
Application of Peleg Model on Hydrothermal Treatment of Tiger Nut Tubers (Cyperus esculentus) and Effect of Dehulling Efficiency on Microbial Load
*Corresponding Author(s):
Djomdi DjomdiDepartment Of Food Science And Nutrition, University Of Ngaoundéré, P.O.Box 455 Ngaoundéré, Cameroon
Tel:+237 699626764,
Email:ngdjomdi@yahoo.fr
Abstract
The present work was carried out with the aim to establish the influence of hydrothermal treatments on physical and microbiological characteristics and Peleg's prediction of soaking behavior of tiger nut tubers. For this purpose, tigernut tubers (Cyperus esculentus) Ø≥1cm, were differently soaked in tree solutions: acid solution (vitamin C) and alkaline solutions (Ca(OH)2 and Kanwa) at different concentrations and temperatures. The effect of these treatments on the water content (swelling), soluble loss, dehulling efficiency and microbial load were investigated. These works show that, water content or swelling of the tiger nut soaked in acid and alkaline solutions can be predicted by Peleg model. This swelling lead to enhance dehulling efficiency of tuber but caused soluble solute loss overall at the highest temperature of soaking in vitamin C solution. Like soaking in water, the constant of Peleg (k1) decrease with the increase in the temperature of soaking, whereas the constant of capacity of Peleg (k2) is approximately 0.02% for all the conditions of soaking. Dehulling efficiency also increased with increasing temperatures (65% at 20°C to 70% at 60°C) for tiger nut tubers soaked in water and affects microbial load. This reduction is considerable at the higher temperatures, reaching a maximum of 4.8log reduction. Soaking and dehulling treatments gave to tiger nut tuber effective decontamination in term of microbial load and obtained good microbiological quality.
Keywords
Acid and alkaline solutions; Cyperus esculentus; Husking; Microorganism; Soaking
INTRODUCTION
Tiger nut tubers (Cyperus esculentus) belong to the foodstuffs having a nutritional potential but which remains under-exploited in Africa [1]. This plant is native of Mediterranean Basin [1]. In Spain and Morocco, it is used in the production of a milky drink called “horchata de chufa”. High level of microorganisms such as bacteria and moulds have been associated with the milky drink [2,3]. Due to this, production and marketing of horchata are limited mainly by its weak shelf life (less than 24 hours) because of its high microbial load (more than 109cfu/ml) [4]. Microbial contamination of tigernut tubers easily occurs and often undetected before the tubers are consumed. The source of microbial contamination could be from infected field workers, contaminated soil, irrigation water, wash tanks, harvesting equipment, fecal materials and transport vehicles. Growth of microorganisms in foods is usually influenced by factors such as product temperature, product-to-headspace, gas volume ratio, initial microbial loads and type of flora, packaging, barrier properties, storage condition and biochemical composition of the food [5]. The water used to apply fungicides and insecticides is a source of microbial contamination of tigernut tubers [6]. According to Okechukwu et al. [7], the use of water polluted with faecal matter to wash knives, polyethene bags and trays could also be a source of microbial contamination of tigernut tubers. Microbial contamination of ready-to-eattigernut tubers is largely attributed to unhygienic practices of vendors and inappropriate storage conditions.
These micro-organisms originate from the rugous surface of the tuber where they are encrusted in the anfractuosities of the collenchyma sleeve [2,4,8,9]. The rough structure of the tuber surface does not allow the easy elimination of the micro-organisms, even when using sodium hypochlorite solution for Selma et al. [4]. This indicates that the micro-organisms are located in the deep incrustations of the tubers surface and are consequently not very accessible to the hypochlorite solution. However, tubers soaked in water for some hours undergo a turgescence which results in an easy elimination of the tuber external skin [10,11]. Since this turgescence is not observed with hypochlorite solution, it could be hypothesized that the processing time using hypochlorite is not sufficiently long to allow the turgescence of the tubers.
The research of cleansing process of tiger nut tubers remains a major concern for a better use of the tubers in the production of vegetable milk. It proves to be important to test treatments having given convincing results with other raw materials requiring a dehulling process. Examples are the dehulling of black bean and maize grains by treatment in alkaline solutions [12,13]. In this respect, the swelling of tiger nut tuber consecutive to soaking in acid or alkaline solutions is likely to facilitate its dehulling and consequently a better elimination of its initial microbial load.
The present work was carried out with the aim to establish the influence of hydrothermal treatments on physical and microbiological characteristics and Peleg's prediction of soaking behavior of tiger nut tubers.
MATERIALS AND METHODS
Preparation of samples
Tiger nut tubers were bought on the market of Guily in the Far-north Region of Cameroon, which is the most producing zone of these foodstuffs. Tubers were sorted by screening on metallic sieve in order to retain tubers with diameter ≥1cm. The tubers were then washed with tap water to remove sands and other undesirable materials (stones, pebbles, dirt materials, rotten stems and broken tubers), then sun dried [10].
Soaking experiments
Soaking was undergone according to the methods of [14,15]. The process consists in soaking the tubers in beakers of 250mL containing 100mL solution of Ca(OH)2, kanwa or vitamin C at 20,40 and 60°C. The beakers were placed in a constant temperature water bath for temperature uniformity during tests. For each experiment, 20g of tubers were immersed in the beaker. During soaking, tubers were periodically removed, superficially dried with a tissue paper and weighed for water retention capacity using an electronic balance (Sartorius, model LC1201S, Germany). The experiment was terminated when tuber moisture content attained an equilibrium value, i.e. when the increment change in sample weight was less than 0.01g. At least three experiments were conducted for every solution concentration and soaking temperature.
The factors of variation of the treatments were:
- - Nature of the solutions: calcium hydroxide, Kanwa and vitamin C.
- - Concentrations of the solutions: 0; 0.1; 0.5; 1 and 1.5% (w/v) [8,9].
- - Temperatures of treatment: 20, 40 and 60°C [10].
The Water absorption capacity of the samples at each time (Ht) was calculated based on the increase in weight of the samples using Peleg model (Equation 1) [15,16]:
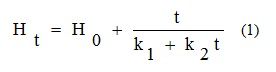
Where: Ht (% DW) is the moisture content at time t
H0 (% DW) is the initial moisture content
k1 (h.%-1) is the Peleg rate constant which is related to absorption rate at the beginning of the process (t=t0)
k2(%-1) is the Peleg capacity constant related to maximum attainable moisture content (t→∞)
The model is linearized into the form:
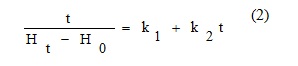
k1and k2 constants are determined from the regression of t/(Ht-H0) vs. time.
Peleg constants (k1 and k2) values obtained were used in Equation (1) to calculate predicted values of moisture content (Hp) which were then compared to the corresponding experimental values in order to appreciate the goodness of fit of the Peleg model. The criterion used to evaluate the goodness of fit was the mean relative percentage deviation (p) between experimental data and theoretical values [17], calculated as:
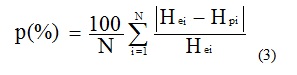
Where: Hei and Hpi are respectively experimental and predicted moisture content values. N is the number of experimental data.
The model is considered acceptable if p values are below 10% [17,18].
Dehulling of tubers
At the end of soaking and swelling process, the dehulling consisted in removing the outer protective casing of the tuber by abrasion through scrubbing of the tubers between the hands. Dehulling efficiency was calculated using the formula suggested by [19], in which the mass percentage of dehulled tubers is compared to that of the whole tubers on dry matter basis.
To account for moisture change during soaking, the mass of tuber samples was measured before and after soaking and dehulling. Moisture Correction Factor (MFC) was calculated using Equation 4 below:
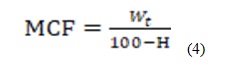
With: Wt(g): whole tuber mass with hulls
H(%): water content of the tubers
In addition, at the end of swelling and after dehulling, the hulls were weighed, dried at 105°C for 24 hours and reweighed to obtain a dry mass (Mh).
The dehulling efficiency is then given by the Equation 5:

Moreover, the solute losses during soaking at different temperatures were calculated according to Evranuz and Gürtas. A known quantity of soaking solution (v) was taken at the end of process, filtered using filter paper Whatman n°3, dried in oven at 105°C for 24 hours and weighed (m).The solute losses were expressed in g/100 g dry weight basis (Equation 6).
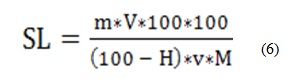
With:
SL (g/100 g DW): Solute loss
m (g): mass of soluble obtained after drying of v aliquot
V (ml): total volume of soaking solution
v (ml): aliquot volume of the soaking solution
H (%): water content of the tubers
M (g): mass of tubers before soaking
Microbiological analyzes
The microbiological analyzes were performed according to standards methods [19]. The research of the total microbial count was carried out by macerating tiger nut tubers in a sterile food processor. 10g of each sample of tiger nut tubers (raw, soaked and dehulled) were crushed in a mortar in asepsis conditions. 1g of the crushed sample was mixed with 9mL of sterile physiological water solution (9g of NaCl in 1L of distilled water) and the mixture used for the preparation of decimal dilutions of 10-2 to 10-9 and the highest three dilutions were taken for analysis.
Enumeration was carried out by Counting of the Colonies (CFU). The count is carried out in triplicates.
All results are expressed as mean values of three separate determinations.
RESULTS AND DISCUSSION
Water absorption of tiger nut tuber in vitamin C, Kanwa and calcium hydroxide solutions
Soaking is a long stage process in which the goal is the swelling of tiger nut tubers. During immersion, there is material transfer made both of soluble between tubers and the solution. For all the soaking solutions, tiger nut tubers showed typical sorption behavior with exponentially water content versus soaking time at all the temperature (Figure 1). As the process continued, water absorption rate steadily decreased due to filling of water into free capillary and intermicellars, and increasing extraction rates of soluble solids loss from the tubers. The absorption ceased when the tubers attained the equilibrium water content.
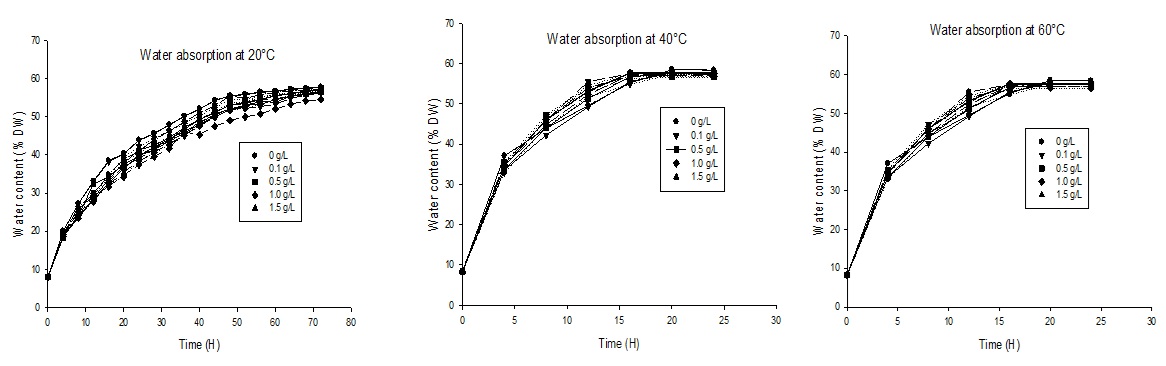 Figure 1: Water absorption of tiger nut soaked in vitamin C ( ? ), Ca(OH)2 ( --- ) and Kanwa ( ……) solutions at different temperatures.
Figure 1: Water absorption of tiger nut soaked in vitamin C ( ? ), Ca(OH)2 ( --- ) and Kanwa ( ……) solutions at different temperatures.
The kinetics absorption of solution by tiger nut tubers at various temperatures and concentrations of vitamin C, Kanwa and Ca(OH)2 showed that the rate of absorption of water increases with the temperature of soaking. Tables 1 and 2 show that we do not have a significant difference (p<0.05) between the constants k1 with the increase of the concentration whatever the treatment applied. But on the other hand we have a significant difference (p<0.05) between constancies k1 with the increase of the temperature whatever the treatment applied. This can be explained by the fact that more the temperature increases, the more one has a fast absorption, which results in a shorter time of soaking (Figure 1).
|
|
|
Ca(OH)2 |
Kanwa |
||||||
|
C (g/L) |
T (°C) |
k1 |
k2 |
r2 |
P (%) |
k1 |
k2 |
r2 |
P (%) |
|
|
20 |
0.2264a |
0.017 |
0.98 |
3.306 |
0.2264a |
0.017 |
0.97 |
3.306 |
|
0 |
40 |
0.1022b |
0.0182 |
0.985 |
3.721 |
0.1022b |
0.0182 |
0.985 |
3.721 |
|
|
60 |
0.0455c |
0.0185 |
0.972 |
5.489 |
0.0455c |
0.0185 |
0.972 |
5.489 |
|
|
20 |
0.3127a |
0.0166 |
0.95 |
3.567 |
0.2693a |
0.0165 |
0.96 |
3.651 |
|
0.1 |
40 |
0.1205b |
0.0179 |
0.968 |
4.128 |
0.1224b |
0.0176 |
0.978 |
4.247 |
|
|
60 |
0.0379c |
0.0187 |
0.978 |
6.053 |
0.049c |
0.0186 |
0.97 |
5.681 |
|
|
20 |
0.3167a |
0.0169 |
0.95 |
3.951 |
0.2649a |
0.0168 |
0.96 |
3.495 |
|
0.5 |
40 |
0.1492b |
0.0173 |
0.968 |
4.6 |
0.1359b |
0.0174 |
0.996 |
3.981 |
|
|
60 |
0.0391c |
0.0189 |
0.978 |
5.651 |
0.0361c |
0.0188 |
0.981 |
5.396 |
|
|
20 |
0.2959a |
0.0169 |
0.96 |
3.563 |
0.2817a |
0.0167 |
0.96 |
3.584 |
|
1 |
40 |
0.1554b |
0.0177 |
0.97 |
3.916 |
0.1398b |
0.0177 |
0.976 |
3.773 |
|
|
60 |
0.0438c |
0.0186 |
0.974 |
5.848 |
0.037c |
0.019 |
0.98 |
5.461 |
|
|
20 |
0.313a |
0.0178 |
0.95 |
3.515 |
0.3252a |
0.0166 |
0.94 |
4.298 |
|
1.5 |
40 |
0.1609b |
0.0178 |
0.97 |
3.718 |
0.1578b |
0.0173 |
0.965 |
4.239 |
|
|
60 |
0.044c |
0.0186 |
0.974 |
6.035 |
0.0391c |
0.0191 |
0.98 |
5.298 |
Table 1 : Water absorption constants and goodness of fit of Peleg model of tigernut tubers soaked in alkaline solutions (Ca(OH)2 and Kanwa) at different temperatures.
Note: Means within the same column followed by same letter are not significantly different using one-way ANOVA test (P?0.05).
|
C (g/L) |
T (°C) |
k1 |
k2 |
r2 |
P (%) |
|
0 |
20 |
0.2264a |
0.017 |
0.97 |
3.303 |
|
40 |
0.1022b |
0.0182 |
0.985 |
3.721 |
|
|
60 |
0.0455c |
0.0185 |
0.972 |
5.489 |
|
|
0.1 |
20 |
0.2283a |
0.017 |
0.97 |
3.317 |
|
40 |
0.1164b |
0.0182 |
0.983 |
3.361 |
|
|
60 |
0.054c |
0.0183 |
0.962 |
6.459 |
|
|
0.5 |
20 |
0.2895a |
0.0167 |
0.96 |
3.521 |
|
40 |
0.1165b |
0.018 |
0.971 |
4.894 |
|
|
60 |
0.0483c |
0.0184 |
0.97 |
6.167 |
|
|
1 |
20 |
0.3115a |
0.0167 |
0.95 |
3.66 |
|
40 |
0.1336b |
0.0177 |
0.977 |
4.089 |
|
|
60 |
0.0416c |
0.0185 |
0.995 |
5.712 |
|
|
1.5 |
20 |
0.2965a |
0.0165 |
0.95 |
3.506 |
|
40 |
0.1378b |
0.0175 |
0.971 |
4.894 |
|
|
60 |
0.0411c |
0.0186 |
0.974 |
5.76 |
Table 2 : Water absorption constants and goodness of fit of Peleg model of tiger nut tubers soaked in vitamin C at different temperatures.
Note: Means within the same column followed by same letter are not significantly different using one-way ANOVA test (P?0.05).
The constant of Peleg (k1) decrease with the increase in the temperature of soaking, whereas the constant of capacity of Peleg (k2) is approximately 0.02% for all the conditions of soaking. There is a relative stability of the values of k2 for all the temperatures and concentration of soaking [10], reported that chemical modification of the tubers under the effect of the temperature (gelatinization) does not affect the capacity of absorption of the tubers. k1 is a constant connected at the rate of transfer of the matter, the weakness of the membrane cellular due to the heating increases the cellular permeability, which involves an increase the rate of absorption of solution by the tubers and which was not constrained at all by the various treatments applied to the tubers during the stage of soaking. This phenomenon was observed by various authors and they attributed it for a plasticizing effect of water at the temperature of gelatinization [10,11,20-23].
The fit of Equation (2) to absorption data showed that, at all soaking conditions, the degree of fit, as judged by the regression coefficient (r2), is about the same. Tables 1 and 2 provides the resulting Peleg constants (k1 and k2), r2 and p values. The use of kinetic data to determine the goodness of fit of the Peleg model resulted in a fit with r2³0.90 at almost all soaking conditions (solutions concentrations, temperature and time). The relative deviation between experimental and predicted data is around 2.798-6.167, which indicates that water absorption of tiger nut tubers during soaking in acid and alkali solution is well described by the Peleg model [23].
It should be noted that the diffusion is not done in only one direction; as the solutions of soaking diffuse in the tubers, those also loss of solutes from tiger nut tubers in the soaking solutions (Figure 2), what implies the solutions of soaking are not sufficiently concentrated to prevent the opposite transfer of matter.
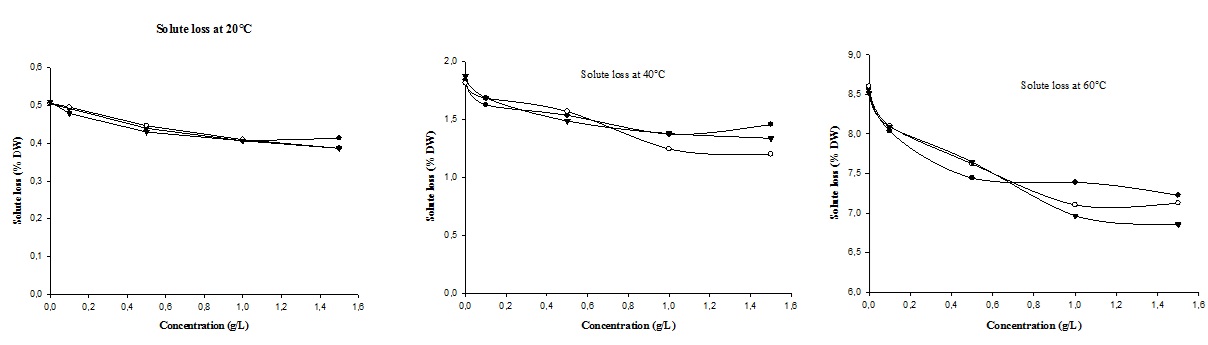 Figure 2: Soluble solids loss of tiger nut tubers soaking at different temperatures.
Figure 2: Soluble solids loss of tiger nut tubers soaking at different temperatures.
The losses of soluble solids at 20 and 40°C were less 0.5 and 2% of the original tubers mass respectively. [21] stipulated that these values can be negligible compared to the water gain. But at 60 we have 8.7% soluble solids loss. Although, hot solutions accelerate the water absorption phenomena, but lead to significant damage in structure and tissue of tiger nut tubers, so there are loss of soluble solids.
Figure 2 showed that soluble solids loss increased with temperature during soaking of tiger nut tubers; and for the higher temperatures (above 40°C), soluble solids loss decreased with increasing of solution concentrations excepted for vitamin C solutions. These results indicate that higher concentration salts slowed down soluble solids loss and higher temperature inactive and decompose vitamin C [24].
This part of work established the suitability of the model of Peleg to describe the behavior of the tubers of C. esculentus L. at the time of soaking. The high temperatures accelerate the phenomenon of absorption of water and loss of soluble solutes. Although the heating contributes to sterilize the tubers, with regard to the microbial load, to optimize the effects of the temperature and the solutions of soaking on the dehulling of tiger nut tubers, with an interest for its microbiological quality seems a scientific and technological concern.
Dehulling efficiency
The tough outer skin of tiger nut tubers consists of thin epidermis wrapped in the tegument, a very thin coat composed mainly of fibers and called fibro-collenchyma sleeve. This coat was removed by alkaline and acid action of soaking solutions.
Figure 3 shows dehulling efficiency of soaking solutions (acid and alkaline) at different temperatures. This figure shows that dehulling efficiency increased with increasing concentration of soaking solutions for all the three solutions tested. There also significant variations in dehulling efficiency among solutions at 20 and 40°C. At these temperatures, vitamin C has the best dehulling efficiency for all the concentrations but at 60°C this activity is reduced compare to the two other solutions. This reduction activity can be attributed to temperature effect on vitamin C, since this temperature lead inactivation and decomposition of vitamin C [25-27]. Vitamin C exhibited the highest dehulling efficiency for all the temperature except at 100°C.
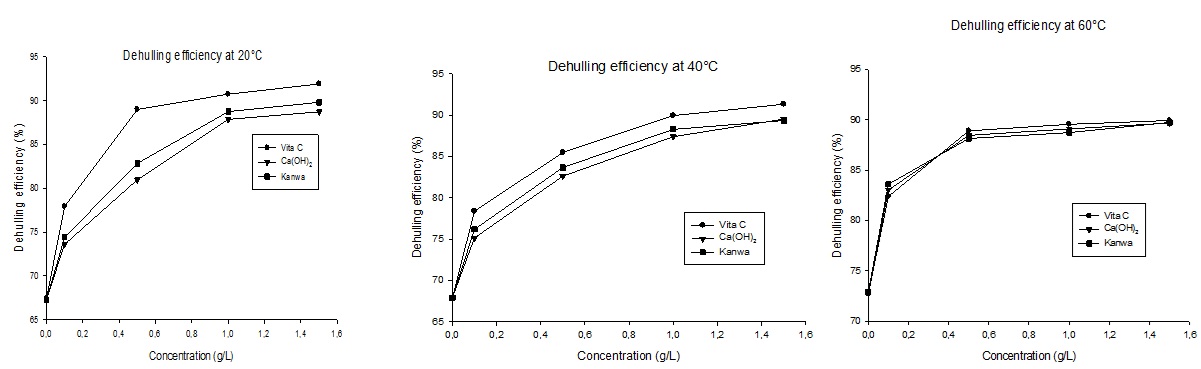 Figure 3: Dehulling efficiency of tiger nut tubers soaked at different temperatures in vitamin C, Ca(OH)2 and kanwa solutions.
Figure 3: Dehulling efficiency of tiger nut tubers soaked at different temperatures in vitamin C, Ca(OH)2 and kanwa solutions.
At the highest concentrations (1.5g/L), most of the solutions had dehulling efficiency of ?85%. Dehulling efficiency also increased with increasing temperatures (?65% at 20°C to ?70% at 60°C) for tiger nut tubers soaked in water (0g/L). Dehulling efficiency appeared most strongly affected by concentrations and temperatures of soaking solutions.
Microbial load of tiger nut tubers before and after treatments
The control sample (raw tubers) has microbial load up to 109 CFU/g, which is very higher compared to microbiological limits standards (106-?107 CFU/g) [28]. Soaking at low temperatures (20 and 40°C) and low concentrations (0.1 and 0.5g/L) of solutions increased microbial load of tiger nut tubers for all the three solutions. Soaking in these conditions stimulates microbial growth by activating microorganisms which are dormant; this is in agreement with work of [29]. On the contrary, high temperature led to reducing microbial load, but the greatest reduction was carried out by dehulling process. Figure 4 shows significant effect of dehulling on the microbial load of tiger nut tubers. This is because all the samples have shown average value from all treatment above 2 log reduction. This result is in agreement with the work of [30], who reported that semi- processed maize grains had lower microbial load.
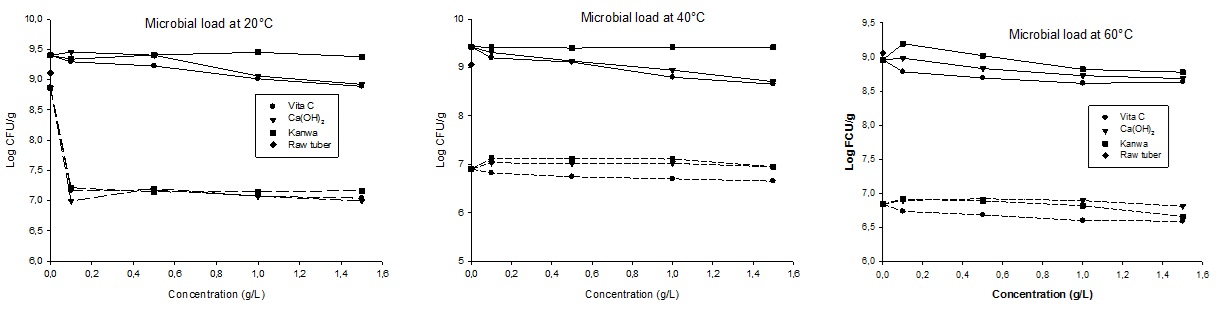 Figure 4: Microbial load of undehulled( ? ) and dehulled ( - - - ) tiger nut tubers soaked in vitamin C, Ca(OH)2 and Kanwa solutions.
Figure 4: Microbial load of undehulled( ? ) and dehulled ( - - - ) tiger nut tubers soaked in vitamin C, Ca(OH)2 and Kanwa solutions.
Like dehulling efficiency, vitamin C solutions has given the best results in term of reduction of microbial load compared to the other soaking solutions. This reduction is considerable at the higher temperatures, reaching a maximum of 4.8 log reduction.
According to [24], the main product resulting from vitamin C decomposition at higher temperature is dehydro-L-ascorbic acid; this component seem to have the same effect like vitamin C in term of dehulling efficiency and microbial load reduction.
Soaking and dehulling treatments gave to tiger nut tuber effective decontamination in term of microbial load and obtained good microbiological quality.
CONCLUSION
As shown in this study, Peleg model can describe soaking behavior of tiger nut tubers in vitamin C, Ca(OH)2 and Kanwa solutions like in simple water. The hydrothermal treatment of tiger nut tubers in acid and alkaline solutions contributed to enhance dehulling efficiency and microbial load reduction. Since there was considerable reduction of microbial load at the higher temperature with vitamin C, these soaking treatments led to important soluble solids loss, on the whole compromising nutritional and organoleptic qualities of tiger nut tubers.
The impact of vitamin C on dehulling efficiency and microbial load demonstrated that research must be continued with regard for the using of alternative acid solutions like acetic acid, lemon extracts for lowering this processing cost.
AUTHOR CONTRIBUTIONS
Conceptualization D; methodology D and RE; software RE; validation D and MP; formal analysis D; Investigation DRD and D; resources D and DRD; data duration RE; writing-original draft preparation D and DRD; writing-review and editing D and RE; supervision MP and RN; project administration D and PM; funding acquisition D. All authors have read and agreed to the published version of the manuscript.
FUNDING
This research received no external funding.
ACKNOWLEDGEMENT
This work was carried out with the help of the Center for International Cooperation in Agronomic Research for Development (CIRAD) in Montpellier/France and the Inter-establishment Agency for Research for Development (AIRD).
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Costa Neto J, Silva R, Amaral P, Leão MR, Gomes T, et al. (2018) Extraction, chemical modification by octenyl succinic and characterization of cyperus esculentus starch. Polímeros 28: 319-322.
- Onovo JC, Ogaraku AO (2007) Studies on some microorganisms associated with exposed tiger nut (Cyperus esculentus ) milk. J Biol Sci 7: 1548-1550.
- Builders PF, Mbah CC, Adama KK, Audu MM (2014) Effect of pH on the physicochemical and binder properties of tiger nut starch. Starch 66: 281-293.
- Selma MV, Valero M, Fernandez PS, Salmeron C (2006) Adaptation of the system of risk analysis and identification and control of critical points (ARICPC) in the management of microbiological quality of tigernut milk. Alimentacion,-Equipos-y-Tecnologia 21: 83-89.
- Okorie SU, Adedokun II, Duru NH (2014) Effect of blending and storage conditions on the microbial quality and sensory characteristics of soy-tiger nut milk beverage. Food Sci Quality Mgt 31: 96-103.
- Akomolafe OM, Awe TV (2017) Microbial contamination and polyethylene packaging of some fruits and vegetables retailed at Akure and Ado Ekiti, South Western Nigeria. J Stored Products Postharv Res 8: 65-72.
- Okechukwu OJ, Orinya CI, Okonkwo EO, Uzoh CV, Ekuma UO, et al. (2016) The microbial contamination of readyto-eat vended fruits in Abakpa main market, Abakaliki, Ebonyi State Nigeria. IOSR J Pharm Bio Sci 11: 71-80.
- Maduka N, Ire FS (2019) A review of some prevention strategies against contamination of cyperus esculentus and tigernut derived products of economic importance. Asian Journal of Advanced Research and Reports 1: 1-13.
- Lorougnon G (1969) Etude morphologique et biologique de deux variétés de C. esculentus Linn. (Cyperacées) Cah. ORSTOM, Ser. Biol. N° 10.
- Djomdi Ejoh R, Ndjouenkeu R (2007) Soaking behaviour and milky extraction performance of tiger nut (Cyperus esculentus) tubers. Journal of Food Engineering 78: 546- 550.
- Mujaffa S, Lee Loy A (2017) The rehydration behavior of microwave- dried amaranth (Amaranthus dubius) leaves. Food Sci Nut 5: 399-406.
- Minka SR, Mbofung CMF, Gandon C, Bruneteau M (1999) The effect of cooking with kanwa alkaline salt on the chemical composition of black beans (Phaseolus vulgaris). Food Chem 64: 145-148.
- Sefa-Dedeh S, Cornelius B, Ohene Afoakwa EO (2014) Effect of nixtamalization on the chemical and functional properties of maize. Food Chemistry 86: 317-324.
- Turhan M, Sayar S, Gunasekaran S (2002) Application of Peleg model to study water absorption in chickpea during soaking. Journal of Food Engineering 53: 153-159.
- Salimi A, Ameri H, Hajighorbani A (2019) Investigating on effect of hot air and water temperature on kinetic of rehydration of celery by using peleg’s model. Latin American Applied Research 49: 249-254.
- Peleg M (1988) An empirical model for the description of moisture sorption curves. Journal of Food Science 53: 1216-1217.
- Wang N, Brennan JG (1991) Moisture sorption isotherm characteristics of potatoes at four temperatures. Journal of Food Engineering 14: 269-282.
- Lomauro CJ, Bakshi AS, Labuza TP (1985) Evaluation of food moisture sorption isotherm equations, Part I: Fruits, vegetable and meat products. Lebensmittel - Wissenschaft und Technologie 18: 111-117.
- Doelhert DC, Wiessenborn DP (2007) Influence of physical grain characteristics on optimal rotor speed during impact dehulling of oats. Cereal Chem 84: 294-300.
- AFNOR (1999) Recueil des normes françaises. Produit dérivé des fruits et légumes. AFNOR, Paris, France.
- Sayar S, Turhan M, Gunasekaran S (2001) Analysis of chickpea soaking by simultaneous water transfer and water-starch reaction. Journal of Food Engineering 50: 91-98.
- Shafaei SM, Masoumi AA, Roshan H (2016) Analysis of water absorption of bean and chickpea during soaking using Peleg model. J Saudi Society of Agri Scie 15: 135-144.
- Kaleta A, Gornicki K, Choinska A, Kosiorek K, Czyzewska A (2017) Modelling of rehydration kinetics of dried carrots using the Peleg model. Agri Forest Eng 69: 13-21
- Marta J, Yuki K, Toshihiro F (2011) Thermal decomposition of vitamin C: An evolved gas analysis-ion attachment mass spectrometry study. Food Chemistry 129: 546-550.
- Ejoh R, Djomdi, Ndjouenkeu R (2006) Characteristics of tigernuts (Cyperus esculentus) tubers and their performance in the production of a milky drink. Journal of Food Processing and Preservation 30: 145-163.
- Djomdi, Kramer JKG, VanderJagt DJ, Ejoh R, Ndjouenkeu R, et al. (2013) Influence of soaking on biochemical components of tiger nut (Cyperus esculentus) tubers cultivated in Cameroon. International Journal of Food Process Engineering 1: 1-15.
- Gilbert RJ, De Louvois J, Donavan T, Little C, Nyen N, et al. (2000) Guidelines for microbiological quality of some ready-to-eat foods sampled at the point of sale. Commun Dis Public Health 3: 163-167.
- Shamsuddeen U, Ahmad MA, Abdulkadir RS (2017) Evaluation of aflatoxin contamination in zea mays (maize) sold in katsina central market, Nigeria. UMYU Journal of Microbiology Research 2: 102-106.
- Muthomi JW, Njenga LN, Gatbumbi JK, Chenug’wa GN (2009) The occurrence of aflatoxins in maize and distribution of mycotoxin-producing Fungi in Eastern Kenya. Plant pathology Journal 8: 113-119.
- Mienda BS (2011) Preliminary report of dehulling effect on the occurrence and distribution of Aspergillus flavus in maize grains stored in Mubi market. Adv Appl Sci Res 2: 612-616.
Citation: Djomdi, Djoulde DR, Ejoh R, Ndjouenkeu R, Michaud P (2020) Application of Peleg Model on Hydrothermal Treatment of Tiger Nut Tubers (Cyperus esculentus) and Effect of Dehulling Efficiency on Microbial Load. J Food Sci Nutr 6: 070.
Copyright: © 2020 Djomdi Djomdi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

