flow of data from clinical observations, national dietary guidelines, biomarkers, and food diaries into AI-powered models. It emphasizes the interplay between federated learning, socioeconomic adaptability, user feedback, and policy-level integration to create culturally appropriate, sustainable, and equitable nutrition interventions.
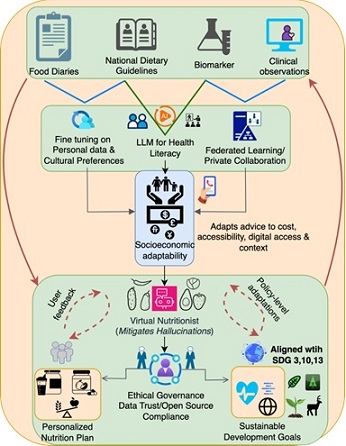
Figure 1: A sociotechnical framework for equitable, personalized nutrition in metabolic care. Large Language Models (LLMs) are fine-tuned through federated learning and cultural preferences. A virtual nutritionist layer validates outputs, while ethical governance ensures compliance with data trust and open-source principles. Feedback loops from users and policy systems enable adaptive updates, aligning outcomes with Sustainable Development Goals (SDGs) 3, 10, and 13.
Scaling Impact and Public Health Relevance
This framework supports both individualized care and population-level strategies. By delivering telehealth-compatible, seasonal meal plans that draw on locally sourced ingredients, the system can reduce barriers to dietary adherence while promoting environmental sustainability. Such AI-driven systems can also assist policymakers in reducing dependence on imported foods, optimizing dietary patterns for both health and ecological outcomes. Integrating knowledge graphs into this ecosystem enables dynamic labeling and sustainable ingredient substitutions aligned with SDGs 12 and 13 [20].
Conclusion and Outlook
The convergence of large language models, federated learning, and nutritional science offers a promising pathway for next-generation clinical nutrition support. By enabling real-time, culturally aware, and privacy-preserving dietary guidance, these frameworks have the potential to strengthen both individual health outcomes and public health nutrition strategies. However, their success hinges on transparent validation, ethical oversight, and collaboration across healthcare, nutrition science, and AI development. As digital tools increasingly shape the future of dietary care, ensuring equity, safety, and trust will be central to their acceptance and impact.
Declarations
Author Contributions
AKG conceptualized the study, developed the framework, and wrote the manuscript.
Conflict of Interest
The author declares no conflicts of interest.
Data Availability
Not applicable.
Funding
This work was supported through the ‘High Tech for a Sustainable Future’ capacity-building programme of the 4TU Federation in the Netherlands.
References
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, et al. (2022) IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice 183: 109119.
- Hilderink H, Duan MJ, Boer GD, Dekker LH, Poos MJJC (2025) Analysis of Life Expectancy for People with Diabetes in the Netherlands, and the Role of Income. The Lancet.
- Li CY, Chang KJ, Yang CF, Wu HY, Chen W, et al. (2025) Towards a holistic framework for multimodal LLM in 3D brain CT radiology report generation. Nat Commun 16: 2258.
- Gavai AK, Hillegersberg JV (2025) AI-driven personalized nutrition: RAG-based digital health solution for obesity and type 2 diabetes. PLOS Digit Health 4: 0000758.
- Gavai A, Bouzembrak Y, Mu W, Martin F, Kaliyaperumal R, et al. (2023) Applying federated learning to combat food fraud in food supply chains. npj Sci Food 7: 46.
- Huang L, Yu W, Ma W, Zhong W, Feng Z, et al. (2025) A survey on hallucination in large language models: Principles, taxonomy, challenges, and open questions. ACM Trans Inf Syst 43: 1-55.
- Omar M, Sorin V, Agbareia R, Apakama DU, Soroush A, et al. (2025) Evaluating and addressing demographic disparities in medical large language models: A systematic review. Int J Equity Health 24: 57.
- Gabison GA, Xian RP (2025) Inherent and emergent liability issues in LLM-based agentic systems: A principal-agent perspective. Arxiv.
- Zhang Z, Li Y, Le NHL, Wang Z, Ma T, et al. (2024) NGQA: A Nutritional Graph Question Answering benchmark for personalized health-aware nutritional reasoning. Arxiv.
- Zhang Z, Wang Z, Ma T, Taneja VS, Nelson S, et al. (2024) MOPI-HFRS: A Multi-Objective Personalized Health-aware Food Recommendation System with LLM-enhanced Interpretation. Arxiv.
- Clusmann J, Kolbinger FR, Muti HS, Carrero ZI, Eckardt JN, et al. (2023) The future landscape of large language models in medicine. Commun Med 3: 141.
- Pavon JM, Schlientz D, Maciejewski ML, Economou-Zavlanos N, Lee RH (2025) Large Language Models in Diabetes Management: The Need for Human and Artificial Intelligence Collaboration. Diabetes Care 48: 182184.
- Rajendran S, Pan W, Sabuncu MR, Chen Y, Zhou J, et al. (2024) Learning across diverse biomedical data modalities and cohorts: Challenges and opportunities for innovation. Patterns 5: 100913.
- Bourne NA, Gill G, Cooley K (2024) Cultural Adaptations Addressing Diversity and Health Access in the Mediterranean Diet: A Realist Synthesis. CANDJ 31: 37-46.
- Buyuktepe O, Catal C, Kar G, Bouzembrak Y, Marvin H, et al. (2023) Food fraud detection using explainable artificial intelligence. Expert Systems 42: 13387.
- Alhanai T, Kasumovic A, Ghassemi MM, Zitzelberger A, Lundin JM, et al. (2025) Bridging the Gap: Enhancing LLM Performance for Low-Resource African Languages with New Benchmarks, Fine-Tuning, and Cultural Adjustments. AAAI 39: 27802-27812.
- Haripriya R, Khare N, Pandey M (2025) Privacy-preserving federated learning for collaborative medical data mining in multi-institutional settings. Sci Rep 15: 12482.
- Abbott LS, Elliott LT (2017) Eliminating Health Disparities through Action on the Social Determinants of Health: A Systematic Review of Home Visiting in the United States, 2005-2015. Public Health Nursing 34: 2-30.
- Gavai AK, Bouzembrak Y, Xhani D, Sedrakyan G, Meuwissen MPM, et al. (2025) Agricultural data privacy: Emerging platforms & strategies. Food and Humanity 4: 100542.
- Gavai A, Vadalkar S, Sharma M (2025) Ai-Driven Knowledge Graphs for Future Foods: Optimizing Sustainable Protein Alternatives and Reducing Environmental Impact. SSRN Product & Services.
Citation: Gavai AK (2025) AI-Driven Personalized Nutrition for Metabolic Care: A Perspective on Equitable Digital Health Solutions. HSOA J Food Sci Nutr 11: 218.
Copyright: © 2025 Anand K Gavai, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

