
Analysis of Outer Nuclear and External Limiting Membrane Layers Pattern on Spectral-Domain Optical Coherence Tomography in Patients with Posterior Uveitis with or without Cystoid Macular Edema
*Corresponding Author(s):
Nathalie MassambaDepartment Of Ophthalmology, Moran Eye Center, University Of Utah, Salt Lake City, Utah, United States
Tel:+1 8015812668,
Email:Nathalie.massamba@gmail.com
Abstract
Purpose: To evaluate the Spectral Domain Optical Coherence Tomography (SD-OCT) findings, particularly retinal deposit of hyper-reflective material of conical shape patterns and the prevalence of Cystoid Macular Edema (CME) in posterior uveitis.
Methods: We retrospectively reviewed the case records of all consecutive patients with posterior uveitis who presented to our Ophthalmology Department between November 2013 and July 2014. Patients were followed up for a mean of nine months.
Results: Ninety-eight eyes of 88 patients (65 females; mean age, 81.5±1.4 years) were included in our study. Overall, CME was observed in 38 of 98 eyes (39%) at baseline while an additional 24 eyes (25%) developed CME during the follow up period. The study eyes were classified into three groups: group 1 consisted of 38 eyes of 37 patients who had CME at baseline; group 2 consisted of 24 eyes of 24 patients who developed CME during the follow-up period and in whom we observed a thickening of the outer plexiform layer along with small, focal, hyper reflective deposits which appeared in the form of a cone; and group 3 consisted of 36 eyes of 27 patients who neither had CME at baseline nor developed it during follow-up.
Conclusion: A thickening of the outer plexiform layer, visualized as deposit of hyper-reflective material of conical shape patterns on SD-OCT, was associated with the development of CME in posterior uveitis. Further studies are needed to improve our understanding of the pathophysiology as well as the prognostic and management implications of this association in patients with posterior uveitis.
Keywords
INTRODUCTION
Cystoid macular edema is often associated with a reduction in visual acuity and has been suggested as a major cause of decreased visual acuity in patients with uveitis [3,4]. However, limited data is available to explain the impact of CME on visual acuity in different uveitis entities [3,4].
Macular edema is often detectable using bio microscopy and can be visualized as a loss of foveal reflection or the appearance of intra retinal cysts; a large CME may be associated with a serous retinal detachment but is diagnosed more easily by using Fluorescein Angiography (FA) or OCT imaging. In 2004, an expert of consensus panel, the Standardization of Uveitis Nomenclature Working Group, concluded that reporting macular edema in clinical studies, based on clinical observation of definite macular edema, was acceptable but that use of confirmatory ancillary testing was preferable for prospective studies [4]. The panel did not specify whether FA or OCT imaging was preferred. Historically, FA has been the most commonly used method for the evaluation of macular edema in clinical trials of retinal diseases. However, OCT imaging has started to supplant FA for this purpose since it does not require dye injection and directly assesses both Macular Thickness (MT) and the presence of intra retinal cysts in macular edema.
The anatomic MT assessed by OCT and the physiologic Macular Leakage (ML) assessed by FA are different, but related, entities within the spectrum of macular edema. It has already been described that the external retina layer, particularly the outer plexiform layer as the origin of the development of CME, but actually we have little information on the accuracy of the outer retina layer which is reached [5]. Therefore, it would be valuable to identify whether there are circumstances in which the tests are likely to be complementary, rather than redundant, in evaluating uveitis. In the Multicenter Uveitis Steroid Treatment (MUST) trial, a large group of patients with intermediate uveitis, posterior uveitis and pan uveitis underwent both protocol-driven FA and Spectral Domain OCT imaging regardless of whether macular edema was suspected. They concluded that “given the observed superiority of OCT in obtaining gradable images, along with its lower cost and non-invasive nature, in most cases OCT seems the most appropriate first test to evaluate the macula, unless some other specific indication for FA exists” [6]. Thus, the aim of this study was to evaluate Spectral Domain Optical Coherence Tomography (SD-OCT) findings, specifically in the outer plexiform and inner nuclear layers and the prevalence of CME in eyes with posterior uveitis, irrespective of etiology.
METHODS
The inclusion criteria were: (1) age≥18 years old and (2) presence of newly diagnosed active posterior uveitis as evaluated and confirmed by fundus bio microscopy, Fluorescein Angiography (FA), Indocyanine Green Angiography (ICG) and SD-OCT (Spectralis HRA + SD-OCT, Heidelberg Engineering, Heidelberg, Germany). The baseline visit consisted of evaluating the presence of CME prior to the initiation of any treatment while subsequent examinations evaluated the occurrence of CME during follow-up. The initial treatment for most of the study patients consisted of oral corticosteroids associated with an immunosuppressive agent, in line with our standard protocol.
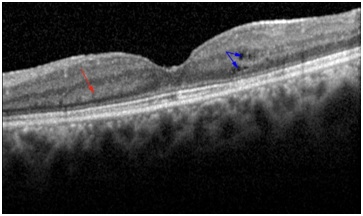
At each post-treatment visit (one month, three months and six months), the study patients were routinely asked to provide detailed medical and ocular history and to undergo a comprehensive ophthalmic examination which included Best Corrected Visual Acuity (BCVA) measurement using ETDRS charts, slit-lamp bio microscopy, fundus bio microscopy, Intraocular Pressure (IOP) measurement, laser flare photometry and SD-OCT. All study eyes had been assessed using multimodal imaging since this is routine practice in our department to confirm the diagnosis of CME, and monitor disease progression. Our review identified three groups of eyes: group 1 consisted of eyes with CME at baseline; group 2 consisted of eyes with a deposit of hyper-reflective material of conical shape pattern on SD-OCT at baseline that developed CME during follow-up or intra retinal cysts. Cysts are the edema caused in the macular area of the eye and group 3 consisted of eyes that neither had CME at baseline nor develop it during follow-up. Multimodal imaging was qualitatively examined by two independent observers (NM, JL) in order to confirm the presence of CME associated with posterior uveitis. All patients in group 1 were excluded from further analysis. The presence of this hyper-reflective material was defined as the presence of small, focal and hyper reflective material deposited as a cone above the outer plexiform layer (Figure 1).
STATISTICAL ANALYSIS
RESULTS
The three groups were classified as follow: group 1 consisted of 38 eyes of 37 patients with CME at baseline, group 2 consisted of 24 eyes of 24 patients who developed CME during post-treatment follow-up and group 3 consisted of 36 eyes of 27 patients who neither had CME at baseline nor develop it during follow-up.
In details, group 1 was composed of patients with severe cystoid macular edema with disorganization of the internal and external retinal layers and it was difficult to visualize the deposit of hyper-reflective material of conical shape in this group. While in group 2, we were able to visualize a few cysts in the external retina layers associated with deposit of hyper-reflective material of conical shape and evolved towards the formation of intra retinal cysts CME. Therefore, our analysis was targeted only on patients in group 2.
This group was composed of 24 eyes of 24 patients (14 females and 10 males, mean 45.3 years old), a deposit of hyper-reflective material of conical shape pattern was identified on SD-OCT at baseline in all the patients (100%). 9 out of 24 (37.5%) eyes had few cystoid areas (no CME) associated with the deposit of hyper-reflective material of conical shape at baseline while the remaining 15 eyes (62.5%) had developed cystoid areas by the final study visit (Table 1).
The characteristic appearance of the material in the form of deposit of hyper-reflective material of conical shape in the outer plexiform layer was observed in group 2 patients and the mean follow-up time to a changing pattern was 23 weeks after the baseline examination. Figures 2 and 3 demonstrated the longitudinal changes and CME development. We found several etiologies of posterior uveitis including Irvine-Gas’s syndrome, Vogt-Koyanagi-Harada syndrome, systemic lupus erythematosus, multifocal choroiditis, birdshot retinochoroidopathy, uveitis with positive HLA-B27, Behçet disease and sarcoidosis.
|
EZ |
|
Number Baseline |
Number Final Study |
Percentage Baseline |
Percentage Final Study |
P |
|
|
Normal |
28 |
14 |
83.30% |
66.60% |
|
||
|
Disrupted |
4 |
9 |
16.70% |
30.30% |
|
||
|
None |
0 |
1 |
0% |
3.10% |
|
||
|
BCVA |
|
0.18 logMAR |
0.45 logMAR |
|
|
p=0.00057 |
|
|
CMT |
|
241 µm |
304 µm |
|
|
p=0.01 |
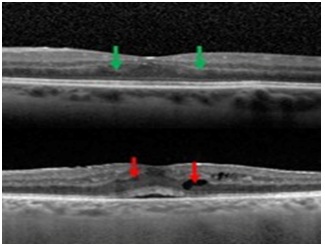
Figure 2: A 45-year-old female from India presented with posterior uveitis due posterior scleritis SD-OCT of LE showed green arrow, we visualized the deposit of hyper-reflective material having a conical shape with a wide large. 5 months later the SD-OCT involve towards to cysts (red arrow) and a sub retinal fluid.
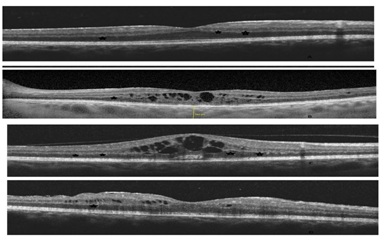
(b) Development of cysts four months later despite treatment
(c) The last study visit showing the change of hyper reflective deposit material conical shape towards CME
(d) SD-OCT after 4 months of treatment by oral prednisolone
In group 2, we did not find any correlation between decreased VA and CMT, the results just failed to reach statistical significance (p=0.06). We found no correlation between EZ disruption and CMT as compared with those having a normal EZ (p=0.78). However, there was a statistically significant correlation between EZ status and VA: eyes with disrupted EZ had low VA (0.45 log MAR) compared with eyes that had a normal EZ (0.1 log MAR; p=0.01) (Table 1).
DISCUSSION
Macular edema is a severe vision-threatening complication of uveitis and therefore, detecting and monitoring this condition is of particular importance [7,8]. The underlying pathogenic mechanism for the development of macular edema is not yet clearly understood. Inflammatory cell infiltration and protein secretion, retinal cell damage, vitreoretinal traction, and epiretinal membrane formation may all be contributing factors. The blood-retinal barrier may break down as a result of Retinal Pigment Epithelium (RPE) changes, capillary endothelium damage or loss of auto regulatory control of blood flow [9]. Quantitative assessment of macular thickening using OCT is useful in predicting the response to treatment of all types of macular edema. In this connection, Sivaprasad et al., demonstrated the value of SD-OCT in predicting response to treatment [10].
In our study, we specifically evaluated in detail the 24 eyes of 24 patients (mean age 43.5 years) with a deposit of hyper-reflective material of conical shape pattern, on SD OCT, at baseline that had developed CME by the final study visit. Of the 24 such eyes, 9 (38%) eyes already had cystoid areas at baseline while the other 15 eyes (62%) had developed cystoid areas by the end of the study. The mean time to develop CME in these eyes was 23 weeks. Considering these results, we agree with Sivaprasad et al., [10] who postulated that detailed interpretation of OCT images at each follow-up visit of patients with inflammatory CME may be an additional tool to determine the prognosis of CME and its response to treatment.
Rangasamy and colleagues observed monocytes/macrophages adhering to the outer surface of retinal capillaries, most likely in the active process of extravasation [5]. The presence of activated monocytes/macrophages in the extravascular space is evidence of the ability of leukocytes to breach the blood retina barrier by diapedesis and establish an inflammatory reaction within the retinal tissues. Their cytological data could explain the thickening of the outer plexiform layer observed in our group 2 eyes [5]. They did not perform any retinal imaging studies but taking their results into consideration along with our observations, we could postulate that the outer plexiform layer is probably the initial site of CME.
To the best of our knowledge, there are no previous publications concerning the thickening of the outer retinal layers in CME associated with posterior uveitis. Markomichelakis et al., [11] described three basic OCT patterns of uveitis macular edema including sponge-like retinal swelling (diffuse), CME and Serous Retinal Detachment (SRD) [12]. Quantitative assessment of macular thickening using OCT is useful in predicting the response to treatment of all types of macular edema. In this connection, Sivaprasad et al., [10] demonstrated the value of SD-OCT in predicting response to treatment.
We observed in our group 2 eyes that VA was better at the beginning of the study than at the end despite the presence of the deposit of hyper-reflective material of conical shape pattern, on SD-OCT; at baseline demonstrating that the deposit of hyper-reflective material of conical shape pattern may not per se does affect VA. Our study suggests a correlation between decreased visual VA and increased CMT, as demonstrated by Payne et al., [12], although our results just failed to reach statistical significance (p=0.06). We found a correlation between EZ disruption and decreased visual acuity probably resulting from the loss or alteration of the photoreceptors.
This might be an early biomarker of the onset of the cystoid macular edema. It could correspond to the stretching of the Henle fibers or could also be a primary injury of the outer plexiform layer which will evolve towards the formation of intra retinal cysts, and then the development of the CME. We could not affirm which theory is the more valid, nevertheless the results obtained in our study plead in favor of the second theory.
We observed various etiologies of posterior uveitis in our study patients excepting infectious causes which we had excluded in order to avoid selection bias. Many of these etiologies characteristically present with CME at the time of diagnosis; therefore, if CME was present at baseline, these eyes were included in our group 1. Although HLA-B27 associated uveitis is typically anterior in nature, we found that some eyes presented with CME and were therefore included in our study.
We acknowledge that this study has several limitations, particularly the retrospective data collection and imaging analysis of CME progression during follow-up, the relatively short follow-up period and the inclusion of both eyes of some patients in our analysis. Further and prospective studies are necessary to improve our knowledge regarding the physiopathology of this condition, particularly the role of the outer plexiform layer in the development of CME associated with posterior uveitis and the management of this pathology.
Several studies have described the natural history, management and prognosis of CME, but only limited data are available regarding the specific sequence of events leading to the formation of CME [7,8]. Therefore, the aim of our study was to analyze SD-OCT findings, particularly in the outer retina layers and to determine the overall prevalence of CME in patients with posterior uveitis. The etiology of posterior uveitis in our study was heterogeneous and we observed that the deposit of hyper-reflective material of conical shape patterns on SD-OCT was visualized in several different etiologies. Nevertheless, we believe that a selection bias remains since each etiology has its own peculiar characteristics. We believe that there is still insufficient evidence to conclude that the increase in the thickness of the outer plexiform layer, visualized as a deposit of hyper-reflective material of conical shape pattern on SD-OCT, initiates and evolves into CME in posterior uveitis. Further studies are needed to confirm our observations and hypothesis.
AUTHOR’S CONTRIBUTION
REFERENCES
- Cunha-Vaz J, Coscas G (2010) Diagnosis of macular edema. Ophthalmologica 1: 2-7.
- Fardeau C, Champion E, Massamba N, LeHoang P (2016) Uveitic macular edema. Eye (Lond) 30: 1277-1292.
- Rothova A, Suttorp-van Schulten MS, Treffers WF, Kijlstra A (1996) Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 80: 332-336.
- Bodaghi B, Cassoux N, Wechsler B, Hannouche D, Fardeau C, et al. (2001) Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore) 80: 263-270.
- Rangasamy S, McGuire PG, Franco Nitta C, Monickaraj F, Oruganti SR, et al. (2014) Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One 9: 108508.
- Kempen JH, Sugar EA, Jaffe GJ, Acharya NR, Dunn JP, et al. (2013) Fluorescein Angiography versus Optical Coherence Tomography for Diagnosis of Uveitic Macular Edema. Ophthalmology 120: 1852-1859.
- Nussenblatt RB (1990) The natural history of uveitis. Int Ophthalmol 14: 303-308.
- Lardenoye CW, van Kooij B, Rothova A (2006) Impact of macular edema on visual acuity in uveitis. Ophthalmology 113: 1446-1449.
- Freeman G, Matos K, Pavesio CE (2001) Cystoid macular oedema in uveitis: an unsolved problem. Eye 15: 12-17.
- Sivaprasad S, Ikeji F, Xing W, Lightman S (2007) Tomographic assessment of therapeutic response to uveitic macular edema. Clin Exp Ophthalmol 35: 719-723.
- Markomichelakis NN, Halkiadakis I, Pantelia E, Peponis V, Patelis A, et al. (2004) Patterns of macular oedema in patients with uveitis: qualitative and quantitative assessment using optical coherence tomography. Ophthalmology 111: 946-953.
- Payne JF, Bruce BB, Lee LB, Yeh S (2011) Logarithmic transformation of spectral-domain optical coherence tomography data in uveitis-associated macular edema. Invest Ophthalmol Vis Sci 52: 8939-8943.
Citation: Massamba N, Jennifer ML, Lehoang P, Sellam A, Bodaghi B (2017) Analysis of Outer Nuclear and External Limiting Membrane Layers Pattern on Spectral-Domain Optical Coherence Tomography in Patients with Posterior Uveitis with or without Cystoid Macular Edema. J Ophthalmic Clin Res 4: 037.
Copyright: © 2017 Nathalie Massamba, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

