
Alginate Edible Coating and Cold Storage for Improving the Physicochemical Quality of Cape Gooseberry (Physalis Peruviana L.)
*Corresponding Author(s):
Catarina Pedro CarvalhoCorpoica, C I La Selva, Rionegro, Antioquia, Colombia
Tel:+57 5745311856, 44055
Email:cpassaro@gmail.com
Abstract
The Cape gooseberry is an exotic tropical fruit and, nowadays, is the second most exported fruit from Colombia. Therefore, the high demand for quality required research for a better understanding of fruit behavior. Furthermore, postharvest quality properties play an important role in meeting consumer demands. Cold storage and edible coatings are reported as efficient technologies for extending shelf life and preserve the quality of fruits in postharvest. As there are no reports of studies about the effect of these technologies on shelf life, quality and antioxidant activity in Cape gooseberry, this work aim to evaluate the use of alginate 1% during 21 days of storage at 2ºC as an alternative for postharvest handling of this fruit. Cape gooseberry exhibits a high respiration rate and ethylene production at 20ºC. Alginate coat decreased significantly the metabolism activity of fruit during the cold storage without change significantly the fruit organoleptic quality and showing total phenolic, carotenoid contents and antioxidant activity. Alginate is an efficient edible coat for preserve the quality and bioactivity of Cape gooseberry during 21 days of storage at 2ºC.
Keywords
INTRODUCTION
Cape gooseberry (Physalis peruviana L.) is a tropical fruit of the Solanaceous family, native of the Andean region that bears cherry tomato-like fruit, being Colombia and South Africa the biggest producers and exporters and Germany and Netherlands the principal importers. Physalis have few cultivars and rather genotypes that has been selected in different countries and adapted to the different climates of the specific regions (ecotypes). The main ecotypes commercialized are associated to the production country: ‘Colombia’, ‘Kenya’, ‘South Africa’ and ‘Ecuador’. This fruit is one of the most promising exotic fruits and many interesting functional products could be developed from these berries [1].
Nevertheless, is necessary to implement appropriate technologies and improve postharvest handling operations, in order to obtain fruit of excellent quality and guarantee it for marketing, avoiding high product losses [2].
Normally, Cape gooseberry fruit is exported in fresh to Europe with the calyx because this protect the fruit and enhanced the shelf life, but USA import this fruit without the calyx because of the cold quarantine treatment (T-107-b) required, being necessary to replace this protection for enhanced the shelf life. In this sense, edible coatings are known to increased storage period and preserve de quality of many fruits [3]. Alginate is a natural polysaccharide extracted from brown sea algae (Phaeophyceae) and it is composed of two uronic acids: β-D-mannuronic acid and α-L-guluronic acid. Sodium alginate is composed of block polymers of sodium poly (L-guluronate), sodium poly (D-mannuronate), and alternating sequences of both sugars. Alginate is known as a hydrophilic biopolymer that has a coating function because of its well-studied unique colloidal properties, which include its use for thickening, suspension forming, gel forming, and emulsion stabilizing [4]. As edible coating, sodium-alginate has been effective on maintaining postharvest quality of tomato [5] and plum cultivars [6].
Cape gooseberry has a high nutritional composition and biologically active health-promoting components [7]. It has been used as a good source of provitamin A, minerals, vitamin C and vitamin B complex. It also contains high levels of antioxidant compounds as well as minerals such phosphorous and iron. In traditional Colombian medicine is widely used as an anti-inflammatory medicinal plant. Cape gooseberry fruit is a climacteric fruit and its ripening is regulated by ethylene [8]. Reports indicate that the fruit contains high level of antioxidant compounds [7,9]. Due to a high antioxidant capacity of this fruit species, its popularity above all as a promising raw material, which can be used for human nutrition.
Storage of fruit for consumption exposes the physicochemical, color, antioxidant capacity and sensory characteristics to detrimental factors that may lead to alterations in concentrations and health-related quality, being important to investigate the effect of alginate coating and cold storage on the bioactive compounds that are present in the fruit. Since there is no literature on the use of alginate on Cape gooseberry quality and antioxidant properties, the aim of this work was to evaluate the effect of an edible coating based on alginate on the quality and antioxidant activity of Cape gooseberry during cold storage.
MATERIALS AND METHODS
Plant material and edible coating
Once at laboratory, 21 homogeneous lots (based on color and size) of ten fruits each were performed at random. Three lots were used to determine the fruit properties at harvest (day 0) and the 18 remaining were split into two groups for the following treatments in triplicate: 0% (control) and 1% (w/v) alginate coating.
Alginate (alginic acid sodium salt from brown algae purchased from Sigma, Madrid, Spain) was prepared according to a previous paper [5] (at 1% concentration w/v, by dissolving alginate in hot water (45ºC) with continuous shaking until the solution became clear. After cooling to 20ºC, glycerol at 20% (v/v) was added as a plasticiser, and treatments were performed by dipping the fruit twice in fresh coating solutions for 1min to ensure the uniformity of the coating of the whole surface. Control fruit were dipped in distilled water. After treatments, fruit were dried for 30 min using an air-flow heater at 25ºC. After drying, the lots were weighed, and then stored at 2ºC during 21 days. Three lots for control and treated fruits were sampled at random after 7, 14 and 21 days of storage.
Respiration rate and ethylene production
Quality parameters
After that the 10 fruits of each replicate were cut in small pieces to obtain a homogeneous sample. Total Soluble Solids (TSS) were determined in duplicated in the juice obtained from 5g of each sample with a digital refractometer Atago PR-101 (Atago Co. Ltd., Tokyo, Japan) at 20°C, and expressed as ºBrix (mean ? SD. Total Titratable Acidity (TTA) was determined in duplicated in the same juice by automatic titration (785 DMP Titrino, Metrohm) with 0.1N NaOH up to pH 8.1, using 1mL of diluted juice in 25mL distilled H2O, and results (mean ± SD) expressed as g citric acid equivalent 100g-1 fresh weight. The maturity index was calculated as the quotient TSS/TTA. The remaining samples from each replicate were quickly frozen in liquid N2 and stored at-20ºC until the following determinations were performed.
Antioxidant analysis
Phytochemical analysis
Total phenolics were extracted as previously reported [10], using water:methanol (2:8) containing 2mM NaF (to inactivate polyphenol oxidase and prevent phenolic degradation) and quantified in duplicated in each extraction by using the Folin-Ciocalteu reagent [13]. Results (mean ± SD) were expressed as mg gallic acid equivalent 100g-1 fresh weight.
Statistical analysis
RESULTS
Figure 1, shows the ethylene production and respiration rate of Cape gooseberry during ripening at 20°C without coating, showing that this fruit has a climacteric ripening-pattern reaching the ethylene peak (ca. 350nL g-1 h-1) after 2 days of storage at 20ºC. Respiration rate increased at the end of storage (day 9) until ca. 200mg kg-1 h-1, maybe due to overripe of the fruit.
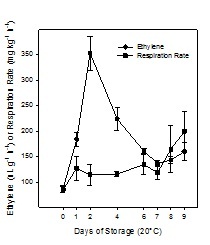 Figure 1: Ethylene production and respiration rate of Cape gooseberry during storage at 20°C without coating. Data are the mean ± SD (n = 3) and vertical bars represent the standard deviation.
Figure 1: Ethylene production and respiration rate of Cape gooseberry during storage at 20°C without coating. Data are the mean ± SD (n = 3) and vertical bars represent the standard deviation.
The ethylene production and respiration rate of control and alginate-coated Cape gooseberry stored during 21 days at 2ºC are presented in figure 2. At day 0 in control fruits, ethylene production was 88.0nL g-1 h-1 and decreased during the first 7 days of storage although ethylene production peaked at day 14 which could be associated to occurrence of climacteric peak, as has been observed at 20°C at day 2 (Figure 1). Respiration rate was 85.6mg kg-1 h-1 at day 0 and remained without significant changes during the first 14 days of storage. In alginate-coated fruits, the same behavior was observed, although ethylene production and respiration rate were significantly lower in all sampling dates (Figure 2).
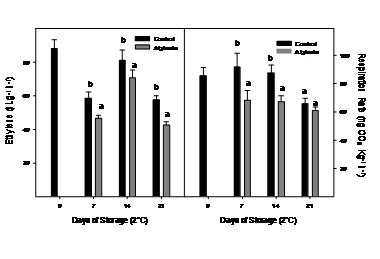 Figure 2: Ethylene production and respiration rate of Cape gooseberry coated with alginate and storage during 21 days at 2ºC. Data are the mean ± SD (n = 3) and vertical bars represent the standard deviation. Same letters represent non significant differences (p>0.05) between treatments.
Figure 2: Ethylene production and respiration rate of Cape gooseberry coated with alginate and storage during 21 days at 2ºC. Data are the mean ± SD (n = 3) and vertical bars represent the standard deviation. Same letters represent non significant differences (p>0.05) between treatments.
Fruit color index did not change significantly after 21 days of cold storage in control fruit storage, observing no significant differences between coated and non-coated fruit (Figure 3).
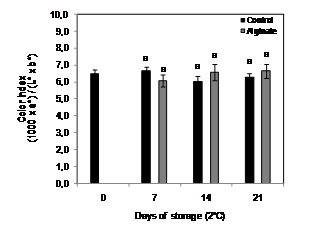 Figure 3: Color evolution of Cape gooseberry coated with alginate and storage during 21 days at 2ºC. Data are the mean ± SD (n = 3) and vertical bars represent the standard deviation. Same letters represent non-significant differences (p>0.05) between treatments.
Figure 3: Color evolution of Cape gooseberry coated with alginate and storage during 21 days at 2ºC. Data are the mean ± SD (n = 3) and vertical bars represent the standard deviation. Same letters represent non-significant differences (p>0.05) between treatments.
No significant changes were observed for total soluble solids and total acidity during storage and generally neither between treatments (Figure 4).
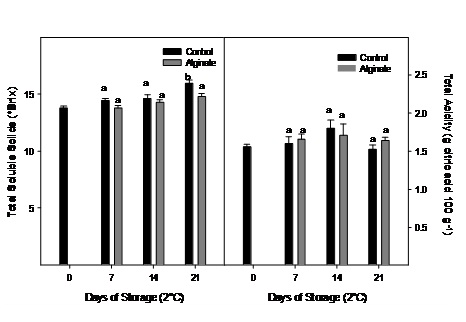 Figure 4: Total soluble solids and acidity evolution of Cape gooseberry coated with alginate and storage during 21 days at 2ºC. Data are the mean ± SD (n = 3) and vertical bars represent the standard deviation. Same letters represent non significant differences (p>0.05) between treatments.
Figure 4: Total soluble solids and acidity evolution of Cape gooseberry coated with alginate and storage during 21 days at 2ºC. Data are the mean ± SD (n = 3) and vertical bars represent the standard deviation. Same letters represent non significant differences (p>0.05) between treatments.
Maturity index did not change significantly during cold storage and no differences were observed between treatments (Figure 5). Fruit firmness was significantly reduced in the first week of storage, nevertheless no significant differences between alginate edible coat and control fruit were registered.
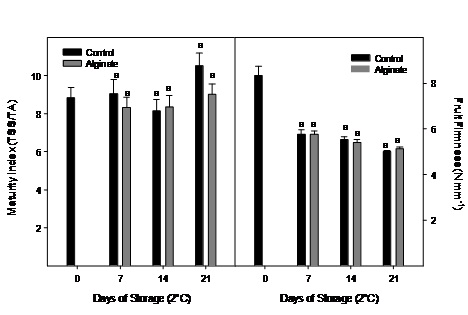
Figure 5: Maturity index and fruit firmness evolution of Cape gooseberry coated with alginate and storage during 21 days at 2ºC. Data are the mean ± SD (n = 3) and vertical bars represent the standard deviation. Same letters represent non significant differences (p>0.05) between treatments.
No significantly changes were observed for total phenolic content during storage in the control fruit, while the fruit coated with alginate presented values significantly higher during storage for all sampling dates (Figure 6). Carotenoids content did not change significantly in control fruits during 21 days of cold storage. Alginate coated fruits had significantly higher carotenoid content at the end of the storage (Figure 6).
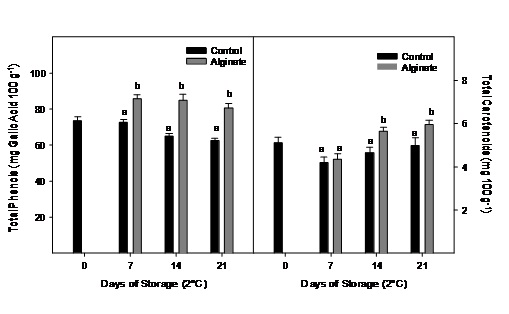
Figure 6: Total phenolics and carotenoids evolution of Cape gooseberry coated with alginate and storage during 21 days at 2ºC. Data are the mean ± SD (n=3) and vertical bars represent the standard deviation. Same letters represent non significant differences (p>0.05) between treatments.
Hydrophilic antioxidant activity was higher than the lipophilic fraction in Cape gooseberry, but both decreased significantly during storage, especially during the first week (Figure 7). Fruits coating with alginate registered a higher hydrophilic antioxidant activity compared to control fruits after 21 days of storage.
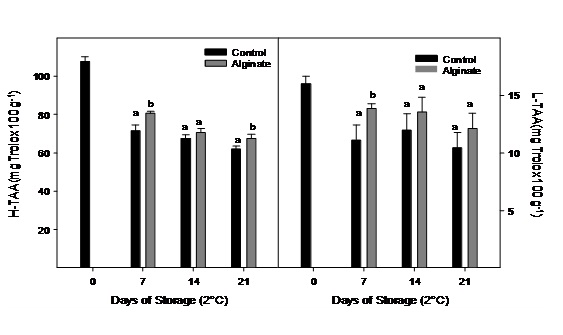 Figure 7: Hydrophilic and lipophilic antioxidant activity of Cape gooseberry coated with alginate and storage during 21 days at 2ºC. Data are the mean ± SD (n = 3) and vertical bars represent the standard deviation. Same letters represent non significant differences (p>0.05) between treatments.
Figure 7: Hydrophilic and lipophilic antioxidant activity of Cape gooseberry coated with alginate and storage during 21 days at 2ºC. Data are the mean ± SD (n = 3) and vertical bars represent the standard deviation. Same letters represent non significant differences (p>0.05) between treatments.
DISCUSSION
The ethylene production and respiration rate of Cape gooseberry results confirm previous report in which this fruit can be classified as a fruit with extremely high climacteric rise in both ethylene (peak of 350nL g-1 h-1) and CO2 (peak of 134.5mg kg-1 h-1) production [8,14] when compared with other climacteric fruits, such as apricot with a variation of 4-6nL ethylene g-1 h-1and 30-50mg CO2 kg-1 h-1 during ripening at 20°C [15], avocado reach near 100nL ethylene g-1 h-1 [16], or plum with a variation of 0.10 to 200nL ethylene g-1 h-1 and 16 to 14mg CO2 kg-1 h-1 [17]. According to Gutiérrez et al., [18] the Cape gooseberry fruits are considered climacteric fruit, because after physiological maturity have an increased respiratory rate.
Alginate coating significantly reduced the ethylene and respiration rates in Cape gooseberry fruits during cold storage, has been previously reported in other climacteric fruits such as tomato [5] and 4 plum cultivars [19]. This can be explained by the fact that alginate coatings increase the skin resistance to gas diffusion by blocking the pores on the fruit surface, resulting in a modified internal atmosphere of relatively high CO2 and low O2 [20]. The elevated internal CO2 could be responsible for the ethylene inhibition by a reduction of the 1-Aminocyclopropane-1-Carboxylic Acid (ACC) synthase activity [5,21]. Additionally, alginate has a moderate permeability to CO2 (500cm3 CO2 m-2 bar-1 day-1) probably reducing the pass of CO2 through the fruit skin [22].
Although, the fruit color index was not significant different between alginate coating fruit and control fruit, this parameter tended to be higher in coated fruit at the end of cold storage (day 14 and 21), as observed by Díaz-Mula et al., [6] where alginate coating were effective on delaying the evolution of color for sweet cherry fruits. Rojas-Graü et al., [23] reported alginate and gellan-based coatings as good carriers for anti browning agents in fresh-cut Fuji apples. Ali et al., [24] reports a significant delay in changes of color development for tomato fruit coated with 10% gum arabic. Balaguera et al., [25] observed an evolution of IC from 4 to 7 in Cape gooseberry at 16°C during 22 days of storage, while in our study the values remain between 6 and 7.
The TSS was near 15° Brix and the TA near to 1.5 during the 21 days at 2°C, similar to the values observed by Balaguera et al., [25] for Physalis, nevertheless they observed a marked decreased of MI (near 7 to near 4) during storage at ambient temperature because the decreased in TA. As these parameters remain constant at 2°C the MI did not show significant differences during cold storage period and the fruits of this study showed a higher MI of near 9. Garzón-Acosta et al., [26] observed lower maturity index in Cape gooseberry fruits stored at 1°C compared to room temperature probably due to the retarding effect of low temperature in the fruit metabolism.
The fruit firmness is regarded as one of the main attributes of quality and often limits the postharvest life. It reflects the changes in cell structure, cell cohesion and some biochemical changes [27].
Alginate coating did not retain significantly the cape gooseberry fruit firmness during cold storage respect to control fruit, although the firmness decline drastically from 8.3 to 5.7Nmm-1 in 7 days of cold storage maybe do to the high MI of fruit (8.87). Gutiérrez et al., [18] observed a continuous decrease in Cape gooseberry firmness during the postharvest, however fruits with low MI at harvest retain higher values of firmness during storage than fruits harvested with greater MI. Nevertheless, fruit firmness in this study decreased but remain high reaching values of 5Nmm-1 after 21 days in cold storage, because according the same authors after eight days of storage at 20°C, the firmness of the fruits of Cape gooseberry can reach about 3 or 4N.
Majumder and Mazumdar [28], found high activity of the enzyme Poligalacturonase (PG) in Physalis fruits at 30 days after anthesis with a continuous increase during the maturation process, coinciding this with ethylene synthesis and high respiratory rate. The PG apparently plays an important role in the solubilization of pectin substances that lead to gradual softening in fruit ripening in Physalis.
In other fruits, such as plums and tomatoes firmness retention and delayed acidity loses have been observed in alginate-coated fruits during storage [5,6], which could be related that these fruits had lower values of ethylene production at the climacteric peak (8-20nL g-1 h-1) compared with Cape gooseberry (? 350nL g-1 h-1).
Phenolic compounds are secondary metabolites, widely distributed in plants. They are important components of many fruits and vegetables not only for their major influence on sensory qualities of the fruit (color, flavour, taste), but also for their antioxidant, anticarcinogenic, antimicrobial, antiallergic, antimutagenic and anti-inflammatory properties [29].
As observed by different authors the total phenols in Cape gooseberry decreased significantly during cold storage in control fruits. The composition of the antioxidant phenolics in fruit increases during ripening, meanwhile during shelf life, antioxidants are rapidly reduced [30,31].
Total carotenoids and phenols content did not change significantly during storage but was significantly higher for alginate coated fruits at the end of storage, with higher hydrophilic antioxidant activity in day 21 of storage respect to control fruits. However, Fischer et al., [32] and Severo et al., [33] observed a significantly increase of carotenoids and phenols content during cold storage at Passive Modified Atmospheres (PMA) in Cape gooseberry fruits, although antioxidant capacity decreased after the second day of storage and remained lower in fruits as observed in our study for control fruits. Strawberries treated with chitosan also maintained better fruit quality with higher levels of phenolics, anthocyanins, flavonoids [34]. Tomatoes fruit coated with 10% gum arabic maintained total antioxidant capacity, total phenolics and total carotenoids during storage as compared to the uncoated control and fruit treated with 5% gum arabic concentration [24].
Chitosan coatings significantly increased the content of total phenolics and antioxidant activity in apricot fruits, as 0.5% chitosan showed maximum total phenolics (82.65mg GAE/100g), content similar to those reached by Cape gooseberry with alginate in this study [35]. According to Benhamou [36], chitosan also has a potential of inducing phenolic contents in plants. The phenols content decreased at the end of storage as reported by Macheix et al., [37] which might be due to breakdown of cell structure in order to senescence phenomena during storage.
The carotenoids content significantly increased in alginate coating fruit although it maintained constant in control fruit. No significant change in color fruit were observed for control fruit and for alginate coating fruit the CI increased slightly without significant differences. According to Balaguera et al., [38] the color change during postharvest fruit of Physalis depends, among other factors, on the stage of maturity at harvest. As the MI of the fruits studied was high the fruit color and the carotenoid content evolution will be expected to be low. Studies realized by Fischer et al., [39] indicate that β-carotene increased in Cape gooseberry fruit until the fruit purchased the orange color and then dropped and rise again in the state of over ripeness. High antioxidant capacity has been demonstrated for Cape gooseberry juice [7], and the synergistic effect of different antioxidants has also been suggested. Furthermore, a high level of phenols was reported for the fruit [9]. In general, alginate coating preserved a higher antioxidant activity respect to control fruit in Cape gooseberry during cold storage as reported by Ali et al., [24] for gum arabic (10%) edible coating, where the antioxidant capacity of tomato fruits was preserved for up to 20 days during storage at 20ºC without any negative effects on postharvest quality.
The total content of antioxidants in a fruit depends on the species and cultivar and can be affected by many factors, such as environmental growing conditions, harvest time, ripening stage, storage and processing conditions [7].
In our studies Cape gooseberry showed high content of total phenols (76mg Gallic Acid 100g-1), carotenoids (5.6mg 100g-1) and hydro-antioxidant capacity of (110mg Trolox 100g-1) similar to values reported by other authors [9,40].
The antioxidant activity of Cape gooseberry seems to be related to hesperidin, tannic acid, quercetin and gallic acid [41]. Gironés et al., [42] only found quercetin and Kaempferol in this fruits. Maturity degree and fruit size affect the fruit’s chemical characteristics and antioxidant activity [43].
Both antioxidant activities decreased markedly in the first 7 days of cold storage, maybe due to the high MI of fruit and the high production of ethylene and respiration rate. Valdenegro et al., [31] observed that unripe fruit of Cape gooseberry presented a high antioxidant level, and a clear increment in antioxidant capacity and polyphenol contents was observed throughout ripening with maximum values at the ripe stage. Nevertheless, after harvest the antioxidant capacity was rapidly reduced during the shelf-life period (20°C) and ethylene treatment increased this reduction.
CONCLUSION
Cape gooseberry exhibits a high respiration rate and ethylene production at 20ºC compared with other fruits and the alginate coat decreased significantly the metabolism activity of fruit during the cold storage. Alginate did not changed significantly the organoleptic quality of Cape gooseberry and total phenols, carotenoids and antioxidant activity was higher for this treatment, during 21 days of storage at 2ºC. Antioxidant activity decreased drastically after one week of storage. The edible coating preserves the total phenolic and carotenoid content during cold storage. Alginate is an efficient edible coat for preserve the quality and bioactivity of Cape gooseberry during storage. These findings represent an alternative for postharvest handling of fresh Cape gooseberry fruit preserving their natural and health contents.
REFERENCES
- Ramadan MF, Moersel JT (2007) Impact of enzymatic treatment on chemical composition, physicochemical properties and radical scavenging activity of goldenberry (Physalis peruviana L.) juice. J Sci Food Agri 87: 452-460.
- Novoa RH, Bojaca M, Galvis JA, Fischer G (2006) Fruit maturity and calyx drying influence post-harvest behavior of Cape gooseberry (Physalis peruviana L.) stored at 12ºC. Agron Colomb 24: 77-86.
- Debeaufort F, Quezada-Galloa JA, Voilleya A (1998) Edible films and coatings: tomorrow’s packagings: a review. Crit Rev Food Sci Nutr 38: 299-313.
- Acevedo CA, López DA, Tapia MJ, Enrione J, Skurtys O, et al. (2012) Using RGB image processing for designing an alginate edible film. Food and Bioprocess Technology 5: 1511-1520.
- Zapata PJ, Guillén F, Martínez-Romero D, Castillo S, Valero D, et al. (2008) Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. J Sci Food Agric 88: 1287-1293.
- Díaz-Mula HM, Serrano M, Valero D (2011) Alginate coatings preserve fruit quality and bioactive compounds during storage of sweet cherry fruit. Food and Bioprocess Technology 8: 2990-2997.
- Ramadan MF (2011) Bioactive phytochemicals, nutritional value, and functional properties of Cape gooseberry (Physalis peruviana): An overview. Food Res Int 44: 1830-1836.
- Castillo B, Llanos WJ, Londoño MT (2013) Characterization of the mechanical properties of the Cape gooseberry fruit (Physalis peruviana L.). Agronomia Colombiana 31: 76-82.
- Puente LA, Pinto-Muñoz CA, Castro ES, Cortés M (2010) Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: a review. Food Res Int 44: 1733-1740.
- Serrano M, Díaz-Mula HM, Zapata PJ, Castillo S, Guillén F, et al. (2009) Maturity stage at harvest determines the fruit quality and antioxidant potential after storage of sweet cherry cultivars. J Agric Food Chem 57: 3240-3246.
- Arnao MB, Cano A, Acosta M (2001) The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 73: 239-244.
- Hurst JW (2002) Methods of Analysis for Functional Foods and Nutraceuticals. (6th edn), CRC PRESS, London, UK.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 299: 152-178.
- Trinchero GD, Sozzi GO, Cerri AM, Vitella F, Franshina A (1999) Ripening-related changes in ethylene production, respiration rate and cell-wall enzyme activity in goldenberry (Physalis peruvianaL.), a solanaceous species. Postharvest Biology and Technology 16: 139-145.
- Crisosto CH, Kader AA (2002) Apricots. In: The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks, USDA Agriculture Handbook 66, USA.
- Woolf AB, White A, Arpaia ML, Gross KC (2004) Avocado USDA-ARS Agriculture Handbook 86, USA.
- Crisosto CH, Kader AA (2002) Plum and Fresh Prune. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks, USDA Agriculture Handbook 66, USA.
- Gutierrez MS, Trinchero GD, Cerri AM, Vilella F, Sozzi GO (2008) Different responses of goldenberry fruit treated at four maturity stages with the ethylene antagonist 1-methylcyclopropene. Postharvest Biology and Technology 48: 199-205.
- Valero D, Díaz-Mula HM, Zapata PJ, Guillén F, Martínez-Romero D, et al. (2013) Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biology and Technology 77: 1-6.
- Maftoonazad N, Ramaswamy HS, Marcotte M (2008) Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int J Food Sci Tech 43: 951-957.
- de Wild HPJ, Balk PA, Fernandes ECA, Peppelenbos HW (2005) The action site of carbon dioxide in relation to inhibition of ethylene production in tomato fruit. Postharvest Biology and Technology 36: 273-280.
- Perez-Gago C, Rojas C, del Rio MA (2003) Effect of hydroxylpropyl methylcellulose-lipid edible composite coating on plum (cv. Autumn giant) quality during storage. J Food Sci 68: 879-883.
- Rojas-Graü MA, Tapia MS, Rodríguez FJ, Carmona AJ, Martin-Belloso O (2007) Alginate and gellan-based edible coatings as carriers of antibrowning agents applied on fresh-cut Fuji apples. Food Hydrocolloids 21: 118-127.
- Ali A, Maqbool M, Ramachandran S, Alderson PG (2010) Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest BiolTechnol 58: 42-47.
- Balaguera HE, Martínez CA, Herrera A (2014) The role of the calyx in the postharvest behavior of Cape gooseberry (Physalis peruviana L.) fruits ecotype Colombia. Revista Colombiana de Ciencias Hortícolas 8: 181-191.
- Garzón-Acosta CP, Villarreal-Garzón DM, Fischer G, Herrera AO, Sanjuanelo DW (2014) Deficiencies of phosphorus, calcium and magnesium affect the postharvest quality of Cape gooseberry (Physalis peruviana L.) Fruits. ActaHort (ISHS) 1016: 83-88.
- Morais PLD, Miranda MRA, Lima LCO, Alves JD, Alves RE, et al. (2008) Cell wall biochemistry of sapodilla (Manilkara zapota) submitted to 1-methylcyclopropene. Braz J Plant Physiol 20: 85-94.
- Majumder K, Mazumdar B (2002) Changes of pectic substances in developing fruits of cape-gooseberry (Physalis peruviana L.) in relation to the enzyme activity and evolution of ethylene. Scientia Horticulturae 96: 91-101.
- Alesiani D, Canini A, D’Abrosca B, DellaGreca M, Fiorentino A, et al. (2010) Antioxidant and antiproliferative activities of phytochemicals from Quince (Cydonia vulgaris) peels. Food Chemistry 118: 199-207.
- Amira EA, Behija SE, Beligh M, Lamia L, Manel I, et al. (2012). Effects of the ripening stage on phenolic profile, phytochemical composition and antioxidant activity of date palm fruit. J Agric Food Chem 60: 10896-10902.
- Valdenegro M, Fuentes L, Herrera R, Moya-León MA (2012) Changes in antioxidant capacity during development and ripening of goldenberry (Physalis peruviana L.) fruit and in response to 1-methylcyclopropene treatment. Postharvest Biology and Technology 67: 110-117.
- Fischer G, Almanza-Merchán PJ, Miranda D (2014) Importancia y cultivo de la uchuva (Physalis peruviana L.). Revista Brasileira de Fruticultura 36: 001-015.
- Severo J, Lima CSM, Coelho MT, Rufato ADR, Rombaldi CV, et al. (2010) Atividade antioxidante e fitoquímicos em frutos de physalis (Physalis peruviana L.) durante o amadurecimento e o armazenamento. R BrasAgrociência Pelotas 16: 77-82.
- Wang SY, Gao H (2013) Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (Fragaria x aranassa Duch.). LWT - Food Science and Technology 52: 71-79.
- Ghasemnezhad M, Shiri MA, Sanavi M (2010) Effect of chitosan coatings on some quality indices of apricot (Prunus armeniaca L.) during cold storage. Caspian J Env Sci 8: 25-33.
- Benhamou N (1996) Elicitor-induced plant defence pathways. Trends in Plant Science 1: 233-240.
- Macheix JJ, Fleuriet A, Billot J (1990) Fruit phenolics. CRC Press Inc., Florida, USA.
- Balaguera HE, Ramírez LV, Herrera AH (2014) Fisiología y bioquímica del fruto de uchuva (Physalis peruviana L.) durante la maduración. In: Carvalho CP, Moreno DA (eds.). Physalis peruviana: fruta andina para el mundo. Imp. Limencop.
- Fischer G, Ebert G, Lüdders P (2000) Provitamin A carotenoids, organic acids and ascorbic acid content of cape gooseberry (Physalis peruviana L.) ecotypes grown at two tropical altitudes. Acta Horticulturae 531: 263-267.
- Bravo K, Sepulveda-Ortega S, Lara-Guzman O, Navas-Arboleda AA, Osorio E (2014) Influence of cultivar and ripening time on bioactive compounds and antioxidant properties in Cape gooseberry (Physalis peruviana L.). Journal of Science Food and Agriculture.
- Namiesnik J, Vearasilp K, Kupska M, Ham K-S, Kang S-G, et al. (2013) Antioxidant activities and bioactive components in some berries. European Food Research and Technology 237: 819-829.
- Gironés-Vilaplana A, Baenas N, Villaño D, Speisky H, García-Viguera C (2014) Evaluation of Latin-American fruits rich in phytochemicals with biological effects. Journal of Functional Foods 7: 599-608.
- Licodiedoff S, Koslowski LAD, Ribani RH (2013) Flavonols and antioxidant activity of Physalis peruviana L fruit at two maturity stages. Acta Scientiarum 35: 393-399.
Citation: Carvalho CP, Villaño D, Moreno DA, Serrano M, Valero D (2015) Alginate Edible Coating and Cold Storage for Improving the Physicochemical Quality of Cape Gooseberry (Physalis Peruviana L.). J Food Sci Nutr 1: 002.
Copyright: © 2015 Catarina Pedro Carvalho, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

