
Aqueous Extraction Optimization of the Antioxidant and Antihyperglycemic Components of Boscia Senegalensis Using Central Composite Design Methodology
*Corresponding Author(s):
Njintang Yanou NicolasDepartment Of Food Science And Nutrition, University Of Ngaoundere, Ngaoundere, Cameroon
Tel:+237 699870979,
Email:njintang@yahoo.fr
Abstract
The objective of this study was to optimize the extraction conditions of antioxidant and antihyperglycemic properties of Boscia Senegalensis decoction using response surface methodology. A Central Composite Design was performed to determine the effect of powder to water ratio (range 0.3/10 - 4/10 g/mL), extraction time (range 3 - 38 min) and extraction temperature (range 25 - 95°C) on total polyphenol content, DPPH free radical scavenging, Ferric ion-reducing power and glycemic index of decoction. Desirability function was established to achieve the best possible combination of factors to a maximum value of total phenolic content, DPPH free radical scavenging, total reducing power of the decoction and a low level of glycemic index. The results revealed that the variation of all the response variables as a function of the factors fit very well the quadratic model with adjusted R2 varying from 82 to 93%. The range variation observed were: Total polyphenol content 0.47 to 2.04 mg galliceq/100mL, glycemic index 55.1 - 89.6%, DPPH antiradical scavenging activity 30.2 - 67.2%, Ferric ion-reducing power 0.29 - 0.95 mg vitamin C eq/100mL decoction. The highest variation of responses 38% was observed on the phenolic content while the lowest 12% was observed on the glycemic index. Significant linear correlation (r = -0.90; p<0.01) was observed between the total polyphenol content and the glycemic index of the decoction. Computed desirability function estimated with accuracy the optimal conditions at 55°C extraction temperature, 3/10 g/mL powder to water ratio and 10 min extraction time. At this optimum point the polyphenol content, total reducing power, DPPH free radical scavenging and glycemic index were respectively 2.34 mg galliceq/100 mL, 0.41 mg vitamin C eq/100 mL, 59.0% and 51.6%. In conclusion the response surface methodology successfully conducted to production of decoction of Boscia Senegalensiswith the highest polyphenol content, antioxidant properties and the lowest glycemic index.
Keywords
INTRODUCTION
Belonging to the Capparidaceae family, Boscia Senegalensis is a wild plant which largely grows under the 20th parallel of the soudanian area of Africa from Senegal, through the Northern Burkina Faso, Nigerian and Niger border, the southern lake of Chad and ended in the Western Sudan. It is an evergreen under shrub plant, usually 1 to 2 m mean height [1]. Largely called in these regions as “Buldumhi” in Fulfulde and “Ndandam” in Wolof [2], Boscia plant produces acidic fruits that are commercialized either for human nutrition or for medicine. The fruits are usually soaked in water for some days to a week to remove the acidity before consumption. The utilization of Boscia seeds for food is generally limited in rural households, notably in Burkina Faso, where populations experienced a food shortage period occurring early in the rainy season and lasting until the next crop harvest period. Rather, Boscia seeds are mostly used for their medicinal properties. In Chad, for instance the seeds are regularly used traditionally for the treatment of diabetes and associate diseases including obesity and coronary heart diseases [3]. One active principle in Boscia has been identified as a glucocapparin, a sulfonated glucose which exhibited not only hypoglycemic effect, but also cytotoxicity [3]. It is demonstrated that Boscia is rich in phenols and antioxidant properties [2], and these molecules may also justify the use of Boscia in traditional medicine. Traditionally the decoction used in patient treatment consisted of mixing the seed powder (about 200 g) with water (about 1L) followed by boiling for about 30 min. However the conditions under which Boscia decoctions are prepared varied from one healer to another and the optimal condition to achieve the extraction of active principle is unknown and need to be investigated.
Optimization of aqueous extraction of plant material has been widely investigated on several food materials including dry seeds, herbs, etc., Generally the most important factors are extraction time and temperature, powder mass to water volume ratio. In particular the extraction of phenolic compounds and antioxidant principles from plant materials has been shown to depend on such factors, but the global and interaction effects of these factors may depend on the type of matrix and this has not yet been investigated on Boscia powder.
The general objective of the present work was to determine the conditions of aqueous extract production with optimal hypoglycemic effect. More specifically the effect of extraction time, temperature and water to flour ratio on total phenolic compounds, antioxidant and hypoglycemic properties of Boscia was first studied, and the determination of optimal extraction condition using multiresponse optimization methodology was done.
MATERIAL AND METHODS
Sampling and production of Boscia Senegalensis flour
Aqueous extraction of Boscia Senegalensis powder
| Run | Independent variables | Dependent variables | |||||
| Coded and actual level | |||||||
| Temperature(°C) | Time(min) | Ratio(g/mL) | DPPH free Scavenging(%) | Total reducing power(Eq mg VitC/ 100 mL of extract) | Total phenolics content(Eq mg gallic acid/100 mL of extract) | Glycemic index(%) | |
| 1 | -1 (40) | -1 (10) | -1 (1/10) | 45.433 | 0.348 | 1.127 | 67.95 |
| 2 | 1 (80) | -1 (10) | -1 (1/10) | 49.557 | 0.693 | 1.468 | 62.27 |
| 3 | -1 (40) | 1 (30) | -1 (1/10) | 32.933 | 0.705 | 1.598 | 66.26 |
| 4 | 1(80) | 1 (30) | -1 (1/10) | 48.905 | 0.702 | 1.161 | 76.68 |
| 5 | -1 (40) | -1 (10) | 1 (3/10) | 46.862 | 0.598 | 1.224 | 72.62 |
| 6 | 1 (80) | -1 (10) | 1 (3/10) | 64.862 | 0.952 | 1.273 | 68.52 |
| 7 | -1 (40) | 1 (30) | 1 (3/10) | 39.823 | 0.466 | 0.853 | 76.35 |
| 8 | 1 (80) | 1 (30) | 1 (3/10) | 67.215 | 0.411 | 0.467 | 89.63 |
| 9 | -1,73 (25) | 0 (20) | 0 (2/10) | 30.215 | 0.291 | 1.159 | 79.26 |
| 10 | 1.73 (95) | 0 (20) | 0 (2/10) | 56.443 | 0.595 | 0.621 | 86.19 |
| 11 | 0 (60) | -1.73 (3) | 0 (2/10) | 48.861 | 0.748 | 1.853 | 64.72 |
| 12 | 0 (60) | 1.73 (38) | 0 (2/10) | 41.911 | 0.768 | 0.687 | 81.44 |
| 13 | 0 (60) | 0 (20) | -1.73 (0.3/10) | 48.518 | 0.637 | 2.041 | 55.09 |
| 14 | 0 (60) | 0 (20) | 1.73 (4/10) | 63.131 | 0.361 | 1.294 | 69.72 |
| 15 | 0 (60) | 0 (20) | 0 (2/10) | 32.733 | 0.414 | 0.692 | 83.47 |
| 16 | 0 (60) | 0 (20) | 0 (2/10) | 50.395 | 0.484 | 0.847 | 79.66 |
| 17 | 0 (60) | 0 (20) | 0 (2/10) | 38.066 | 0.510 | 0.882 | 78.30 |
Table 1: Matrice of central composite design of independent variables (actual and coded levels) for extraction of antioxidant properties of Boscia Senegalensis flour.
Determination of the response variable
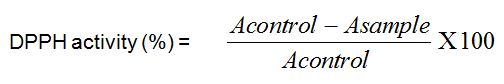
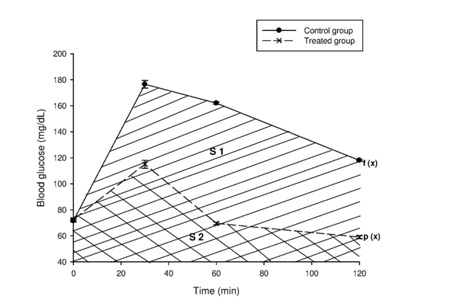 Figure 1: Tolerance to glucose test for 2 rats groups respectively administered and non-administered Boscia decoction. Hatched zone illustrates the Area Under Curve (AUC) for calculation of glycemic index.
Figure 1: Tolerance to glucose test for 2 rats groups respectively administered and non-administered Boscia decoction. Hatched zone illustrates the Area Under Curve (AUC) for calculation of glycemic index.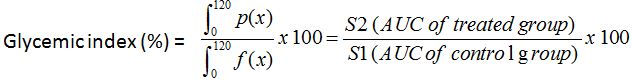
The AUC was determined by integration using GraphPad prism Software version 5. All the rats were treated as per the National Institute of Health guidelines of care and use of laboratory animals (1996). Before experiments, the rats had free access to standard diet and water. They were kept for acclimatization under standard laboratory conditions at room temperature (23 ± 1°C; 55 ± 5% humidity) in individual metabolic cages. All the animals used were fasted overnight before administration of extract and/or glucose. After the administration of the extract and/or glucose till the end of the experiment they were not given access to water and food.
Experimental design, statistical analysis and optimization procedure
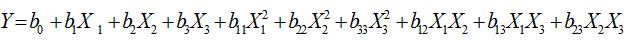
Statistical analysis was performed using Response surface methodology in Minitab.16 software. The significant (p<0.05) terms in the model were found by Analysis Of Variance (ANOVA) for each response. The model adequacies were checked by lack-of-fit test, R2, adjusted-R2 (adj-R2), and p-values as outlined by previous studies [9,10].
The desirability method [11,12] was used as optimization tool to find the best combination of factors that result in Boscia Senegalensis decoction with maximum values of DPPH free radical scavenging, total reducing power, total phenolic compounds, but low level of glycemic index.
RESULTS
Model fitting
| Source | DPPH Free Scavenging (%) | Reducing power (mg Vit C eq/100mL) | Total phenolics (mg galliceq/100mL) | Glycemic index (%) | |||||||||||||
| DF | Coefficients | Sum of squares | F-ratio | P-value | Coefficients | Sum of squares | F-ratio | P-value | Coefficients | Sum of squares | F-ratio | P-value | Coefficients | Sum of squares | F-ratio | P-value | |
| Linear | |||||||||||||||||
| 1 | 7.922 | 878.76 | 10.71 | 0.082 | 0.083 | 0.97 | 17.44 | 0.004 | -0.097 | 0.133 | 3.32 | 0.111 | 0.480 | 3.236 | 0.15 | 0.732 | |
| 1 | -2.134 | 63.76 | 0.78 | 0.471 | -0.019 | 0.005 | 0.95 | 0.362 | -0.216 | 0.656 | 16.36 | 0.004 | 1.950 | 53.250 | 2.53 | 0.252 | |
| 1 | 4.802 | 323.72 | 3.94 | 0.185 | -0.035 | 0.017 | 3.18 | 0.117 | -0.202 | 0.572 | 14.26 | 0.007 | 4.961 | 344.614 | 16.36 | 0.055 | |
| Quadratic | |||||||||||||||||
| 1 | 1.218 | 18.11 | 0.22 | 0.684 | -0.005 | 0.003 | 0.001 | 0.981 | 0.003 | 0.001 | 0.01 | 0.959 | 8.256 | 831.06 | 39.53 | 0.024 | |
| 1 | 1.904 | 44.21 | 0.54 | 0.539 | 0.104 | 0.132 | 23.83 | 0.001 | 0.129 | 0.205 | 5.11 | 0.058 | 4.862 | 288.277 | 13.71 | 0.06 | |
| 1 | 5.383 | 353.41 | 4.31 | 0.173 | 0.018 | 0.004 | 0.72 | 0.424 | 0.262 | 0.838 | 20.83 | 0.002 | 3.420 | 142.652 | 6.79 | 0.121 | |
| Interaction | |||||||||||||||||
| 1 | 2.655 | 56.40 | 0.69 | 0.494 | -0.094 | 0.071 | 12.82 | 0.009 | -0.030 | 0.184 | 4.60 | 0.069 | -3.555 | 101.104 | 4.81 | 0.159 | |
| 1 | 3.161 | 79.98 | 0.97 | 0.427 | -0.005 | 0.002 | 24.16 | 0.001 | -0.151 | 0.224 | 5.60 | 0.049 | -2.102 | 35.364 | 1.68 | 0.324 | |
| 1 | 1.058 | 8.96 | 0.11 | 0.772 | -0.129 | 0.134 | 0.04 | 0.846 | -0.167 | 0.007 | 0.18 | 0.684 | -1.602 | 20.544 | 6.79 | 0.121 | |
| 40.398 | 0.469 | 0.807 | 60.51 | ||||||||||||||
| Lack of ?t | 5 | 11.34 | 0.03 | 0.998 | 0.034 | 2.74 | 0.288 | 0.260 | 5.10 | 0.172 | 0.191 | 1.00 | |||||
| Pure error | 2 | 164.12 | 0.005 | 0.020 | 42.044 | ||||||||||||
| Total | 16 | 1944.64 | 0.514 | 3.049 | 1497.98 | ||||||||||||
| R2 | 90.97 | 92.42 | 90.78 | 97.18 | |||||||||||||
| Adj-R2 | 89.37 | 82.68 | 88.94 | 93.55 | |||||||||||||
Effect of factors on phenolic compounds, glycemic index and antioxidant properties of Boscia Senegalensis decoction
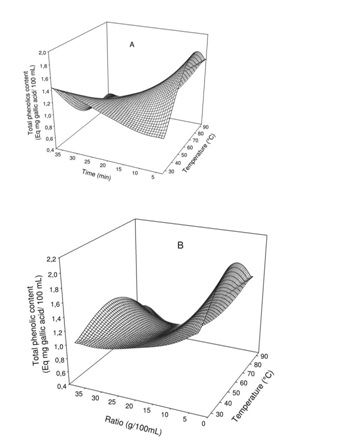 Figure 2: Effect of extraction temperature vs time (A) and ratio (B) on total polyphenol content of Boscia Senegalensis decoction.
Figure 2: Effect of extraction temperature vs time (A) and ratio (B) on total polyphenol content of Boscia Senegalensis decoction.The change in phenolic content showed significant interaction with either the extraction time or the mass to water ratio. Generally at lower extraction time, increase in extraction temperature led to significant (p<0.05) increase in phenol of the decoction up to a maximum around 70°Cfrom which further increase in temperature resulted in decrease while no significant effect of temperature was observed at higher extraction time. In addition while at medium mass to water ratio range (1.5 - 2.5 g/10mL) extraction temperature had no significant effect, at lower and higher mass to water ratio increase in temperature led to significant increase in phenols up to a maximum around 80°C from which further increase in temperature resulted in decrease of phenol content.
The change in glycemic index of Boscia decoction with the extraction factors is presented in figure 3. Generally the glycemic index increased with increase in extraction time and temperature.
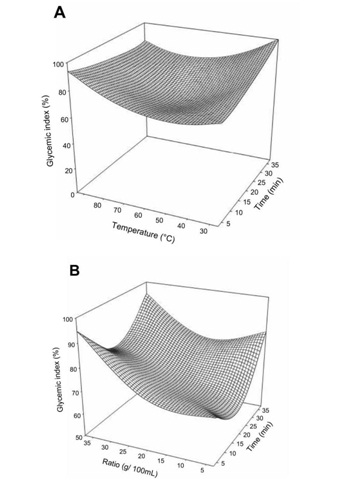 Figure 3: Effect of extraction temperature vs time (A) and ratio (B) on glycemic index of Boscia Senegalensis decoction.
Figure 3: Effect of extraction temperature vs time (A) and ratio (B) on glycemic index of Boscia Senegalensis decoction.Generally the extraction conditions had no significant effect on the DPPH free radical scavenging activity of the decoction (Table 2). However for the total reducing power (Figure 4), temperature was the most important factor with the increase inducing significant increase in the total reducing power. The mass to water ratio showed a quadratic influence with minimum reducing power at 2.0 to 2.5 g/10 mL. Meanwhile the effect of decoction time was mostly observed at higher temperature where an increase led to significant decrease in total reducing power. We did not found significant correlation between the phenols and neither the DPPH scavenging activity (r = - 0.17) or the total reducing power (r = 0.11). This could be due to the interference of the method of quantification of phenolic with aromatic amino acid of the proteins. In fact the Folin-Ciocalteu method, although widely applied to plant extracts, is not specific for phenolic compounds and does suffer interference [13] from other compounds such as proteins which were revealed very high in the Boscia Senegalensis decoction. In addition no significant linear relation was observed between the DPPH scavenging activity and the total reducing power of the Boscia Senegalensisdecoction.
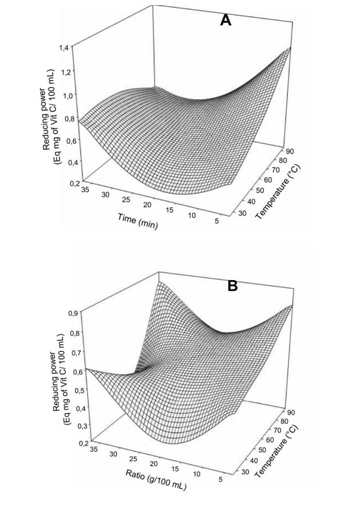 Figure 4: Effect of extraction temperature vs time (A) and ratio (B) on Ferric iron-reducing power of Boscia Senegalensis decoction.
Figure 4: Effect of extraction temperature vs time (A) and ratio (B) on Ferric iron-reducing power of Boscia Senegalensis decoction.Optimization procedure
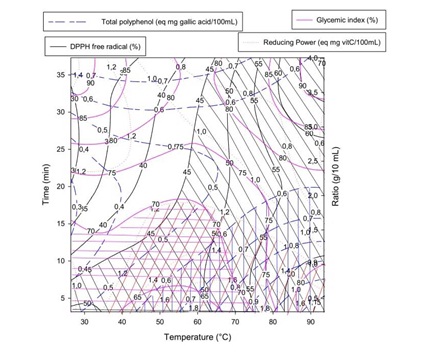 Figure 5: Overlaid plotting for multi response optimization of antioxidant and hypoglycemic properties of Boscia Senegalensis decoction. Each color corresponds to the contour plot along with hatched zone for optimal response for a given variable.
Figure 5: Overlaid plotting for multi response optimization of antioxidant and hypoglycemic properties of Boscia Senegalensis decoction. Each color corresponds to the contour plot along with hatched zone for optimal response for a given variable.Optimum was achieved graphically by identifying zones of maximum DPPH free radical scavenging, total reducing power, total phenolic content and minimum of glycemic index as stripped in the contours plots. The optimal extraction zone corresponded to the range temperature of 40 - 90°C, ratio of 0.5/10 - 3/10 g/mL and time of extraction of 5 - 10 min. As expected the computed optimal conditions of Boscia Senegalensis decoction extraction given in table 3 were within the optimum zone determined graphically. By the graphical method, we observed that optimal zones of all response variables were superposed suggesting a high desirability of the optimization procedure.
| Factors | Low | High | Optimum |
| Time (min) | 3 | 38 | 10 |
| Temperature (°C) | 25 | 95 | 55 |
| Ratio (g/mL) | 0.3/10 | 4/10 | 3/10 |
The computed optimal condition was determined using the desirability function depicted in figure 6. The desirability function describes the variation in probability to achieve the optimization of the response variables. All the factors significantly (p<0.05) influenced the desirability with temperature having the most important effect. Significant interaction between the 3 factors can observed with the response surface all lower than 1. The optimization procedure using desirability function indicated that the overall optimum region had a desirability of 0.90.
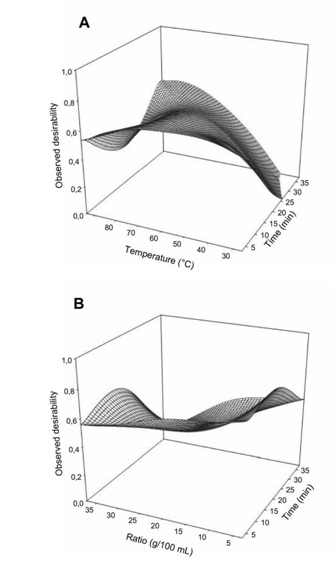 Figure 6: Effect of extraction temperature vs time (A) and ratio (B) on the desirability for maximization of antioxidant properties and minimization of glycemic index of Boscia Senegalensis decoction.
Figure 6: Effect of extraction temperature vs time (A) and ratio (B) on the desirability for maximization of antioxidant properties and minimization of glycemic index of Boscia Senegalensis decoction.Prediction of the optimal zone
| Parameters | Predicted values | Experimental values |
| Phenols content (Eq mg gallic acid/100 mL of extract) | 2.02 | 1.98±0.05 |
| Glycemic index (%) | 54.71 | 50.20±2.25 |
| Total reducing power ((Eq mg Vit C/ 100 mL of extract) | 0.34 | 0.28±0.04 |
| DPPH free radical scavenging (%) | 62.02 | 60.12±0.15 |
| Desirability | 0.90 |
Table 4: Predicted responses at optimum conditions.
Mean ± SD, n = 3
DISCUSSION
Phenolic compounds can be defined as any compound containing a benzene ring with one or more hydroxyl groups [14]. It has already been admitted that these compounds possess antioxidant and hypoglycemic activities [15]. The extraction procedure of phenolics in plant materials has been reviewed by Khoddami et al., [16]. Among solvent usually employed for phenolic extraction, water has been shown to be efficient in extracting phenolic compound [17], flavonoids and proanthocyanidins. We showed in this study that the yield of extraction of phenolic compounds depended on extractions conditions such as the temperature, the time and the ratio of powder to solvent. According to some authors longer extraction time increase the chance of oxidation of phenolic unless reduction agent are added to the solvent [16].However, some authors reported longer time as 30-120 min as optimal extraction time of polyphenolics [17,18]. The increase in phenol extraction with increase in temperature has also been reported in other food materials [18] and may be a consequence of increase in agitation which not only increases the diffusivity of solvent into granules, but also the mass transfer and solubility of molecules. We observed in this study a decrease of phenolic extraction at higher temperature which may result from their loss by volatilization and thermal, chemical and enzymatic decomposition [18], or by complexation with other compounds such as protein which remain bound to the matrix and subsequent insolubilisation [12]. But this may depend on the type of phenols as is the case of stems the optimal time and temperature was observed for 270 min and 55°C [19]. As for the effect of solvent to sample ratio, basically its increase promotes phenolic extraction, but it is advised to determine the optimum ratio so as to minimize the solvent input and saturation effects [16].
The rate of extraction of phenolic may impact the in vivo and in vitro activities of the decoction as they might be active principles as reported for other plants resources [8]. Fortunately we found in this study a significant (r = - 0.93; p<0.001) and linear relationship between the total phenols content and the glycemic index. The negative correlation implied that as the phenols content increased in the decoction, the glycemic index decreased. This indeed highlighted the role of phenols in the hypoglycemic activity of Boscia decoction claimed by consumers and healers. Mahamat et al., [3] recently reported a sugar complex, glucocapparin, as the active molecule in Boscia decoction. Although this may not be rejected, it comes from this study that phenols may partly play a role either alone or in interaction with other hydrophilic compounds such as polysaccharides [20]. One major mechanism by which phenols might regulate glucose concentration was reported in the intestine through inhibition of the membrane transport Na+ - dependent D-glucose [21]. In this respect dietary phenolic compound favor the dissipation of the Na+ electrochemical gradient which provides the driving force for active glucose accumulation [21].
Generally the glycemic index increased with increase in extraction time and temperature, suggesting that higher extraction time and temperature had detrimental effects on the biological activity of the decoction.
Another beneficial aspect of dietary phenols is their antioxidant activity in biological systems. Recent studies on the antioxidant activity of Boscia demonstrated their high antioxidant activity potential [2]. Unfortunately we did not found significant correlation between the phenols and neither the DPPH scavenging activity (r = - 0.17) or the total reducing power (r = 0.11). In addition no significant linear relation was observed between the DPPH scavenging activity and the total reducing power of the Boscia decoction. This may be a result of thermal degradation of Boscia phenols at higher temperature as significant decrease in phenol was observed at temperature higher than 70°C. In addition other components such as polysaccharides in the aqueous extract may be responsible of the activity [20], and this needs to be investigated.
The conditions for production of aqueous extract with high phenols content and low glycemic index were determined 10 min and 55°C. In these conditions our decoction contained 2.02 mg phenols/L of decoction, exhibited about 62% scavenging activity. The glycemic index of the decoction was lower than 55, meaning our Boscia decoction may be classified as low glycemic index potential as compared to intermediate (55-69) and high (70 -100) glycemic potential molecules [22]. All these values give our Boscia decoction a high potential to contribute to the management of diabetes and associated metabolic disorders.
CONCLUSION
The extraction conditions have significant effects on the antioxidant and hypoglycemic properties of Boscia Senegalensis decoction, but the behavior varied from one response variable to another. While the DPPH activity do not varied significantly with any of the studied factor, the total reducing power increases significantly in a quadratic manner with increase in the extraction time and temperature. In addition the total phenolic content linearly decreases with the increase in extraction time and powder to water ratio concomitantly with an increase in glycemic index. Globally decoction with high total phenolic content exhibits low glycemic index thus suggesting a potential role of Boscia phenol in the management of diabetes. With a desirability of 90%, the optimal condition to maximize the phenol, DPPH antiradical scavenging activity and total reducing power, and to minimize the glycemic index is 55°Cextraction temperature, 3/10 g/mL powder to water ratio and 10 min extraction time. In these conditions, the Boscia Senegalensis extract is expected to exhibit higher biological activity. However the functionality of the decoction will depend on the dose and in this respect the toxicity as well as the glucosinolate content of the Boscia extract need to be investigated. In addition, study of the biological activity of the extract including antioxidant activity, α and β glucosidase inhibition and effect on the blood biochemical components needs to be investigated. Investigations on the antioxidant and hypoglycemic effect of isolate molecules in the aqueous extract of Boscia are also needed.
REFERENCES
- Arbonnier M (2009) Arbres arbustes et lianes des zones sèches d’Afrique de l'Ouest.
- NgomVougat RRB, Foyet HS, Garabed RB, Ziebe R (2015) Antioxidant activity and phytochemical constituent of two plants used to manage foot and mouth disease in the Far North Region of Cameroon. J Intercult Ethnopharmacol 4: 40-46.
- Mahamat NAS, Yaya M, Dijoux-Franca MG, Gbenou J, Moudachirou M (2012) In vitro antihyperglycaemic effect of glucocapparin isolated from the seeds of Boscia Senegalensis (Pers.) Lam. ex Poiret. African Journal of Biotechnology 11: 6345-6349.
- Musa KH, Abdullah A, Jusoh K, Subramaniam V (2011) Antioxidant Activity of Pink-Flesh Guava (Psidium guajava L.): Effect of Extraction Techniques and Solvents. Food Analytical Methods 4: 100-107.
- Ebrahimzadeh MA, Nabavi SF, Nabavi SM, Eslami B (2010) Antihemolytic and antioxidant activities of Allium paradoxum. Central European Journal of Biology 5: 338-345.
- Namjooyan F, Azemi ME, Rahmanian VR (2010) Investigation of antioxidant activity and total phenolic content of various fractions of aerial parts of Pimpinella Barbata (DC.) Boiss. Jundishapur Journal of Natural Pharmaceutical Products 5: 1-5.
- Ihediohanma NC (2011) Determination of the Glycemic Indices of Three Different Cassava Granules (Garri) and the Effect of Fermentation Period on Their Glycemic Responses. Pakistan Journal of Nutrition 10: 6-9.
- Eseyin O, Ebong EEP, Awofisayo O, Agboke A (2010) Effect of Telfairia occidentalis on oral glucose tolerance in rats. African Journal of Pharmacy and Pharmacology 4: 368-372.
- Mirhosseini H, Chin-Ping Tan, Hamid NSA, Yusof S (2008) Optimization of the contents of Arabic gum, xanthan gum and orange oil affecting turbidity, average particle size, polydispersity index and density in orange beverage emulsion. Food Hydrocolloid 22: 1212-1223.
- Karazhiyan H, Razavi SMA, Phillips GO (2011) Extraction optimization of a hydrocolloid extract from cress seed (Lepidium sativum) using response surface methodology. Food Hydrocolloid 25: 915-920.
- Makanjuola SA, Enujiugha VN, Omoba OS, Sanni DM (2015) Optimization and prediction of antioxidant properties of a tea- ginger extract. Food Sci Nutr 3: 443-452.
- Mang DY, Abdou AB, Njintang NY, Djiogue EJM, Panyo EA, et al. (2015) Optimization of vegetable milk extraction from whole and dehulled Mucunapruriens (Var Cochinchinensis) flours using central composite design. J Food SciTechnol 52.
- Zhao H, Chen W, Lu J, Zhao M (2010) Phenolic pro?les and antioxidant activities of commercial beers. Food Chem 119: 1150-1158.
- Chirinos R, Betalleluz I, Humána A, Arbizub C, Pedreschi R, et al. (2009) HPLC-DAD characterisation of phenolic compounds from Andean oca (Oxalis tuberosa Mol.) tubers and their contribution to the antioxidant capacity. Food Chem 113: 1243-1251.
- Oboh G, Ademosun AO, Akinleye M, Omojokun OS, Boligon AA, et al. (2015) Starch composition, glycemic indices, phenolic constituents, and antioxidative and antidiabetic properties of some common tropical fruits. Journal of Ethnic Foods 2: 64-73.
- Khoddami A, Wilkes AM, Roberts TH (2013) Techniques for Analysis of Plant Phenolic Compounds. Molecules 18: 2328-2375.
- Kua SF, Ibrahim J, Ooi CKW, Nan KI, Hashim N, et al. (2015) Optimisation of phenolic extraction and quantification of phenolics in palm kernel cake. Renewable Bioresources 3.
- Dent M, Dragovi-Uzelac V, Peni M, Brncic M, Bosiljkov T, et al. (2013) The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol Biotech 51: 84.
- Tan MC, Tan CP, Ho CW (2013) Effects of extraction solvent system, time and temperature on total phenolic content of henna (Lawsonia inermis) stems. International Food Research Journal 20: 3117-3123.
- Sun C, Chen Y, Li X, Tai G, Fan Y, et al. (2014) Anti-hyperglycemic and anti-oxidative activities of ginseng polysaccharides in STZ-induced diabetic mice. Food Funct 5: 845-848.
- Stringer MD, Zahradka P, Taylor CG (2015) Glucose transporters: cellular links to hyperglycemia in insulinresistance and diabetes. Nutrition Reviews 76: 140-154.
- Chen H, Shaw MJ, Moyer-Mileur JL (2010) The new glucose revolution: Is the authoritative guide to the glycemic index the right dietary solution for lifelong health? J Nutr Metab 2: 73-81.
Citation: Faustin D, Selestin SD, Nicolas NY (2017) Aqueous Extraction Optimization of the Antioxidant and Antihyperglycemic Components of Boscia Senegalensis Using Central Composite Design Methodology. J Food Sci Nutr 3: 015.
Copyright: © 2017 Dongmo Faustin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

