
Journal of AIDS Clinical Research & STDs Category: Clinical
Type: Research Article
Attentional Circuits in People Living with HIV and Apathy: Differential Alterations
*Corresponding Author(s):
Martin J Mazzoglio Y NabarFaculty Of Medicine, University Of Buenos Aires, Buenos Aires City, Argentina
Tel:(5411) 5950-9500,
Email:mazzoglioynabar@hotmail.com
Received Date: Jun 27, 2019
Accepted Date: Jul 10, 2019
Published Date: Jul 17, 2019
Abstract
Depressive symptoms are prevalent and of great importance, with high negative impact. People living with HIV (PLHIV) have particular characteristics in their depressive disorder, such as the presence of symptoms of apathy, which conditions their response to treatment, evolution, and residual symptomatology. The objective was to determine alterations in the attentional domain in PLHIV diagnosed with Depression Disorder with and without apathy to determine differential parameters. A cohort of people living with HIV was studied with neuropsychological tests to evaluate the attention domain. There were differences in attentional subtypes between people who had apathy and those who did not. Apathy in PLHIV and depression disorders present specific and differential alterations in the attentional domain.
Keywords
Apathy; Attention; Depression; HIV
INTRODUCTION
Depressive symptoms are prevalent and of great importance, with the high negative impact of the quality of life of people dealing with chronic diseases. People Living With HIV (PLHIV) present some specific characteristics in their depressive mood disorders: increased apathy, pharmacological resistance, sub-diagnosis, unfinished treatments, higher suicidality rates, and neurocognitive alterations [1-7].
OBJECTIVES
To determine alterations in the attentional domain in PLHIV diagnosed with Depression Disorder with and without apathy in order to determine differential parameters.
MATERIALS AND METHODS
A cross-sectional study in a sample of 38 PLHIV with undetectable viral load and normal levels of CD4 in peripheral blood was studied. The sample included members of both genders (9 women and 29 men) with depressive disorder (F32.9-DSM IV), 16 diagnosed with apathy and 22 who were not, in HAART (Highly Active Antiretroviral Treatment) without therapeutic failure in the past two (2) years and not using protease inhibitors; no psychopharmacological treatment (with the exception of anxiolytic medication) or HIV induced dementia (American Academy of Neurology) or infectious comorbidities (hepatitis C, central nervous system infections or central vascular infections). The PLHIV were studied by the same interdisciplinary team consisting of psychologist and psychiatrist at the Faculty of Medicine of the Buenos Aires University.
The instruments used were MINI-International Neuropsychiatric Interview[8], Hamilton Depression Rating Scale [9,10], Apathy Evaluation Scale clinical version [11], Neuropsychiatric Inventory as well as several neuropsychological assessment tools (Stroop Color Word Test, Trail Making Test Part A and B, WAIS Digit-Symbol Coding, Digit Span and Vocabulary, BTS-1 and BTS-3) [12-18].
The clinical version of the AES (AES-C) is one of the most widely used instruments to assess apathy in an interdisciplinary context, although there is also a self-administered version for caregivers (with different scores and cuts). It consists of 17 items classified on a scale of 4 Likert-type points; scores less than 26 are considered as "clinical" apathy.The questions refer to cognitive, behavioral, emotional and other aspects without specification, each aspect constitutes a subscale.It should be noted that the score has an inverse correlation with severity.
Statistical parameters were applied to find significant differences between the groups of patients compared to the control group of PLHIV without depression and without apathy. The statistical significance was defined as p <0.05. Legal and ethical measures were considered according to Helsinki declaration, international regulations and compliance with the ANMAT Provision 6677/10.
The instruments used were MINI-International Neuropsychiatric Interview[8], Hamilton Depression Rating Scale [9,10], Apathy Evaluation Scale clinical version [11], Neuropsychiatric Inventory as well as several neuropsychological assessment tools (Stroop Color Word Test, Trail Making Test Part A and B, WAIS Digit-Symbol Coding, Digit Span and Vocabulary, BTS-1 and BTS-3) [12-18].
The clinical version of the AES (AES-C) is one of the most widely used instruments to assess apathy in an interdisciplinary context, although there is also a self-administered version for caregivers (with different scores and cuts). It consists of 17 items classified on a scale of 4 Likert-type points; scores less than 26 are considered as "clinical" apathy.The questions refer to cognitive, behavioral, emotional and other aspects without specification, each aspect constitutes a subscale.It should be noted that the score has an inverse correlation with severity.
Statistical parameters were applied to find significant differences between the groups of patients compared to the control group of PLHIV without depression and without apathy. The statistical significance was defined as p <0.05. Legal and ethical measures were considered according to Helsinki declaration, international regulations and compliance with the ANMAT Provision 6677/10.
RESULTS
Table 1 shows the subgroups evaluated of people living with HIV (PLHIV). PLHIV and depressive disorder presented a high prevalence of apathy (42.11%), with its characteristics (Graph 1 and 2). Processing speed was found to be slower, correlated with the intensity of the depression (r2>0.90) but without significant differences with the group that showed apathy (p=0.03) (Graph 3). In patients who presented apathy, higher statistical alterations were found, according to decreasing differential affectation, in both sustained and selective attention (p<0.05) (Graph 4 and 5). Selective attention was found to be significantly altered in the group with depressive disorder and apathy (p<0.05) (Graph 6).
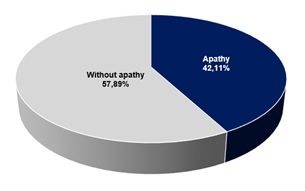 Graph 1: Prevalence of PLVIH with depressive disorder and apathy.
Graph 1: Prevalence of PLVIH with depressive disorder and apathy.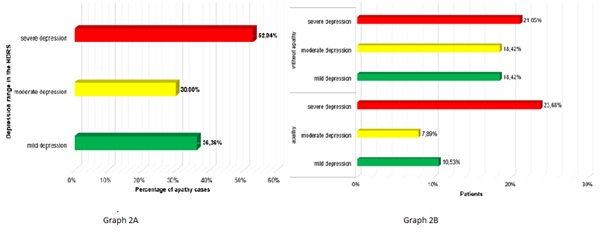 Graph 2A and 2B: Prevalence of cases of apathy according to depression rango in the HDRS.
Graph 2A and 2B: Prevalence of cases of apathy according to depression rango in the HDRS.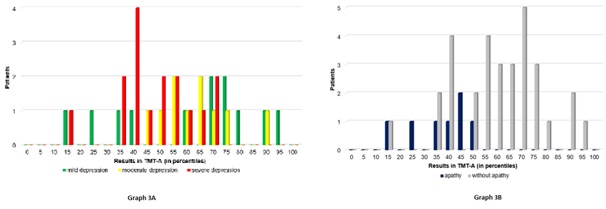
Graph 3: Results obtained in the TMT-A.
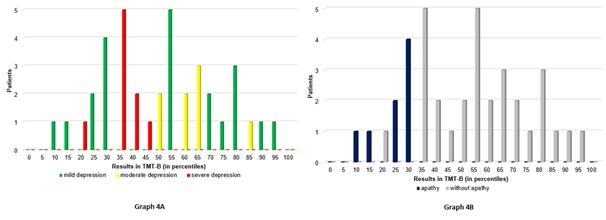 Graph 4: Results obtained in the TMT-B
Graph 4: Results obtained in the TMT-B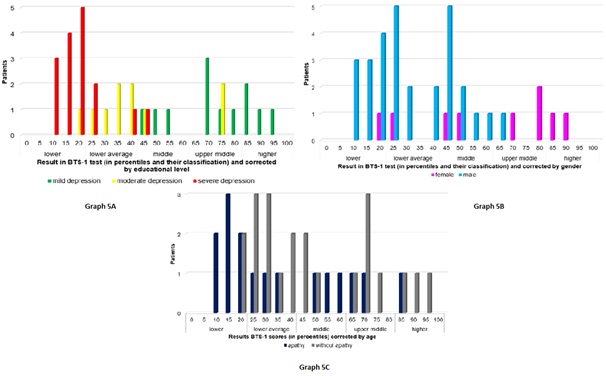 Graph 5: Results obtained in the BTS-1
Graph 5: Results obtained in the BTS-1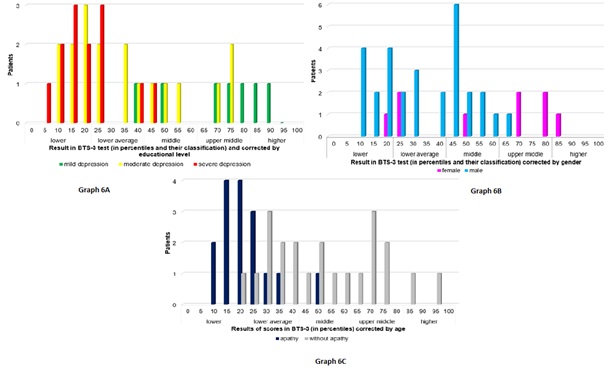 Graph 6: Results obtained in the BTS-3
Graph 6: Results obtained in the BTS-3|
Variables |
Apathy |
Variables |
Apathy |
||||
|
plus |
minus |
plus |
minus |
||||
|
Depressive Disorder |
plus |
16 |
22 |
Depressive Disorder |
plus |
42.11% |
57.89% |
|
|
minus |
0 |
30 |
|
minus |
0% |
100% |
Table 1: Distribution of patients in groups.
DISCUSSION
HIV is a neurotrope virus wich enters the nervous system through the mononuclear phagocytic system in primoinfection. HIV per se produced Dementia as complication in 12% cases despite Highly Active Antiretroviral Therapy (HAART), characterized by cognitive and motor dysfunction. Nevertheless in immunocompromised individuals with low or undetectable viral loads, cognitive deterioration also occurs and mainly affects declarative memory, motivation and attention. Neurocognitive alterations have a subcortical pattern, alterations in different cognitive domains can be observed in negativized persons that affect daily life activities in different proportions since primary infection.
Depressive symptoms has multifactorial etiology. The early detection of psychopathological disorders improves the effectiveness in the interdisciplinary approach and treatment. The prevalence of depressive disorder in People Living With HIV (PLHIV) is greater than the non-infected population and presents clinical-semiological specificities that generate cases of underdiagnosis (for which it does not receive treatment), incorrect or inconclusive treatments (due to erroneous medication due to the mechanism of action or interactions with HAART), increased suicidality, mortality due to AIDS-related complications, and may be associated or be a trigger for neurocognitive disorders. The diagnosis of depressive disorder and its therapeutic approach is hampered by the high prevalence of apathy that leads to a torpid evolution with partial response to SSRI antidepressants given the neurobiological substrate is not mainly serotonergic; difficulties for the psychopharmacological approach with the current treatment guidelines.
Apathy represents the loss of motivation, with behavioral, emotional, and cognitive implications. From the work of Marin et al., it acquired its entity, and it was the work of Stuss & van Reekum, modified by Levy et al., who described its three underlying neuroanatomical pathways[19-22]. The commitment of the dorsomedial-subcortical prefrontal circuit was associated with executive disorders (planning and organization of new objectives); as well as the cingular-subcortical loop linked to the motivation and its disturbance.
The presence of apathy in people living with HIV is a prevalent cause that makes diagnosis difficult and generates a torpid evolution of the depressive disorder, lack of response to psychopharmacological treatment (antidepressants inhibitors of serotonin reuptake) and can associate or trigger neurocognitive alterations [23,24]. The neurocognitive disorder of people living with HIV has subcortical characteristics, and at the beginning of the picture, attention alterations were observed with pathway dysfunction related to the anterior cingulate cortex and the dopaminergic pathways [25-28].
The early detection of apathy and its correct clinical discrimination improves the effectiveness in the care approach of these patients and their evolution, as well as could detect the onset of cognitive alterations in other cognitive domains [2,3,5,7,29].
As described, depressive symptoms and their comorbidity with apathy generate neurocognitive alterations that affect the activities in PLHIV. The attentional domain is one of the first to be affected, especially prior to hippocampal-dependent mnemonic circuits. In this research attention and its subtypes were studied, understood as a fundamental and determining mental activity for the development of other cognitive functions. Although there are many models that describe attention, the Posner and Petersen model that conceptualizes it in networks and systems was used. This model describes 3 main functions: orientation network, surveillance network and the executive network [30]; at the same time that it specifies 2 attentional systems: posterior and anterior. The posterior attention system is responsible for the selective attention and orientation, while the previous focal or executive attention.
This model describes the anatomical networks related to each attentional network and relates the neurotransmitters involved to each attentional network. Likewise, there is ample evidence in reports and experiences carried out with this model; for this reason it is useful for the clinical context.
On the basis of these descriptions in apathy, and the main alteration of the dopaminergic pathways (not unique), their comorbidity with depressive symptoms worsens synergistically the clinical manifestations since not only different structures but different biochemical pathways are compromised. It should be noted that in primoinfection, and later in the evolution of the infection, immunohistopathological investigations found proteins and particles of the HIV capsid in the basal nuclei, for this reason it has been postulated that it has an important role in the neurocognitive disruption that it occurs slowly in PLHIV.
Depressive disorder and apathy present different neurocognitive alterations within which the attention domain is highly important because of the commitment and repercussion on the other cognitive functions, as well as the involvement in daily life activities and autonomy that have an impact on the clinical evolution of people. In our work we have identified that there are specific alterations in attentional subtypes such as sustained, selective and divided attention. In PLHIV, its detailed study and approach are more relevant since this specific alteration can constitute a differential diagnostic parameter and of evolutionary follow-up, in order to focus on the early detection of the picture to establish an effective interdisciplinary treatment and reduce complications in the evolution.
Depressive symptoms has multifactorial etiology. The early detection of psychopathological disorders improves the effectiveness in the interdisciplinary approach and treatment. The prevalence of depressive disorder in People Living With HIV (PLHIV) is greater than the non-infected population and presents clinical-semiological specificities that generate cases of underdiagnosis (for which it does not receive treatment), incorrect or inconclusive treatments (due to erroneous medication due to the mechanism of action or interactions with HAART), increased suicidality, mortality due to AIDS-related complications, and may be associated or be a trigger for neurocognitive disorders. The diagnosis of depressive disorder and its therapeutic approach is hampered by the high prevalence of apathy that leads to a torpid evolution with partial response to SSRI antidepressants given the neurobiological substrate is not mainly serotonergic; difficulties for the psychopharmacological approach with the current treatment guidelines.
Apathy represents the loss of motivation, with behavioral, emotional, and cognitive implications. From the work of Marin et al., it acquired its entity, and it was the work of Stuss & van Reekum, modified by Levy et al., who described its three underlying neuroanatomical pathways[19-22]. The commitment of the dorsomedial-subcortical prefrontal circuit was associated with executive disorders (planning and organization of new objectives); as well as the cingular-subcortical loop linked to the motivation and its disturbance.
The presence of apathy in people living with HIV is a prevalent cause that makes diagnosis difficult and generates a torpid evolution of the depressive disorder, lack of response to psychopharmacological treatment (antidepressants inhibitors of serotonin reuptake) and can associate or trigger neurocognitive alterations [23,24]. The neurocognitive disorder of people living with HIV has subcortical characteristics, and at the beginning of the picture, attention alterations were observed with pathway dysfunction related to the anterior cingulate cortex and the dopaminergic pathways [25-28].
The early detection of apathy and its correct clinical discrimination improves the effectiveness in the care approach of these patients and their evolution, as well as could detect the onset of cognitive alterations in other cognitive domains [2,3,5,7,29].
As described, depressive symptoms and their comorbidity with apathy generate neurocognitive alterations that affect the activities in PLHIV. The attentional domain is one of the first to be affected, especially prior to hippocampal-dependent mnemonic circuits. In this research attention and its subtypes were studied, understood as a fundamental and determining mental activity for the development of other cognitive functions. Although there are many models that describe attention, the Posner and Petersen model that conceptualizes it in networks and systems was used. This model describes 3 main functions: orientation network, surveillance network and the executive network [30]; at the same time that it specifies 2 attentional systems: posterior and anterior. The posterior attention system is responsible for the selective attention and orientation, while the previous focal or executive attention.
This model describes the anatomical networks related to each attentional network and relates the neurotransmitters involved to each attentional network. Likewise, there is ample evidence in reports and experiences carried out with this model; for this reason it is useful for the clinical context.
On the basis of these descriptions in apathy, and the main alteration of the dopaminergic pathways (not unique), their comorbidity with depressive symptoms worsens synergistically the clinical manifestations since not only different structures but different biochemical pathways are compromised. It should be noted that in primoinfection, and later in the evolution of the infection, immunohistopathological investigations found proteins and particles of the HIV capsid in the basal nuclei, for this reason it has been postulated that it has an important role in the neurocognitive disruption that it occurs slowly in PLHIV.
Depressive disorder and apathy present different neurocognitive alterations within which the attention domain is highly important because of the commitment and repercussion on the other cognitive functions, as well as the involvement in daily life activities and autonomy that have an impact on the clinical evolution of people. In our work we have identified that there are specific alterations in attentional subtypes such as sustained, selective and divided attention. In PLHIV, its detailed study and approach are more relevant since this specific alteration can constitute a differential diagnostic parameter and of evolutionary follow-up, in order to focus on the early detection of the picture to establish an effective interdisciplinary treatment and reduce complications in the evolution.
CONCLUSION
Apathy in people living with HIV and depression disorders present specific and differential alterations in the attentional domain. Attentional dysfunction of sustained, divided and selective attention were specific in people with depressive disorder and apathy, with higher affectation in the anterior attentional circuit and would be related with the latter cognitive disruption as a prodrome. We believe it is necessary to increase the sample to obtain substancial significance among the results.
REFERENCES
- Castellon SA, Hinkin CH, Myers HF (2000). Neuropsychiatric disturbance is associated with executive dysfunction in HIV-1 infection. J Clin Exp Neuropsychol 6:336–347.
- Castellon SA, Hinkin CH, Wood S (1998). Apathy, depression, and cognitive performance in HIV-1 infection. J Neuropsychiatry Clin Neurosci 10:320–329.
- Gorman AA, Foley JM, Ettenhofer ML (2009). Functional Consequences of HIV-Associated Neuropsychological Impairment. Neuropsychol Rev 19: 186–203.
- Kamat R, Cattie JE, Marcotte TD (2015). Incident Major Depressive Episodes increase the severity and risk of apathy in HIV infection. J Affect Disord 175: 475–480.
- Marin RS, Butters MA, Mulsant BH (2003). Apathy and Executive Function in Depressed Elderly. J Geriatr Psychiatry Neurol 16: 112.
- Paul R, Flanigan TP, Tashima K (2005). Apathy correlates with cognitive function but not CD4 status in patients with human immunodeficiency virus. J Neuropsychiatry Clin Neurosci 17: 114-118.
- Tate D (2003). Impact of apathy on quality of life in HIV. AIDS Patient Care STDS 17: 115-120.
- Sheehan DV, Lecrubier Y, Sheehan KH (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59: 22-33.
- Hamilton M (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry23:56-62.
- Ramos-Brieva JA, Cordero A (1998). A new validation of the Hamilton Rating Scale for Depression. J Psychiatr Res 22:21-8.
- Marin RS (1996). Apathy: Concept, Syndrome, Neural Mechanisms, and Treatment. Seminars in Clinical Neuropsychiatry 1:304-314.
- Cummings J, Mega M, Gray K, Rosenberg-Thompson S, Carusi D A, et al.(1994) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44: 2308-2314.
- Golden CJ (1994) Stroop: Test de colores y de palabras. Madrid, TEA Ediciones.
- Fernández A, Marino J, Alderete A (2002). Estandarización y validez conceptual del test del trazo en una muestra de adultos Revista de neurología 25: 83-88.
- Wechsler D (2002). Wais III Test de Inteligencia para Adultos. Buenos Aires, Paidós.
- Wechsler Memory Scale (3rd).Psychological Corporation, San Antonio.
- RM Paterno, RM Brenlla ME, Eusebio CA, Destefano GB, Montagut IA, et al. (2015) Tonglet EC. Batería de Tests con Símbolos-1. Ed Paidos, Buenos Aires, Argentina.
- RM Paterno, RM Brenlla ME, Eusebio CA, Destefano GB, Montagut IA, et al. (2015) Tonglet EC. Batería de Tests con Símbolos-1. Ed Paidos, Buenos Aires, Argentina.
- Marin RS (1996). Apathy: Concept, Syndrome, Neural Mechanisms, and Treatment. Semin Clin Neuropsychiatry 1: 304-314.
- Stuss D, Van Reekum R (2000). Differentiation of states and causes of apathy. Neuropsychol Emotion. 340-363
- Levy R, Czernecki V (2006). Apathy and the basal ganglia. J Neurol 253: 54-61.
- Levy R, Dubois B (2006). Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 16: 916-928.
- Kroenke K, Spitzer RL (2002). The PHQ-9: A New Depression Diagnostic and Severity Measure. Psych Ann 32: 509-515.
- Shapiro ME, Mahonney JR, Zingman BS(2013). Apathy correlates with cognitive performance, functional disability, and HIV RNA plasma levels in HIV-positive individuals. J Clin Exp Neuropsychol 35: 934-945.
- Chang L, Ernst T (2000). Relationship among brain metabolites, cognitive function and viral loads in antiretroviral-naive HIV patients. Neuro Image 17: 1638-1648.
- Ances BM, Roc MS, Wang J (2006). Caudate blood flow and volume are reduced in HIV + neurocognitively impaired patients. Neurol 66: 862-866.
- Paul RH (2005). Apathy is associated with volume of the nucleus accumbens in patients infected with HIV. J Neuropsychiatry Clin Neurosci 17: 167-171.
- Kamat R, Brown GG, Bolden K (2014). Apathy is associated with white matter abnormalities in anterior, medial brain regions in persons with HIV infection. J Clin Exp Neuropsychol 36: 854-866.
- Barclay TR, Hinkin CH, Castellon SA (2007). Age-Associated Predictors of Medication Adherence in HIV Positive Adults: Health Beliefs, Self-Efficacy, and Neurocognitive Status. Health Psychol. 26: 40-49.
- Fernández-Duque D, Posner MI (2001). Brain imaging of attentional networks in normal and pathological states. J Clin Exp Neuropsychol 23:74-93.
Citation: Mazzoglio Y Nabar MJ, Tornese EB, Terren EEL and Iturry M(2019) Attentional Circuits in People Living with HIV and Apathy: Differential Alterations. J AIDS Clin Res Sex Transm Dis 6: 025.
Copyright: © 2019 Martin J Mazzoglio Y Nabar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
