
Assessment of the Stability and Viability of a Probiotic Formulation with Strains of Lactiplantibacillus LH05 and LH06 Isolated from Breast Milk
*Corresponding Author(s):
Aguilar-Uscanga BRDoctorate In Sciences In Microbiology And Molecular Biotechnology, Human Milk Research Laboratory, University Center For Exact Sciences And Engineering, University Of Guadalajara, Guadalajara, Mexico
Email:blanca.aguilar@academicos.udg.mx / agublanca@gmail.com
Fajardo-Robledo NS
Pharmaceutical Research And Development Laboratory, University Center For Exact Sciences And Engineering, University Of Guadalajara, Guadalajara, Mexico
Email:nicte.fajardo@academicos.udg.mx
Abstract
The prophylactic use of human-origin probiotics has gained recognition as adjuvant therapy for gastrointestinal disorders, though maintaining their stability and viability during processing and storage remains challenging. This study developed and characterized a lyophilized formulation containing Lactiplantibacillus LH05 and LH06 strains isolated from breast milk combined with Fructooligosaccharides (FOS) and encapsulated in Hydroxypropyl Methylcellulose (HPMC) capsules. We assessed post-lyophilization viability, storage stability (25°C vs 4°C for 6 months), gastrointestinal stress resistance (pH 1.2-6.8, 0.3-0.5% bile salts, digestive enzymes), and functional properties (antimicrobial activity against Escherichia coli ATCC 25922 and Salmonella enterica serovar Typhi ATCC 6539, cell surface hydrophobicity). The probiotic formulation contained lyophilized strains with Nutraflora® FOS and magnesium stearate in HPMC capsules. Post-lyophilization viability reached 90% (p < 0.05 by Student's t-test). HPMC-encapsulated strains maintained > 6 log CFU/g at 4°C for 6 months, however significant viability reduction occurred at 3 and 6 months (p < 0.01). The strains demonstrated gastric acid resistance (pH 3.0), bile tolerance (0.3-0.5%), and potent antimicrobial activity (inhibition zones >6 mm). While FOS enhanced viability (90% maintenance), it incompletely preserved all probiotic attributes. HPMC encapsulation effectively protected bacterial viability (>106 CFU/g) during refrigerated storage, demonstrating strong potential for targeted delivery applications.
Keywords
Encapsulation; FOS; Human-milk; Lactiplantibacillus; Probiotics; Viability
Introduction
Human derived probiotics, particularly those isolated from breast milk, have gained significant relevance in recent years due to their isolation source, which is associated with an evolutionary adaptation to the human host, resulting in greater therapeutic specificity and efficacy [1-3]. In this context, it has been reported that Lactobacillus strains present in breast milk facilitate early mucosal colonization in infants, promoting a resilient gut microbiome, immunological homeostasis, and systemic benefits [4]. Among these, Lactiplantibacillus (formerly L. plantarum), isolated from human milk in a Mexican population, has demonstrated functional properties, including the alleviation of symptoms in patients with constipation-predominant irritable bowel syndrome, preservation of epithelial barrier integrity (intestinal and blood-brain barrier), and neuroprotective effects mediated by the attenuation of oxidative stress and neuroinflammation in animal models of parkinsonism [5-7].
Probiotic potential and biological activities are strain dependent, including the effectiveness as nutraceutical compounds [8]. Therefore, the proper selection of probiotic strains, especially those derived from human sources, should be based on rigorous safety and efficacy criteria to ensure their therapeutic effectiveness and their application in food and clinical products [9].
However, this therapeutic potential requires the development of stable formulations that guarantee cellular viability (>106-108 CFU/g) during processing and storage [10-12]. Nevertheless, it has been documented how biopharmaceutical factors such as thermal stress during manufacturing, oxidative damage, and suboptimal storage conditions can significantly compromise cellular viability and, consequently, probiotic functionality [13,14]. This scenario has driven the development of novel formulations aimed at preserving both microbial viability and the functional properties of these strains [15,16].
Among available pharmaceutical forms, oral capsule formulations have emerged as the system of choice due to their ease of production, precise dosing, and high patient compliance [17]. These formulations typically incorporate excipients with specific functions: some act as diluents (e.g., microcrystalline cellulose) or lubricants (e.g., magnesium stearate) [18], while others provide bulk for low-dose drugs [19]. In this work, we propose using Fructooligosaccharides (FOS) as an alternative diluent due to their rapid aqueous solubility that enhances active ingredient release while leveraging their additional capacity as prebiotic substrates that selectively stimulate lactic acid bacteria growth in the colon [20-22]. This probiotic-prebiotic synergy (symbiotic effect) has been shown to significantly enhance physiological benefits in vivo [23,24].
Polymer-based encapsulation represents an additional strategy to protect probiotic cells from adverse upper gastrointestinal tract conditions [25]. In this regard, enteric polymers like Hydroxypropyl Methylcellulose (HPMC) have proven particularly effective, as their pH-dependent dissolution properties shield microorganisms from gastric acidity while enabling controlled release in the small intestine, thereby improving bioavailability and therapeutic efficacy [17,26].
Although Fructooligosaccharides (FOS) have demonstrated cryoprotective potential, their application in breast milk-derived probiotic strains remains unexplored. This study developed an HPMC capsule formulation containing lyophilized Lactiplantibacillus LH05 and LH06 strains (human milk isolates) with FOS. The formulation capitalizes on FOS's dual function as a water-soluble diluent and prebiotic substrate, combined with magnesium stearate as lubricant. We evaluated the system's ability to maintain cellular viability during room temperature and refrigerated storage, along with the strains' functional properties post-lyophilization.
Materials And Methods
Chemicals and Bacterial Strain
The strains Lactiplantibacillus LH05 and LH06 were isolated from human milk of healthy Mexican mothers. Taxonomic identification at the genus and species level was confirmed by 16S rRNA gene sequencing at the National Center for Genetic Resources (CNRG, Mexico). Both strains are deposited in the CNRG microbial culture collection under the accession numbers Lactiplantibacillus LH05 CM-CNRG 1117 and Lactiplantibacillus LH06 CM-CNRG 1118. Indicator strains E. coli ATCC and Salmonella spp. were obtained from the Clinical Microbiology Laboratory of the University of Guadalajara. Magnesium stearate (commercial brand: ALDROPAZ), Nutraflora® (Ingredion), and de Man, Rogosa, and Sharpe (MRS) agar (DIFCO®) were purchased for use in this study.
Activation and Biomass Production of Lactiplantibacillus LH05 and LH06 Strain
The Lactiplantibacillus LH05 and Lactiplantibacillus LH06 cells were reactivated in 10mL of MRS broth supplemented with 1% Nutraflora. Incubation was done at 37°C for 18 hours under anaerobic conditions. The biomass production of each strain was carried out separately.
Preparation of Freeze-Dried Cells
The Lactiplantibacillus LH05 and Lactiplantibacillus LH06 cells, previously produced, were recovered by centrifugation to 5,000×g, 10 minutes, 4°C. The cell pellet was washed with sterile physiological saline (0.85% NaCl) to remove medium components. Nutraflora® was added at 10% (w/v) as a cryoprotectant (2:1; cells/FOS). The cell pellets were frozen at -80°C and freeze-dried in a Scientz-SC10N freeze dryer at -60°C and 10Pa for 72 hours. The resulting freeze-dried biomass was stored at -20°C until viability testing, powder flow property evaluation, and stability assays.
Enumeration of Bacteria and Percentage of Viability
To determine cell concentration and viability, 1g of lyophilized bacterial cells was reconstituted in 10mL of sterile 0.9% NaCl solution. Serial dilutions were prepared in saline, and 1mL aliquots were plated using the pour-plate technique on MRS agar. Following in performed 37°C for 48 hours, colony counts were performed in duplicate. Only plates containing between 20 and 350 colonies were considered for viable cell enumeration, with results expressed as log CFU/mL. Cell survival rate was calculated according to equation (1) [11,27], which determines the percentage of viable cells:
 Where Log CFU refers to the cell count after freeze-drying, and Log CFU refers to the cell count before freeze-drying.
Where Log CFU refers to the cell count after freeze-drying, and Log CFU refers to the cell count before freeze-drying.
Probiotic Properties
To assess probiotic potential, Lactiplantibacillus strains LH05 and LH06 were cultured in 10mL of MRS broth for 24 hours at 37°C under anaerobic conditions. Following incubation, a 9-log dilution was prepared, and the culture was centrifuged at 5000×g for 5 minutes at 4°C. The resulting cell pellet was then used for subsequent analyses
Bile Salt Tolerance Test
The bile salt tolerance test was conducted based on a previously reported protocol [27], with slight adjustments. Briefly, the harvested cells were resuspended in 5mL of MRS broth containing 0.4% and 0.5% bile salts and incubated at 37°C for 4 hours. Post-incubation, viable cell counts were determined by plating on MRS agar.
pH Resistance Test
The acid resistance of the bacterial strains was analyzed following a previously established method [28], with slight adjustments. The cell biomass was suspended in 5mL of MRS broth adjusted to pH 2 and 3, then incubated at 37°C for 4 hours. After incubation, 1mL of the culture was spread onto MRS agar plates and incubated at 37°C for 24-48 hours to determine viable cell counts.
Evaluation of Enzymatic Resistance
Resistance to enzymatic degradation was evaluated according to the protocol outlined in [29], with minor modifications. Initially, the cells were washed with 2mL of phosphate-buffered saline (PBS: 8g/L NaCl, 0.2g/L KCl, 1.44g/L Na2HPO4, 0.24g/L KH2PO4 in deionized water, pH adjusted to 7.4 with HCl). The pellet was then resuspended in 1mL of PBS. The enzymes used, all from SIGMA-ALDRICH®, were proteinase (Aspergillus melleus, Type XXIII), and trypsin (pancreatic, Type II-S), which were mixed in their respective buffers. The cell pellet was added to each enzyme solution and incubated at 37°C for 2 hours. After incubation, the mixture was centrifuged at 5000g for 10 minutes, and the cell pellet was washed with 1mL of PBS. The bacterial cells were mixed with each enzyme solution and incubated at 37°C for 2 hours. Following incubation, the samples were centrifuged at 5000×g for 10 minutes, and the pellet was washed with 1mL of PBS. Finally, the cells were resuspended in sterile 0.9% and NaCl, plated on MRS agar, and incubated at 37°C for 24 hours before colony enumeration.
Hydrophobicity
The hydrophobicity of the bacterial cells was assessed following a previously described protocol [30], with slight modifications. Briefly, the cell biomass was washed with 2mL of 0.2M PBS buffer and suspended in 4mL of fresh PBS. Subsequently, 0.8mL of toluene was added to the bacterial suspension and vortexed for 2 minutes. After incubating the mixture at room temperature for 20 minutes, the aqueous layer was carefully removed, and its absorbance at 600nm was measured using a UV-Vis spectrophotometer (Scinco S-3100, Lab ProPlus Software, Korea). The reduction in optical density of the aqueous phase was used to quantify cell surface hydrophobicity, calculated as percentage hydrophobicity (%H) using equation (2):
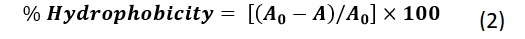
Where A0 represents the initial absorbance before toluene treatment and A is the absorbance after extraction.
Antimicrobial Effect
The antimicrobial potential of lyophilized bacterial cultures was tested against indicator strains (E. coli ATCC and Salmonella enterica subsp.) using the pour-plate method [28]. Mueller-Hinton agar plates were inoculated with 6 log CFU/mL of each test organism. Wells were punched into the agar and filled with 100μL of either bacterial supernatant (grown in MRS broth) or positive control solution. After incubation at 37°C for 24 hours, the plates were examined for inhibition zones. Antimicrobial activity was confirmed by the presence of clear zones around the wells, indicating growth inhibition of the pathogenic strains. Carbenicillin (1mg/mL, 50μL) served as the positive control.
Development of Formulation of Lactiplantibacillus LH05 and Lactiplantibacillus LH06
Preformulation Assay: Determination of Flow Properties
The flow properties of the lyophilized Lactiplantibacillus LH05 and LH06 were evaluated according to the method described in the 12th edition of the Mexican [31].
Bulk Density
A volume of 100mL of powder was transferred into a 100mL graduated glass cylinder without compacting the sample. The lyophilized bacteria and Nutraflora® were weighed with a precision of 0.1%, and the apparent volume (V) was recorded. The apparent density was calculated using equation (3): 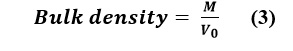
Where M is the mass of the sample and V0 is the apparent volume.
Tapped Density
The same sample used for the apparent density evaluation was employed. After covering the opening of the cylinder, it was lifted to a height of 10±5cm and tapped 250 times onto a flat, soft surface at a constant rate. The tapped volume (V) was recorded, and the tapped density was calculated using equation (4):
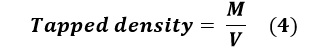
Where M is the mass of the sample and V is the apparent volume.
Flowability Assessment
The Carr and the Hausner ratio were calculated to determine the flowability of the powder, using the following equations:
Carr ratio: 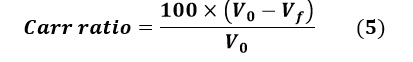
Hausner ratio:
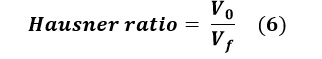
Where V0 is the apparent volume and Vf is the final settled volume.
Encapsulation of Lactiplantibacillus LH05 and LH06
For capsule filling, gastro-resistant HPMC capsules size #00 (brand Capsuline®) were used. The components of the encapsulated mixture included freeze-dried Lactiplantibacillus LH05 and LH06, Nutraflora® as a prebiotic and diluent, and magnesium stearate which served as both lubricant and antistatic agent for the freeze-dried powder while ensuring uniform capsule filling [32]. The dose of each Lactiplantibacillus strain was 10log CFU per gram, with a percentage distribution of 35.9% for Lactiplantibacillus LH05, 35.6% for Lactiplantibacillus LH06, 28.1% Nutraflora®, and 0.3% magnesium stearate. The average weight of each capsule was 478.43mg. The components were mixed manually, and the capsules were aseptically dosed using a manual encapsulator (Profiler®), previously disinfected with 70% ethanol. The capsules were packaged in polypropylene bottles with airtight closures and stored at a temperature of 2-4°C for subsequent evaluation.
Development of Dissolution Profiles for Lactiplantibacillus LH05 and LH06 Capsules
To evaluate the in vitro viability of the Lactiplantibacillus LH05 and LH06 and the performance of the pharmaceutical form, a dissolution profile was developed following the conditions established in 12th edition of the FEUM [33], using a Type I apparatus and a Distek 2100C dissolution tester. Two dissolution media were prepared to simulate gastrointestinal conditions: a 0.1 N hydrochloric acid solution, pH 1.2 (acidic medium), which simulates gastric fluids without enzymes, and a pH 6.8 buffer solution simulating intestinal fluid without enzymes (basic medium). Each dissolution vessel was filled with 900mL of each medium, and the temperature was maintained at 37±0.5°C. Samples of 2mL were taken every 30 minutes without replenishing the medium.
To evaluate the viability of the formulation, a two-stage dissolution profile was developed. In the first stage, the capsules were evaluated in the acidic medium at 50 rpm for 2 hours to assess the integrity and tolerance of the capsules in this medium. In the second stage, the same capsules were consecutively evaluated in the basic medium at 100 rpm for 2 hours [34].
The samples extracted from the dissolution tester were stored at 4°C, and at the end of the test, the cell viability of the Lactiplantibacillus LH05 and LH06 in the sample was assessed using the plate count method. The plates were incubated at 37°C under anaerobic conditions for 24 and 48 hours.
Stability of Lactiplantibacillus LH05 and LH06 Capsules
The stability of the capsules, stored in polypropylene bottles with airtight closures, was evaluated in terms of cell viability through the dissolution profiles described above. The evaluations were performed under two storage conditions: 1 month at room temperature and 1, 3, and 6 months at 2-4°C. Cell viability of the formulation in the capsules was evaluated monthly using the plate count technique with MRS agar and incubated for 24 to 48 hours at 37°C under anaerobic conditions. The percentage of survival was calculated using the equation (1).
Scanning Electron Microscopy of lyophilized cells
The lyophilized samples of Lactiplantibacillus LH05 and LH06 were mounted on slides using conductive tape and coated with gold for 60 seconds using a sputter coater (Denton Vacuum, LLC, model DESK V). Morphological imaging was performed using a scanning electron microscope (JEOL, model JSM6610LV) operating at 5.0 kV with a working distance of 11mm.
Statistical Analysis
The stability test values for the Lactiplantibacillus LH05 and LH06 capsules were expressed as the mean ± SEM from four independent experiments. As heteroscedasticity was identified in the data using Levene’s test, a Welch’s ANOVA was applied to evaluate differences between groups. Subsequently, Dunnett’s post hoc test was used to compare each group with its respective control. Separate analyses were conducted to assess the effect of three main factors: storage time (0, 1, 3, and 6 months), measurement time (30, 60, 90, and 120 minutes), and capsules (n=6). Additionally, statistical assumptions were verified using the Shapiro-Wilk test to assess data normality and Levene’s test to check for homogeneity of variances. All statistical analyses were performed using R software (version 4.4.2). Microsoft Excel was used to capture data, organize information, and generate tables. Results were considered statistically significant with p values < 0.05. GraphPad 8.0.1 software was also used for data visualization.
Results
Analysis of Probiotic Activity and Viability of Freeze-Dried Lactiplantibacillus LH05 and Lactiplantibacillus LH06
The freeze-dried strains Lactiplantibacillus LH05 and LH06, lyophilized with FOS, demonstrated strong acid tolerance at pH 3.0, exhibiting survival rates of 90.7% (LH05) and 91.9% (LH06). However, exposure to a more acidic environment (pH 2.0) completely inhibited growth, indicating limited gastric survivability under extreme conditions.
Both strains also exhibited high bile salt resistance, maintaining survival rates between 95.9% and 98.3% at concentrations of 0.4% and 0.5%, suggesting robust intestinal persistence. Additionally, they displayed exceptional enzymatic stability, retaining viability above 94% after exposure to proteinase and amylase.
In antimicrobial assays, LH05 and LH06 produced inhibition zones of 7 mm against Escherichia coli and 6 mm against Salmonella spp. Cell surface hydrophobicity, an indicator of mucosal adhesion potential, was measured at 10.8% for LH05 and 7.8% for LH06. Despite this variation, cell viability remained above 90% (Table 1). Scanning Electron Microscopy (SEM) analysis demonstrated that lyophilized Lactiplantibacillus cells maintained their characteristic bacillar morphology, with sizes ranging between 3-5µm. The cells exhibited clustered distribution with smooth, homogeneous surfaces, indicating effective preservation during the lyophilization process. Although one structurally damaged cell was observed per microscopic field (indicated by arrows in Figure 1), most cells retained their morphological integrity. These results confirm that the employed lyophilization protocol adequately preserves cellular structure, supporting the probiotic potential of these strains [35].
|
Cells Freeze-Dried |
pHa |
% Bile Saltsb |
Enzymec |
Antimicrobial Effectd |
% H*e |
||||
|
2.0 |
3.0 |
0.4 |
0.5 |
Proteinase |
Amylase |
E. coli |
Salmonella |
||
|
L. LH05 |
0 |
90.7 |
95.9 |
96.8 |
95.9 |
94.9 |
7mm |
6mm |
10.8 |
|
L. LH06 |
0 |
91.9 |
98.3 |
97.7 |
97.9 |
96.7 |
7mm |
6mm |
7.8 |
Table 1: Probiotic activity of Lactiplantibacillus LH05 and LH06 cells freeze-dried with FOS.
Note: a,b,c Values denote the survival percentage of Lactiplantibacillus LH05 and LH06 post-lyophilization, determined by the pour-plate method (MRS agar) under stress conditions: pH tolerance, bile salt resistance, and enzymatic activity; [28]a,b; [27]d; [29]c; [30]e;*% H: hydrophobicity.
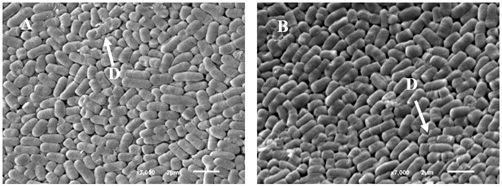 Figure 1. Scanning electron micrographs of lyophilized Lactiplantibacillus LH05 and LH06 with FOS powder: (A) Lactiplantibacillus LH05 cells; (B) Lactiplantibacillus LH06 cells. Scale bar=2µm. D. White arrows indicate cellular damage.
Figure 1. Scanning electron micrographs of lyophilized Lactiplantibacillus LH05 and LH06 with FOS powder: (A) Lactiplantibacillus LH05 cells; (B) Lactiplantibacillus LH06 cells. Scale bar=2µm. D. White arrows indicate cellular damage.
Flow Properties of Freeze-Dried Lactiplantibacillus LH05 and LH06
Table 2 presents the results of flow properties, including the Hausner ratio and Carr index, according to the values specified in the 12th edition of the FEUM [31]. The powders exhibit acceptable flowability. The effect of the Nutraflora® mixture, magnesium stearate, and the freeze-dried Lactiplantibacillus LH05 and LH06 was demonstrated by the good flow properties, as suggested by the Hausner and Carr ratio.
|
Items |
Carr Ratioa |
Hausner Ratiob |
|
Lactiplantibacillus LH05 |
20 |
1.25 |
|
Lactiplantibacillus LH06 |
27.7 |
1.38 |
|
FOS + magnesium stearate + freeze-dried Lactiplantibacillus LH05 and LH06 |
17.6 |
1.21 |
Table 2. Flow Properties of the powders.
Note: a12 to 17 indicates good flow, 18 to 22 indicates acceptable flow; b1.19 to 1.34 indicates acceptable flow [31].
Capsule Evaluation
Storage at 2-4°C in polypropylene bottles allowed the HPMC gastro-resistant capsules to maintain their physical integrity, with no changes in appearance or odor. During the dissolution test at pH 1.2, the capsules preserved their hermetic seal and did not release the formulation even after 120 minutes, as confirmed using a Neubauer chamber. This demonstrates the capsules' ability to withstand the stomach's acidic pH, preventing the premature release of Lactiplantibacillus LH05 and LH06. Since lyophilized Lactiplantibacillus LH05 and LH06 cannot tolerate such a low pH (pH 2.0), it was essential to use capsules capable of protecting them from this acidic environment, ensuring they reach the stomach intact. At pH 6.8, when evaluating the release percentage, measured as the logarithm of cellular viability per capsule (% log CFU/capsule), dissolution peaked at 90-120 minutes, both at 1, 3, and 6 months (Figure 2). This behavior reinforces the stability of the capsules over time, ensuring the controlled release of Lactiplantibacillus LH05 and LH06 in the gastrointestinal tract.
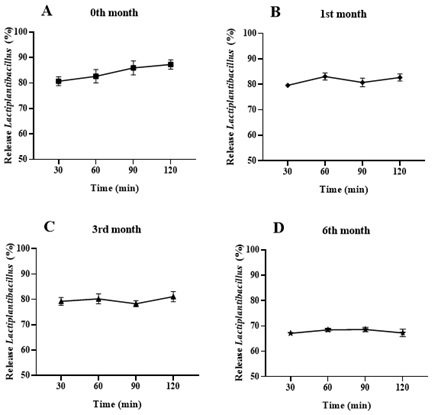
Figure 2. Dissolution percentage of capsules containing Lactiplantibacillus LH05 and LH06, stored at 4°C. Dissolution profile of capsules stored at 4°C for 0 (), 1 (♦), 3 (Δ), and 6 (*) months. Panels A-D show the release (log CFU/capsule) of Lactiplantibacillus LH05 and LH06 from gastro-resistant capsules at 0 (A), 1 (B), 3 (C), and 6 (D) months, at pH 6.8 over 120 minutes. Data are presented as the mean±SEM (n=6).
Stability of Lactiplantibacillus LH05 and LH06 in Formulation and Encapsulation
No survival of the encapsulated strains was observed when stored at room temperature. Statistically significant differences (p < 0.05) were recorded compared to the initial dissolution profile, showing a 62% loss in bacterial viability (Figure 3).
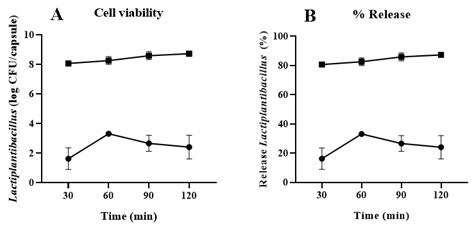 Figure 3. Cell Viability and Percentage of Release of Capsules Containing Lactiplantibacillus LH05 and LH06 Stored at Room Temperature. Dissolution profile for 0 () and 1 () month. (A) Cell viability (log CFU/capsule) and (B) percentage release of Lactiplantibacillus LH05 and LH06 from gastro-resistant capsules at 0 and 1 months at 6.8 over 120 minutes. Data are expressed as the mean±SEM (n=6). The Student’s t-test was applied for group comparisons, with a significance level of (p < 0.05).
Figure 3. Cell Viability and Percentage of Release of Capsules Containing Lactiplantibacillus LH05 and LH06 Stored at Room Temperature. Dissolution profile for 0 () and 1 () month. (A) Cell viability (log CFU/capsule) and (B) percentage release of Lactiplantibacillus LH05 and LH06 from gastro-resistant capsules at 0 and 1 months at 6.8 over 120 minutes. Data are expressed as the mean±SEM (n=6). The Student’s t-test was applied for group comparisons, with a significance level of (p < 0.05).
Regarding the stability of the strains in the formulation contained within the capsules, it was observed that the viability, expressed as Colony-Forming Units (CFU) of Lactiplantibacillus LH05 and LH06 showed no significant differences (p>0.05) in the number of viable colonies between 0 and 1 month of storage at 2-4°C, maintaining an average viability of 8.7±0.18 and 8.3±0.14 log CFU/mL, respectively. However, a significant decrease in cellular viability (p < 0.05) was observed at 3 and 6 months of storage (Figure 4), with a maximum viability observed at 3 months (8.1 log CFU/mL) and 6 months (6.8 log CFU/mL). Despite this decrease, the obtained value remains within the viable range of 6 log CFU, which is the recommended threshold for food or probiotic formulations intended for human consumption [10-12].
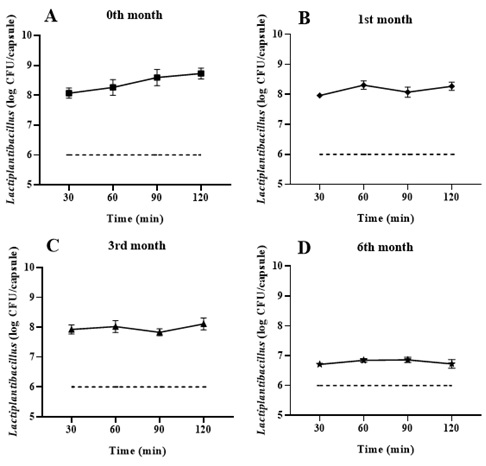 Figure 4. Cell viability of capsules containing Lactiplantibacillus LH05 and LH06, stored at 4°C. Dissolution profile for 0 ()1 (♦), 3 (Δ), 6 (*) months and the minimum (---), suggested by [10-12]. Panels A-D show cell viability (log CFU/capsule) of Lactiplantibacillus LH05 and LH06 from gastro-resistant capsules at A (0), B (1), C (3), and D (6) months, at pH 6.8 over 120 minutes. Data is expressed as the mean±SEM (n=6). A Welch ANOVA was applied for the comparison between groups, followed by a Dunnett's test for pairwise comparisons, with a significant value of (p < 0.05).
Figure 4. Cell viability of capsules containing Lactiplantibacillus LH05 and LH06, stored at 4°C. Dissolution profile for 0 ()1 (♦), 3 (Δ), 6 (*) months and the minimum (---), suggested by [10-12]. Panels A-D show cell viability (log CFU/capsule) of Lactiplantibacillus LH05 and LH06 from gastro-resistant capsules at A (0), B (1), C (3), and D (6) months, at pH 6.8 over 120 minutes. Data is expressed as the mean±SEM (n=6). A Welch ANOVA was applied for the comparison between groups, followed by a Dunnett's test for pairwise comparisons, with a significant value of (p < 0.05).
SEM images of the probiotic formulation revealed morphological changes in Lactiplantibacillus cells after 6 months of storage, showing slight surface roughness (Figure 5). Furthermore, physical interactions were observed between the formulation's excipients: magnesium stearate particles (0.3% w/w) formed a discontinuous layer around FOS aggregates, acting as a barrier that limited direct contact between this prebiotic and the lyophilized bacterial cells.
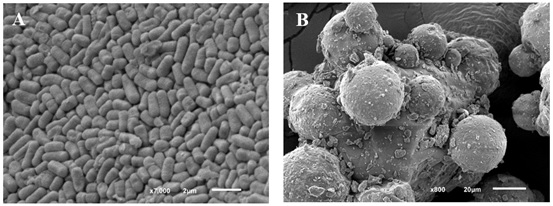 Figure 5. Scanning electron microscopy of FOS + magnesium stearate formulation containing freeze-dried Lactiplantibacillus LH05 and LH06. A) Lactiplantibacillus LH05 and LH06 cells. B) FOS-magnesium stearate interaction. Scale bars: A=2µm, B=20µm.
Figure 5. Scanning electron microscopy of FOS + magnesium stearate formulation containing freeze-dried Lactiplantibacillus LH05 and LH06. A) Lactiplantibacillus LH05 and LH06 cells. B) FOS-magnesium stearate interaction. Scale bars: A=2µm, B=20µm.
Discussion
Probiotic viability is a critical determinant of efficacy in dietary supplements, particularly following exposure to stressful conditions like lyophilization. The selection of an appropriate cryoprotectant is essential to ensure cellular integrity [36]. In this study, the preserved viability of human milk-derived Lactiplantibacillus LH05 and LH06 following FOS-assisted lyophilization confirms its protective effect. This can be attributed to FOS's ability to penetrate cell walls, induce pre-freezing plasmolysis, and provide mechanical protection during lyophilization [37,38].
Previous studies reported comparable survival rates (90%) for L. plantarum using fructooligosaccharides [39], though with lower initial microbial loads (9 log CFU/mL), resulting in post-lyophilization counts inferior to our results (10 log CFU/mL). Similarly, was evaluated L. plantarum microencapsulation and found their optimal lyophilization-resistant formulation combined FOS with Whey Protein Isolate (WPI), achieving 9.8 log CFU/mL [40], (40) comparable to our FOS-only results (10 log CFU/mL). These findings suggest that for human milk-derived Lactiplantibacillus LH05 and LH06, FOS alone may be as effective as more complex formulations, thereby optimizing production costs and processes.
Beyond viability, probiotics must retain functional properties to exert host benefits, including gastrointestinal survival, epithelial adhesion capacity, and antimicrobial activity. In this study, FOS-lyophilized LH05 and LH06 maintained resistance to proteases, trypsin, bile salts, and antimicrobial activity against E. coli and S. typhi, despite lyophilization's potential to damage cell membranes, enzymatic activity, and genetic material [36]. These results align with previous observations in Lactiplantibacillus LH05 and LH06, which demonstrated comparable enzyme resistance and tolerance to low pH and bile salts [35].
We further evaluated hydrophobicity and acid resistance (pH 2.0-3.0) in lyophilized strains. Hydrophobicity, mediated by surface proteins and phospholipids, influences intestinal mucus adhesion [38,41]. Probiotic acid tolerance depends not only on adaptive mechanisms like enzymatic adjustments and genetic modifications but also on membrane composition changes, including increased unsaturated fatty acids to limit proton influx and maintain intracellular pH [42-44], within viability-compatible ranges, even in extreme acidity.
However, reduced pH 2.0 tolerance and decreased hydrophobicity suggest FOS incompletely protects post-lyophilization membrane integrity, supported by SEM observations (Figure 1) of occasional cellular damage. While FOS stabilizes membranes by binding phospholipids during dehydration, this interaction may restrict lipid mobility, potentially affecting adhesion [45]. Notably, hydrophobicity doesn't always directly correlate with probiotic adhesive capacity [46], warranting further in situ adhesion studies.
Effective probiotic formulations require both microbial viability and controlled gastrointestinal release [47]. The HPMC capsule design effectively prevented release under acidic conditions (pH 2.0) while enabling sustained intestinal release of Lactiplantibacillus (90% release at 120 minutes, pH 6.8). This dual release profile is primarily attributed to the pH-dependent gelation properties of HPMC, which forms a stable matrix in acidic environments but undergoes progressive erosion under intestinal pH conditions [48].
The sustained intestinal release profile (30-120 minutes) reflects a balance between capsule erosion (favored by HPMC solubility at pH≥6.8) and FOS dissolution [20], which facilitated Lactiplantibacillus dispersion. SEM analysis confirmed magnesium stearate's primary lubricant role, forming a thin layer over FOS particles, while FOS maintained its diluent function with minimal interaction to lyophilized cells. The FOS-magnesium stearate synergy suggests a protective system maintaining Lactiplantibacillus viability during 6-month storage, likely by efficiently modulating matrix water activity - a critical factor for lyophilized formulation stability [49].
FOS served three key roles as a lyoprotectant, diluent excipient, and prebiotic substrate [50-53]. This multifunctionality is particularly valuable in probiotic formulations where components must contribute to both physical stability and biological functionality [22].
Notably, after 6-month refrigeration, Lactiplantibacillus cells exhibited roughened structures suggesting partial dehydration/oxidative stress, potentially leading to DNA damage a characteristic lyophilized system phenomenon. This indicates molecular degradation mechanisms persist even under optimal refrigeration [54-56], highlighting needs for investigating molecular protection mechanisms, optimizing FOS: Stearate ratios for release kinetics, developing storage stability monitoring methods, and correlating formulation physicochemical parameters with in vivo probiotic viability/functionality.
Conclusion
The formulation of encapsulated probiotics, based on the inclusion of Lactiplantibacillus LH05 and LH06 strains isolated from milk, demonstrated the ability to maintain bacterial viability and phenotypic integrity during mid-term cold storage. This study proposes that a mixture of fructooligosaccharides and magnesium stearate serves as an effective carrier for milk-derived probiotics, with potential prebiotic benefits. However, in vivo studies are required to confirm their probiotic potential.
Use of AI Tools Declaration
The authors declare they have not used Artificial Intelligence (AI) tools in the creation of this article.
Acknowledgment
The authors gratefully acknowledge the financial support provided by L'Oréal-UNESCO-AMC. We are grateful to students Jonathan Emanuel Flores Polanco and Leonel Silva Chocoteco for their participation.
Conflict of Interest
The authors declare no conflict of interest.
Authors Contribution
García-Robles G: Formal analysis, Methodology, Visualization, and Writing original draft. Fajardo-Robledo N.S: Project administration, Conceptualization, Supervision, Funding acquisition, and Writing review & editing. Ruvalcaba-Gómez J.M: Writing review & editing and Resources. Solís-Pacheco J.R: Writing review & editing, Supervision, Resources, Funding acquisition. Villarruel-López A: Resources, Writing review & editing. Aguilar-Uscanga B.R: Project administration, Conceptualization, Supervision, Resources, Funding acquisition, and Writing review & editing.
References
- Mawla AAE, Mehanna NS, Sallam MK, Kamel Z, Mohamed MSM (2024) Potential probiotics from human breast milk with promising cholesterol-reduction and anti-tumour effects. Microbes and Infectious Diseases 5: 1541-1556.
- Bazireh H, Shariati P, Jamalkandi SA, Ahmadi A, Boroumand MA (2020) Isolation of novel probiotic Lactobacillus and Enterococcus strains from human salivary and fecal sources. Front Microbiol 11: 597946.
- Obisesan AO, Abiodun OO, Ayeni FA (2024) Lactic acid bacteria isolated from women’ breast milk and infants’ faeces have appreciable immunogenic and probiotic potentials against diarrheagenic E. coli strains. BMC Microbiol 24: 350.
- Lubiech K, Twaruzek M (2020) Lactobacillus bacteria in breast milk. Nutrients 12: 3783.
- Nápoles-Medina AY, Aguilar-Uscanga BR, Solís-Pacheco JR, Tejeda-Martínez AR, Ramírez-Jirano LJ, et al. (2023) Oral administration of Lactobacillus inhibits the permeability of blood-brain and gut barriers in a Parkinsonism model. Behav Neurol 2023: 6686037.
- Flores-Soto ME, Nápoles-Medina AY, Tejeda-Martínez AR, Solís-Pacheco JR, Chaparro-Huerta V, et al. (2025) Supplementation of the probiotic LLH135 reduces oxidative stress in a model of Hemiparkinsonism. Behav Neurol 2025: 8401392.
- Pacheco JRS, Arreola ARG, Velasco JAVR, Aguilar JGS, López JAA, et al. (2021) Effect of the intake of probiotics isolated from human milk in people with gastritis and irritable bowel syndrome. Journal of Probiotics & Health 9: 228.
- Shi LH, Balakrishnan K, Thiagarajah K, et al. (2016) Beneficial properties of probiotics. Trop Life Sci Res 27: 73-90.
- WHO/FAO (2002) Guidelines for the Evaluation of Probiotics in Food. Food and Agriculture Organization of the United Nations/World Health Organization, London, UK.
- Senadeera SS, Prasanna PHP, Jayawardana NWIA, Gunasekara DCS, Senadeera P, et al. (2018) Antioxidant, physicochemical, microbiological, and sensory properties of probiotic yoghurt incorporated with various Annona species pulp. Heliyon 4: 00955.
- Shu G, Wang Z, Chen L, Wan H, Chen H (2018) Characterization of freeze-dried Lactobacillus acidophilus in goat milk powder and tablet: Optimization of the composite cryoprotectants and evaluation of storage stability at different temperature. LWT 90: 70-76.
- Varela-Pérez A, Romero-Chapol OO, Castillo-Olmos AG, García HS, Suárez-Quiroz ML, et al. (2022) Encapsulation of Lactobacillus gasseri: Characterization, probiotic survival, in vitro evaluation and viability in apple juice. Foods 11: 740.
- Amund OD (2016) Exploring the relationship between exposure to technological and gastrointestinal stress and probiotic functional properties of lactobacilli and bifidobacteria. Can J Microbiol 62: 715-725.
- Mills S, Stanton C, Fitzgerald GF, Ross RP (2011) Enhancing the stress responses of probiotics for a lifestyle from gut to product and back again. Microbial Cell Factories 10: 1-15.
- Šipailiene A, Petraityte S (2018) Encapsulation of probiotics: proper selection of the probiotic strain and the influence of encapsulation technology and materials on the viability of encapsulated microorganisms. Probiotics Antimicrob Proteins 10: 1-10.
- Chávarri M, Marañón I, Villarán MC (2012) Encapsulation technology to protect probiotic bacteria. INTECH Open Access Publisher, Croatia.
- Baral KC, Bajracharya R, Lee SH, Han HK (2021) Advancements in the pharmaceutical applications of probiotics: Dosage forms and formulation technology. Int J Nanomedicine 16: 7535-7556.
- How YH, Yeo SK (2021) Oral probiotic and its delivery carriers to improve oral health: A review. Microbiology 167: 001076.
- Vadaga A (2024) A Text Book of Pharmaceutics for I Year Diploma in Pharmacy. Thinkplus Pharma Publications. India.
- Garleb KA, Snowden MK, Wolf BW, Chow JM (2002) Application of fructooligosaccharides to medical foods as a fermentable dietary fiber. Bioscience and microflora 21: 43-54.
- Bamigbade GB, Subhash AJ, Kamal-Eldin A, Nyström L, Ayyash M (2022) An updated review on prebiotics: Insights on potentials of food seeds waste as source of potential prebiotics. Molecules 27: 5947.
- Basholli-Salihu M, Kryeziu T, Nebija D, Salar-Behzadi S, Viernstein H, et al. (2019) Prebiotics as excipients for enhancement of stability and functionality of Bifidobacterium longum infantis with potential application as symbiotics in food and pharmaceuticals. Pharmazie 74: 326-333.
- Rivière A, Selak M, Lantin D, Leroy F, Vuyst LD (2016) Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front Microbiol 7: 979.
- Kumar SGV, Singh SK, Goyal P, Dilbaghi N, Mishra DN (2005) Beneficial effects of probiotics and prebiotics on human health. Pharmazie 60: 163-171.
- Kil BJ, Yoon SJ, Yun CH, Huh CS (2020) The effect of milk protein on the biological and rheological properties of probiotic capsules. J Microbiol Biotechnol 30: 1870.
- Wang G, Chen Y, Xia Y, Song X, Ai L (2022) Characteristics of probiotic preparations and their applications. Foods 11: 2472.
- Pieniz S, Andreazza R, Anghinoni T, Anghinoni T, Brandelli A (2014) Probiotic potential, antimicrobial and antioxidant activities of Enterococcus durans strain LAB18s. Food Control 37: 251-256.
- Aguilar-Uscanga BR, Solís-Pacheco JR, Plascencia L, Aguilar-Uscanga MG, García HS, et al. (2013) Effect of culture medium on bacteriocin production by Lactobacillus rhamnosus HN001 and Lactobacillus reuteri ATCC 53608. J Microbiol Biotechnol Food Sci 2: 2462-2468.
- Botes M, Loos B, Reenen CA, Dicks LMT (2008) Adhesion of the probiotic strains Enterococcus mundtii ST4SA and Lactobacillus plantarum 423 to Caco-2 cells under conditions simulating the intestinal tract, and in the presence of antibiotics and anti-inflammatory medicaments. Arch microbiol 190: 573-584.
- Re BD, Sgorbati B, Miglioli M, Palenzona D (2000) Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol 31: 438-442.
- Salud SD (2018) Densidad aparente y densidad compactada de polvos. Farmacopea de los Estados Unidos Mexicanos 12 edición. México: Comisión Federal para la Protección contra Riesgos Sanitarios.
- Chendo C, Pinto JF, Paisana MC (2023) Comprehensive powder flow characterization with reduced testing. Int J Pharm 642: 123107.
- Salud SD (2018) Disolución. Farmacopea de los Estados Unidos Mexicanos 12 edición. México: Comisión Federal para la Protección contra Riesgos Sanitarios.
- Zawari M, Poller B, Walker G, Pearson A, Hampton M, et al. (2019) Formulation of Broccoli Sprout Powder in Gastro-Resistant Capsules Protects against the Acidic pH of the Stomach In Vitro but Does Not Increase Isothiocyanate Bioavailability In Vivo. Antioxidants 8: 359.
- Arreola AR, Pacheco JRS, Lacroix M, López EB, Hernández REN, et al. (2020) In vivo assessment and characterization of lactic acid bacteria with probiotic profile isolated from human milk powder. Nutr Hosp 38: 152-160.
- Ge S, Han J, Sun Q, Zhou Q, Ye Z, et al. (2024) Research progress on improving the freeze-drying resistance of probiotics: A review. Trends Food Sci Technol 147: 104425.
- Goderska K (2012) Different methods of probiotics stabilization. IntechOpen, UK.
- Savedboworn W, Teawsomboonkit K, Surichay S, Riansa-Ngawong W, Rittisak S, et al. (2019) Impact of protectants on the storage stability of freeze-dried probiotic Lactobacillus plantarum. Food Sci Biotechnol 28: 795-805.
- Shekh SL, Boricha AA, Chavda JG, Vyas BRM (2020) Probiotic potential of lyophilized Lactobacillus plantarum GP. Annals of Microbiology 70: 1-12.
- Rajam R, Anandharamakrishnan C (2015) Microencapsulation of Lactobacillus plantarum (MTCC 5422) with fructooligosaccharide as wall material by spray drying. LWT-Food Science and Technology 60: 773-780.
- Arellano K, Park H, Kim B, Yeo S, Jo H, et al. (2021) Improving the viability of freeze-dried probiotics using a lysine-based rehydration mixture. Microbiol Biotechnol Lett 49: 157-166.
- Matoba Y, Oda K, Wataeda M (2024) pH-dependent regulation of an acidophilic O-acetylhomoserine sulfhydrylase from Lactobacillus plantarum. Appl Environ Microbiol 90: 00118-00124.
- Wu J, Yan X, Weng P, Chen G, Wu Z (2021) Homology-and cross-resistance of Lactobacillus plantarum to acid and osmotic stress and the influence of induction conditions on its proliferation by RNA-Seq. J Basic Microbiol 61: 576-590.
- Zhu J, Sun Y, Zhang S (2024) Unraveling the genetic adaptations in cell surface composition and transporters of Lactiplantibacillus plantarum for enhanced acid tolerance. J Agric Food Chem 72: 5368-5378.
- Schwab C, Vogel R, Gänzle MG (2007) Influence of oligosaccharides on the viability and membrane properties of Lactobacillus reuteri TMW1. 106 during freeze-drying. Cryobiology 55: 108-114.
- Krausova G, Hyrslova I, Hynstova I (2019) In vitro evaluation of adhesion capacity, hydrophobicity, and auto-aggregation of newly isolated potential probiotic strains. Fermentation 5: 100.
- Liu R, Ci X, Liu L, Wang X, Rifky M, et al. (2024) Chitosan entrapping of sodium alginate/Lycium barbarum polysaccharide gels for the encapsulation, protection and delivery of Lactiplantibacillus plantarum with enhanced viability. International Journal of Biological Macromolecules 260: 129615.
- Fredua-Agyeman M (2024) Surviving process and transit: Controlled freeze drying, storage and enteric coated capsules for targeted delivery of probiotic Lactobacillus acidophilus. Heliyon 10.
- Sielatycka K, Sliwa-Dominiak J, Radaczynska M, Juzwa W, Kaczmarczyk M, et al. (2023) Application of flow cytometry and measurement of water activity to evaluate the stability of probiotic products. Research Square.
- Campos D, Betalleluz-Pallardel I, Chirinos R, Aguilar-Galvez A, Noratto G, et al. (2012) Prebiotic effects of yacon (Smallanthus sonchifolius & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food chemistry 135: 1592-1599.
- Ojwach J, Kumar A, Mukaratirwa S, Mutanda T (2020) Fructooligosaccharides synthesized by fructosyltransferase from an indigenous coprophilous Aspergillus niger strain XOBP48 exhibits antioxidant activity. Bioactive Carbohydrates and Dietary Fibre 24: 100238.
- Chen HL, Wang CH, Kuo YW, Tsai CH (2011) Antioxidative and hepatoprotective effects of fructo-oligosaccharide in d-galactose-treated Balb/cJ mice. Br J Nutr 105: 805-809.
- Altieri C, Bevilacqua A, Sinigaglia M (2011) Prolonging the viability of Lactobacillus plantarum through the addition of prebiotics into the medium. J Food Sci 76: 336-345.
- Santivarangkna C (2016) Advances in probiotic technology. CRC Press, USA.
- Albadran HA, Chatzifragkou A, Khutoryanskiy VV, Charalampopoulos D (2015) Stability of probiotic Lactobacillus plantarum in dry microcapsules under accelerated storage conditions. Food Res Int 74: 208-216.
- Santivarangkna C, Kulozik U, Foerst P (2008) Inactivation mechanisms of lactic acid starter cultures preserved by drying processes. J Appl Microbiol 105: 1-13.
Citation: García-Robles G, Fajardo-Robledo NS, Ruvalcaba-Gómez JM, Solís-Pacheco JR, Villarruel-López A, et al. (2025) Assessment of the Stability and Viability of a Probiotic Formulation with Strains of Lactiplantibacillus LH05 and LH06 Isolated from Breast Milk. HSOA J Food Sci Nutr 11: 222.
Copyright: © 2025 García-Robles G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

