
Autologous Blood Products: When, Where, and How?
*Corresponding Author(s):
Alfonso L SabaterDepartment Of Ophthalmology, Bascom Palmer Eye Institute, University Of Miami Miller School Of Medicine, Miami, FL 33136, United States
Email:asabater@med.miami.edu
Abstract
Autologous blood eye drops have become an interesting, well-known, and widely used therapeutic option for many ocular surface diseases since their introduction into the ophthalmologic field forty-six years ago. The foundation for their use in the treatment of pathologies such as severe keratoconjunctivitis sicca, persistent epithelial defects, ocular chemical burns, recurrent epithelial erosions, Stevens-Johnson syndrome, ocular graft versus host disease, and, lately, in many other ocular surface conditions, relies on the fact that they contain proteins, vitamins, cytokines, and growth factors that participate in the signaling pathways of corneal epithelial healing in similar amounts to those found in normal, healthy tear films. These molecules confer on them a huge advantage over conventional artificial tears that can only lubricate the eyes but lack epitheliotropic properties. Despite some reported controversy regarding their composition and safety, the versatility of autologous blood eye drops in terms of source and final products, as well as their proven efficacy in the treatment of ocular surface diseases resistant to conventional therapy, have slowly but steadily granted them recognition and acceptance in the modern clinical ophthalmologic practice.
Keywords
Autologous serum; Blood-derived products; Ocular surface diseases; Ophthalmology
Background and Rationale
Autologous, meaning self, blood derivate products have been used to treat several ocular surface pathologic conditions for more than four decades now. They were first mentioned in ophthalmologic-related literature in 1975, when a mobile ocular perfusion pump containing a patient’s autologous serum was used to treat conditions such as Stevens-Johnson Syndrome (SJS), ocular pemphigoid, Keratoconjunctivitis Sicca (KCS), chemical burns, and Persistent Epithelial Defects (PED), as well as following a Penetrating Keratoplasty (PK), demonstrating the pump’s efficacy especially in treating patients with severe KCS and in promoting the healing of PEDs [1]. Nine years later, in 1984, a group of investigators used Autologous Serum Tears (ASTs) to treat patients with idiopathic or secondary to Sjögren Syndrome (SS) KCS, for whom other conventional therapies (i.e., artificial tears, ATs) had previously failed. They found statistically significant symptomatic and objective improvement in their entire study population, with no adverse events being reported [2]. Further studies have also shown improvement in patient symptoms and/or at least one clinical measure of ocular surface disease (i.e., corneal or conjunctival staining, tear film breakup time, TBUT, and Schirmer test) when using ASTs to treat severe KCS [3-9] or PED [5,10,11], making them a more widely accepted treatment option for managing these conditions.
The rationale for using blood-derived eye drops to treat ocular surface conditions arises from the fact that the normal tear film contains a wide variety of proteins, vitamins, immunoglobulins, cytokines, and Growth Factors (GFs) that are believed to play an important role in the signaling pathways for corneal healing and the maintenance of its transparency after injury. These molecules are also present in similar, although not the same, concentrations in the blood’s serum, conveying on ASTs a physiological profile that more precisely mimics healthy tears when compared to conventional ATs [11-14]. Of all the molecules found in the human serum, Epithelial Growth Factor (EGF), transformer growth fibroblast β-factor (TGF-β), albumin, Vitamin A, fibronectin, α-2 macroglobulin, platelet-derived growth factor (PDGF-AB), hepatocyte growth factor, substance P, and the insulin-like growth factor (IGF-1) are believed to have the strongest trophic effects during the ocular surface repair mechanisms [13,15,16]. Furthermore, tears and serum have the same pH (7.4) and similar osmolarities (298 and 296, respectively) [17], and they are free of potentially toxic preservatives usually present in conventional ATs that are used to alleviate the dryness caused by KCS [18,19] .
Table 1 shows Newest AST published data.
|
Study |
Year of publication |
Type of study |
Condition |
Product |
Dilution (%) |
Results |
|
Wang et al. [20] |
2019 |
Systematic review and meta-analysis of 7 RCTs and cross-over studies |
KCS |
AST |
20 and 40 |
Improvement in OSDI scores, TBUT, and rose Bengal staining scores. |
|
Shtein et al. [21] |
2020 |
Systematic review of 10 studies |
KCS |
AST |
20, 50, and 100 |
8/10 reported symptomatic improvement. 10/10 showed objective clinical improvement. |
|
Shtein et al. [21] |
2020 |
Systematic review of 4 studies |
PED |
AST |
20, 50, and 100 |
4/4 reported symptomatic improvement. 3/4 showed >90% reduction in size of defects. |
|
Valencia et al. [22] |
2020 |
Clinical Trial |
KCS |
AST |
20 |
Improvement in Schirmer test, TBUT, and increase in density of goblet cells in impression citology. |
|
Ripa et al. [23] |
2020 |
Prospective, observational cohort study |
Localized KCS and KCS secondary to systemic diseases |
AST |
20 |
Despite finding differences in the concentration levels of GF, OSDI scores were reduced in both groups. |
|
Jirsova et al. [24] |
2020 |
Prospective, observational study |
KCS secondary to GVHD and SS |
AST |
20 |
Although a significant increase in aberrant HLA-DR positive conjunctival cells was observed in the GVHD group, improvement in OSDI scores was reported in both populations. |
|
Kirgiz et al. [25] |
2020 |
Prospective, randomized study |
Post cross-linking surgery in patients with keratoconus |
AST |
20 |
Lower mean epithelial closure time and decreased pain scores. |
|
So et al. [26] |
2020 |
Retrospective observational study |
Ocular surface disease in patients using glaucoma eyedrops |
AST |
20 |
Improvement of symptoms even without discontinuing glaucoma eyedrops. |
Table 1: Newest AST published.
RCTs: Randomized controlled trials; KCS: Keratoconjunctivitis sicca; PED: Persistent epithelial defects; SS: Sjögren syndrome; GVHD: Graft versus host disease; AST: Autologous serum tears; OSDI: Ocular surface disease index; TBUT: Tear breakup time; GF: Growth factors. Within the last years, multiple studies have addressed and reaffirmed the clinical efficacy of blood-derived eye drops in the treatment of several complicated ocular surface conditions that are resistant to other treatments, consistently demonstrating positive outcomes.
In 2017, the consensus report of the Asia Dry Eye Society (ADES) defined KCS thus: “Dry eye is a multifactorial disease characterized by unstable tear film causing a variety of symptoms and/or visual impairment, potentially accompanied by ocular surface damage” [27]. A quantitative or qualitative deficiency of tears causes lack of lubrication in the eyes, exposing the ocular surface to damage by environmental factors and compromising the wound healing process [28,29]. Historically, dry eye disease has been classified as aqueous-deficient (due to a failure of lacrimal tear secretion) or evaporative (lipid deficient) [30,31]. In 2020, the ADES proposed a new classification system: “Tear Film-Oriented Diagnosis” (TFOD), which is based on TBUT patterns when using fluorescein. They added an extra category: “Decreased wettability dry eye,” which is thought to be caused by a localized membrane-type mucin deficiency at the cornea. This TFOD classification system involves the three layers of the tear film and surface epithelium, allowing for an easier recognition of the root cause and thus a better understanding and “Tear Film-Oriented Treatment” (TFOT) [32].
In this 2020 ADES consensus, they mention ASTs as a possible effective treatment option forpatients with the severe aqueous-deficient type of dry eye on the basis that ASTs would provide the essential tear components to the ocular surface epithelium. This population includes patients with SS, ocular cicatricial pemphigoid, SJS, chronic Graft Versus Host Disease (cGVHD), and non-Sjögren type dry eye, such as that seen in long-term visual display terminal users [32]. Also in 2020, the American Academy of Ophthalmology (AAO) published a report focused on ASTs and their use in severe KCS and PED. They reviewed ten studies (levels II or III evidence) in which ASTs were used to treat severe KCS that is resistant to conventional treatment. Eight of the studies reported improvement of symptoms, and all studies noted improvement in at least one clinical sign. For PED, the AAO reviewed four studies (levels II or III evidence). All of the studies demonstrated improvement, and three reported an improvement rate of >90% in the size of the defects. Nevertheless, no randomized controlled studies were reviewed, so no level I evidence data was provided [21].
In December 2019, Wang et al., [20] published a systematic review and meta-analysis of seven randomized controlled trials that compared ASTs to ATs in the treatment of dry eye. They concluded that ASTs significantly improved dry eye symptoms (lower Ocular Surface Disease Index OSDI scores), TBUT, and rose Bengal staining score when compared to ATs. However, Schirmer tests and fluorescein staining scores showed no significant difference between the two treatment arms [20]. Another study evaluated the efficacy of ASTs in patients with moderate to severe KCS, finding significant improvement in Schirmer tests and TBUT and an increase in the density of goblet cells in conjunctival impression cytology after one month of therapy [22].
The fact that patients with systemic inflammatory or autoimmune diseases may contain high levels of pro-inflammatory cytokines in their serum that could potentially irritate or damage the ocular surface has always been a subject of concern [33]. Very recently, Ripa et al., [23] conducted a study to compare the levels of inflammatory molecules and the difference in treatment efficacy of ASTs between patients with localized ocular surface disease and patients with systemic diseases. Interestingly, they found that EGF concentration was significantly lower in the group of patients with systemic diseases [23]. EGF is one of the most important GFs that promotes corneal epithelial migration and proliferation to improve the wound healing process [34]. Conversely, the levels of fibronectin, IL-8, IL-17, Vitamin A, and TNF-a (when detectable) were found to be similar in both groups. Despite these differences, the OSDI scores were significantly reduced in both groups, although the reduction was even greater in the group with localized ocular disease versus the group with systemic diseases (36% and 24%, respectively). Researchers concluded that the differences between the levels of EGF may account for the differences in therapeutic and symptomatic outcomes in each group, but that ASTs can be safely and effectively used not only in patients with localized diseases but also in patients with systemic diseases [23].
Similarly in 2011, a study that compared the levels of GF in the serum of patients with SJS and in patients without systemic disease demonstrated that there were no statistically significant differences in the levels of any of the epitheliotropic factors among the groups. Although they did not report the therapeutic outcomes, the results suggested that the epitheliotropic capacity of ASTs from SJS patients could be comparable to those from patients without autoimmune or inflammatory diseases [35]. In the case of cGVHD-related KCS, several studies have also corroborated the safety and effectiveness of ASTs [36-38]. Moreover, an article published in 2020 described symptomatic improvement (measured through OSDI questionnaire) in these patients, even though they also found a significant increase in aberrant HLA-DR positive conjunctival epithelial cells after the use of ASTs for three months [24]. HLA-DR, which is considered to be a marker of inflammation, is a membrane glycoprotein that should only present in the surface of cells of the immune system, such as B lymphocytes, activated T cells, and macrophages. It should not be found in the surface of healthy conjunctival epithelial cells [39-41]. Despite this finding, these investigators concluded that the presence of subclinical inflammation (marked by the expression of aberrant HLA-DR in conjunctival cells) did not outweigh the positive effects of ASTs in GVHD patients [24].
However, in 2014 Hwang et al., [6] studied a population of patients with SS and subdivided it into two groups: primary (idiopathic) SS and secondary SS (i.e., secondary to Systemic Lupus Erythematous, SLE, rheumatoid arthritis, RA, or scleroderma). They measured some proinflammatory cytokine levels, such as TNF-a, IL-1B, IL-6, and IL-18 in the serum of each subpopulation and found significantly higher concentrations of these in the secondary SS group. They also studied the therapeutic outcomes after 4 weeks of treatment with ASTs: in the primary SS group, they found statistically significant improvement in ocular symptoms, ocular surface staining grades, and TBUT; in the secondary SS group, although they also found improvement in these parameters, it was not statistically significant, so they concluded that their results suggest that ASTs might not be effective for the treatment of secondary SS [6]. Due to these discrepancies in the literature, more and larger studies should be conducted before coming to a conclusion regarding the use of ASTs for the treatment of dry eye in patients with systemic inflammatory and/or autoimmune diseases.
Since their introduction, besides their indication for the treatment of severe KCS and for the healing of PED, ASTs have also been used and found to be successful for the treatment of Neurotrophic Keratopathy (NK) [42-44], Recurrent Erosion Syndrome (RES) [45,46], chemical burns [11,47], Superior Limbic Keratoconjunctivitis (SLK) [48], and for ocular surface syndrome after Laser-Assisted in Situ Keratomileusis (LASIK) [49]. More recently, AST use after cross-linking surgery in patients with keratoconus has been reported, demonstrating a significantly lower mean epithelial closure time and decreased pain scores when compared to conventional post-surgical treatment with ATs [25]. Another study suggested that the use of ASTs is effective in improving symptoms of ocular surface damage caused by long-term use of glaucoma eyedrops containing preservatives, even without the interruption of glaucoma treatment [26]. This is a very encouraging finding, as it suggests that ASTs can be used to manage ocular discomfort, which is a very frequent finding (49-59%) and has a great impact on the quality of life in patients with advanced glaucoma who cannot discontinue the use of their medications [50].
Preparation And Other Blood-Derived Products
ASTs are not pharmaceutical but instead biological products. They have not been approved by the United States Food and Drug Administration (FDA). To date, no federal nor international protocol exists, and there are no requirements for the preparation and/or use of ASTs, although some U.S. states do have certain regulations [14,21]. Preparation of this product has varied from study to study [2,3,17]. However, after trying and comparing different ways of preparing ASTs, and posteriorly assessing for the characteristics in each preparation, in 2005 Lie et al. came out with a standardized, optimized protocol to produce them: 50-100ml of whole blood should be obtained from a peripheral vein of the patient and left at room temperature for two hours (without anticoagulants) to achieve complete clotting. Subsequently, the blood is centrifuged at 3000 g for 15 minutes in order to separate the serum from the solid components of the blood. The overlying supernatant is then collected and diluted with Balanced Salt Solution (BSS) to a desired concentration (Figure 1). Investigators determined that following this protocol best supported corneal cell proliferation, migration, and differentiation rates during the healing process [14,51].
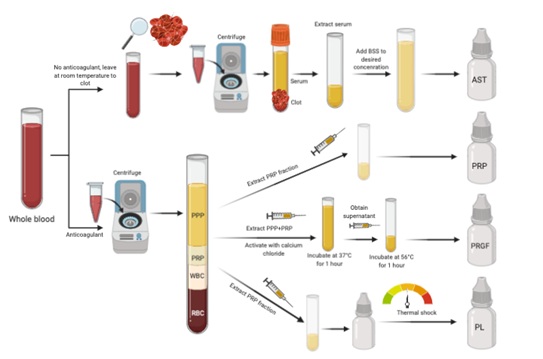 Figure 1: Preparation of autologous blood products.
Figure 1: Preparation of autologous blood products.
PPP: Platelet-poor plasma; PRP: Platelet-rich plasma: WBC: White blood cells; RBC: Red blood cells; BSS: Balanced salt solution; AST: Autologous serum tears; PRGF: Plasma rich in growth factors; PL: Platelet lysate. Steps for the preparation of the different autologous blood eyedrops.
The most frequently used dilution is 20% [52], but higher concentrations (50% to 100%) have also been used to treat different ocular surface conditions, showing positive results and without exhibiting detrimental side effects [10,11,52,53]. ASTs can be stored at -20°C for several months, with the maximum storage interval to be regulated by the local legislation of each country [14,51]. A study in 2016 reported how epitheliotropic factors of ASTs remained stable even after 9 months of storage at -20°C [54].
Although ASTs were the first and to date probably the most used, other autologous blood-derived eye drops have more recently been introduced into ophthalmologic practice: Platelet-Rich Plasma (PRP), Plasma Rich in Growth Factors (PRGF), and Platelet Lysate (PL). Likewise, homologous or allogeneic (meaning non self-derived) blood sources such as healthy donor’s blood and Umbilical Cord Blood Serum (UCBS) have also been proposed as alternatives to obtain these different blood-derived products. This is especially useful in patients with severe, debilitating systemic diseases in which autologous products are not feasible, and may represent an option for patients with elevated amounts of proinflammatory cytokines in the blood [14,15]. The process of preparation of allogeneic serum tears (either from healthy donor’s blood or from UCBS) basically follows the same protocol as that for ASTs, although it first requires obtaining informed consent from the donor or pregnant woman, plus serologic screening for infectious diseases like HIV, Hepatitis B, Hepatitis C, Cytomegalovirus, Toxoplasmosis, Syphilis, and Human T-lymphocyte virus (HTLV) in order to prevent the transmission of these blood-borne diseases [14,55-59].
A concern that could surge when using allogeneic serums is the possibility of an inflammatory reaction due to the presence of anti-A and/or anti-B antibodies. However, even though ABO antigens are known to exist on corneal and conjunctival cells, and a study performed in 2014 used ABO blood type matching in their methods [60], in several older studies [59,61,62] blood type matching was not a consideration and no adverse reactions were noted [55,57,59,61-63]. An advantage of allogeneic over autologous products, especially in the case of UCBS, is the possibility of collecting a large quantity of serum at the same time so that several patients can be supplied with a single sampling [14].
PRP, PRGF, and PL are all considered platelet-derived preparations. They have been widely used in regenerative medicine, orthopedics, and maxillo-facial surgery to stimulate tissue healing [64]. PRP tears, as opposed to ASTs, contain platelets as a part of their composition. To obtain them, whole blood is extracted and mixed with an anticoagulant (3.2% sodium citrate). After its centrifugation for 10 minutes at 1600 rpm, three distinct layers are seen: 1) Platelet Poor Plasma (PPP) on top, 2) PRP in the middle, and 3) red and white blood cells at the bottom. PRP fraction is aspired with a syringe and 3-4mL aliquots are transferred into sterilized bottles with eye drop applicators (Figure 1). Their major effects are mediated by platelet-derived growth factor (PDGF), which is implicated in wound healing and angiogenesis [65,66]. This product has been used to treat severe KCS [67], PED [68], and post-LASIK ocular surface syndrome [69,70], showing beneficial outcomes in all cases. Moreover, solid PRP clots can also be prepared and used in combination with amniotic membranes and autologous fibrin membranes. Among others, as surgical tools in corneal perforations [66].
Besides trisodium citrate as anticoagulant, PRGF tear preparation requires calcium chloride, which activates the coagulation cascade in a controlled way and significantly increases GF concentration. This product is obtained by centrifuging whole blood at 580 g for 8 minutes at room temperature. The whole column of plasma (PPP and PRP) is collected and incubated at 37°C for one hour. Then, the obtained supernatant is heat-treated at 56°C for another hour in order to generate “immunosafe PRGF” by reducing the immunologic components. Finally, the plasma supernatants are filtered, aliquoted in bottles and stored at -20°C until use [71-73] (Figure 1). Currently, companies such as BTI Human Technology offer kits that facilitate the blood processing and the obtaining of PRGF tears (Endoret ophthalmology kit). PRGF’s use has been reported to be successful in the treatment of KCS in several studies [71,74-76], as well as in the treatment of PED [77]. In 2020, Anitua et al., [78] conducted a study with the purpose of analyzing and comparing the biological content and activity of lyophilized (freeze-dried) PRGF tears after their storage at room temperature or at 4°C for 3 months with that of fresh samples. Furthermore, they evaluated the proliferative and migratory inductive potential of these products over primary human keratocytes. Interestingly, they demonstrated that lyophilized PRGF eye drops preserve the main GF levels and their biological activity on keratocytes when either stored at room temperature or at 4°C, and even without lyoprotectant substances for at least 3 months. Another surprising finding was that the number of migratory human keratocytes significantly increased after treatment with lyophilized PRGF tears kept for 3 months when compared to those freshly obtained [78].
PL tears are obtained from PRP, which is diluted to a final concentration of 30% (v/v), and aliquoted into 1.5mL sterile vials. It should then undergo thermal shock by being frozen at -80°C for at least one hour and then thawed at 4°C with the goal of causing platelet lysis and PDGF release. The final product is then stored again at -20°C for a maximum of 45 days [79,80] (Figure 1). PL tears have shown to be effective in promoting rabbit corneal healing in-vitro [79], as well as in in-vivo treatment of KCS secondary to SS [81] and in refractory-to-treatment GVHD-associated KCS [80].
Conclusion and future Perspectives
Despite all the published evidence regarding the role and potential benefit that blood-derived products represent for the treatment of many severe ocular surface diseases, there are still some barriers that must be overcome before seeing more widespread use of these products. First, they are not currently accepted as standard of care for any ocular condition in the USA nor in most European countries [56]. Current treatment options in the USA are determined by disease severity level instead of by the root cause of the ocular surface problem [82]. The use of blood derivate products is recommended only at the worst severity levels [83]. This is further aggravated by the limited accessibility and high costs (not covered by most insurance plans) of these eye drops, which makes their implementation even more of a challenge, and results in ophthalmologists reserving them for either the most severe cases or for those that failed to improve after using conventional, less costly, and more readily available therapies [21]. This in turn limits the possibility of assessing their efficacy in less severe cases.
Likewise, the protocol of preparation, concentration, storage, quality control, and frequency of use of these eye drops varies from study to study. The absence of standardization makes it difficult to compare and determine their real effectiveness. Performing randomized controlled trials to evaluate their use in each different ocular surface pathology would allow for the determination of their optimal concentration and dosage intervals and, thus, conclusive level 1 evidence on their safety and efficacy [13,21]. Another point that must be addressed is what is the ideal concentration range of the different GFs that should, or conversely that should not (in the case of cytokines), be present in these eyedrops to best stimulate cellular proliferation and migration during the healing process. Corneal healing is complex and mediated by GFs and ILs, but this only occurs when their concentration is in the proper range, as scarce amounts may be insufficient but excessive amounts may lead to scarring or stromal haze [13,84].
Various studies have evaluated the concentration ranges of GFs and other molecules in normal, healthy tears. They’ve also suggested that each GF and IL may selectively participate and regulate different cellular mechanisms involved in the healing process [85,86]. Taking these considerations into account, Versura et al., [83] standardized EGF concentration in UCBS eyedrops to 0.15ng/mL daily, as normal tear concentration of this GF ranges between 0.7-9.7ng/mL. They demonstrated the positive effect that tailoring the EGF to this specific concentration has over the corneal healing process in patients with severe KCS [83]. Besides, as each type of blood-derived product differs in its amount of GFs, cytokines, vitamins, and ILs, the selection of the type of product to be used (i.e., autologous vs. homologous or platelet-derivates) should be decided based on the cellular mechanism that is implicated in each ocular surface pathology. Nevertheless, the fact that routine screening and tailoring of the GF can be expensive and very time consuming cannot be ignored [14].
Last but not least, the refrigeration requirements for these biological products to remain stable, preserve their GFs, and prevent bacterial of fungal growth are mostly perceived as tedious and a matter of concern to patients. They are usually indicated 4-6 times per day, and as most people work or spend an important part of the day out of their homes, it can be difficult to carry around these products compared to conventional ATs that do not require refrigeration. For this reason, a more practical version in terms of storage and use is urgently needed, more so for patients for whom long-term therapy is required [87]. However, in 2020 three studies (one using PRGF [78] and two ASTs[87, 88]) demonstrated that the concentration of GFs remained stable for up to 3 months [78,87,88] after lyophilization. Lyophilization is a method of dehydration that involves freezing a product and removing the frozen water through sublimation. It is a well-known way to preserve heat-sensitive elements such as blood components, tissues, proteins, and enzymes [89-93].
The resultant dry, powdered ASTs must be reconstituted with saline solution for use. No significant differences were found between the concentration of GF, osmolarity, pH, and density in fresh serum versus re-dissolved lyophilized ASTs stored at 4°C for one month. Additionally, no differences were found in the proliferation and differentiation of corneal and conjunctival cells in-vitro between either preparation [88]. Still, despite the possibility of keeping the bottles containing the lyophilized blood product that is not yet being used at room temperature, once reconstituted the bottle in use must be kept at 4°C.
For all these reasons, blood-derived tears, either from autologous or allogeneic sources, represent a very interesting but at the same time challenging option for the treatment of many ocular surface diseases. Further perspectives on these products include the production of eye drops that contain tailored amounts of GF for each particular ocular surface disease, as well as more resistant versions that do not require refrigeration at all, although these could increase their already considerable costs. Also, randomized clinical trials following the same protocol for preparation and the same guidelines for use are needed in order to improve the quality of blood-derived tears and provide stronger evidence of their effectiveness, which would ultimately lead to wider use in ophthalmologic clinical practice.
Acknowledgments
We would like to thank Dr. Valerie Gramling from the English Composition Program of the University of Miami Miller School of Medicine for her proofreading services.
Declarations
Funding: Not applicable
Conflicts of interest: Not applicable
Availability of data and material: Not applicable
Code availability: Not applicable.
References
- Ralph RA, Doane MG, Dohlman CH (1975) Clinical experience with a mobile ocular perfusion pump. Arch Ophthalmol 93: 1039-1043.
- Fox RI, Chen R, Michelson JB, Belmont JB, Michelson PE (1984) Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum 27: 459-461.
- Tsubota K, Goto E, Fujita H, Ono M, Inoue H, et al. (1999) Treatment of dry eye by autologous serum application in sjögren's syndrome. Br J Ophthalmol 83: 390-395.
- Celebi ARC, Ulusoy C, Mirza GE (2014) The efficacy of autologous serum eye drops for severe dry eye syndrome: a randomized double-blind crossover study. Graefes Arch Clin Exp Ophthalmol 252: 619-626.
- Cho YK, Huang W, Kim GY, Lim BS (2013) Comparison of autologous serum eye drops with different diluents. Curr Eye Res 38: 9-17.
- Hwang J, Chung SH, Jeon S, Kwok SK, Park SH, et al. (2014) Comparison of clinical efficacies of autologous serum eye drops in patients with primary and secondary Sjogren syndrome. Cornea 33: 663-667.
- Liu Y, Hirayama M, Cui X, Connell S, Kawakita T, et al. (2015) Effectiveness of Autologous Serum Eye Drops Combined With Punctal Plugs for the Treatment of Sjogren Syndrome-Related Dry Eye. Cornea 34: 1214-1220.
- Lopez-Garcia JS,García-Lozano I, Rivas L, Ramírez N, Raposo R, et al. (2014)Autologous serum eye drops diluted with sodium hyaluronate: clinical and experimental comparative study. Acta Ophthalmol 92: 22-29.
- Mahelkova G, Jirsova K, Stangova PS, Palos M, Vesela V, et al. (2017) Using corneal confocal microscopy to track changes in the corneal layers of dry eye patients after autologous serum treatment. Clin Exp Optom100: 243-249.
- Lekhanont K, Jongkhajornpong P, Choubtum L, Chuckpaiwong V (2013) Topical 100% serum eye drops for treating corneal epithelial defect after ocular surgery. Biomed Res Int 2013: 521315.
- Semeraro F, Forbice E, Braga O, Bova A, Salvatore AD, et al. (2014) Evaluation of the efficacy of 50% autologous serum eye drops in different ocular surface pathologies. Biomed Res Int 2014: 826970.
- Quinto GG, Campos M, Behrens A (2008) Autologous serum for ocular surface diseases. Arq Bras Oftalmol 71: 47-54.
- Buzzi M,Versura P, Grigolo B, Cavallo C, Terzi A, et al. (2018) Comparison of growth factor and interleukin content of adult peripheral blood and cord blood serum eye drops for cornea and ocular surface diseases. Transfus Apher Sci 57: 549-555.
- Giannaccare G, Versura P, Buzzi M, Primavera L, Pellegrini M, et al. (2017) Blood derived eye drops for the treatment of cornea and ocular surface diseases. Transfus Apher Sci 56: 595-604.
- Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, et al. (2020) Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res 1: 113-129.
- Pancholi S, Tullo A, Khaliq A, Foreman D, Boulton M (1998) The effects of growth factors and conditioned media on the proliferation of human corneal epithelial cells and keratocytes. Graefes Arch Clin Exp Ophthalmol 236: 1-8.
- Geerling G, Maclennan S, Hartwig D (2004) Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol 88: 1467-1474.
- Noecker R (2001) Effects of common ophthalmic preservatives on ocular health. Adv Ther 18: 205-215.
- Geerling G, Daniels JT, Dart JK, Cree IA, Khaw PT (2001) Toxicity of natural tear substitutes in a fully defined culture model of human corneal epithelial cells. Invest Ophthalmol Vis Sci 42: 948-956.
- Wang L, Cao K, Wei Z, Baudouin C, Labbé A, et al. (2020) Autologous Serum Eye Drops versus Artificial Tear Drops for Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ophthalmic Res 63: 443-451.
- Shtein RM, Shen JF, Kuo AN, Hammersmith KM, Li JY, et al. (2020) Autologous Serum-Based Eye Drops for Treatment of Ocular Surface Disease: A Report by the American Academy of Ophthalmology. Ophthalmology 127: 128-133.
- Castillo SLV, Martín ES, Frade LJG, García-Miguel FJ (2020) Autologous serum eye drops improve tear production, both lachrymal flow and stability tests and conjunctival impression cytology with transfer in dry eye disease. Blood Transfus 19: 45-53.
- Ripa M, Jabbehdari S, Yazdanpanah G, Lukacs E, Karcher B, et al. (2020) The Role of Multisystem Disease in Composition of Autologous Serum tears and ocular surface symptom improvement. Ocul Surf 18: 499-504.
- Jirsova K, Stangova PS, Palos M, Mahelkova G, Kalasova S, et al. (2020) Aberrant HLA-DR expression in the conjunctival epithelium after autologous serum treatment in patients with graft-versus-host disease or Sjogren's syndrome. PLoS One 15: 0231473.
- Kirgiz A, Akdemir MO, Yilmaz A, Kaldirim H, Atalay K, et al. (2020) The Use of Autologous Serum Eye Drops after Epithelium-off Corneal Collagen Crosslinking. Optom Vis Sci 97: 300-304.
- So HR, Park HYL, Chung SH, Kim HS, Byun YS (2020) Effect of Autologous Serum Eyedrops on Ocular Surface Disease Caused by Preserved Glaucoma Eyedrops. J Clin Med 9: 3904.
- Tsubota K, Yokoi N, Shimazaki J, Watanabe H, Dogru M, et al. (2017) New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul Surf 15: 65-76.
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, et al. (1998) The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea 17: 584-589.
- Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC (2004) The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res 78: 409-416.
- Lemp MA (1995) Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J 2: 221-232.
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5: 75-92.
- Tsubota K, Yokoi N, Watanabe H, Dogru M, Kojima T, et al. (2020) A New Perspective on Dry Eye Classification: Proposal by the Asia Dry Eye Society. Eye Contact Lens 46: 2-13.
- Welder JD, Bakhtiari P, Djalilian AR (2011) Limbitis secondary to autologous serum eye drops in a patient with atopic keratoconjunctivitis. Case Rep Ophthalmol Med 2011: 576521.
- Wang L, Wu X, Shi T, Lu L (2013) Epidermal growth factor (EGF)-induced corneal epithelial wound healing through nuclear factor kappaB subtype-regulated CCCTC binding factor (CTCF) activation. J Biol Chem 288: 24363-24371.
- Phasukkijwatana N, Lertrit P, Liammongkolkul S, Prabhasawat P (2011) Stability of epitheliotrophic factors in autologous serum eye drops from chronic Stevens-Johnson syndrome dry eye compared to non-autoimmune dry eye. Curr Eye Res 36: 775-781.
- Azari AA, Karadag R, Kanavi MR, Nehls S, Barney N, et al. (2017) Safety and efficacy of autologous serum eye drop for treatment of dry eyes in graft-versus-host disease. Cutan Ocul Toxicol 36: 152-156.
- Tahmaz V, Gehlsen U, Sauerbier L, Holtick U, Engel L, et al. (2017) Treatment of severe chronic ocular graft-versus-host disease using 100% autologous serum eye drops from a sealed manufacturing system: a retrospective cohort study. Br J Ophthalmol 101: 322-326.
- Rocha EM, Pelegrino FS, de Paiva CS, Vigorito AC, de Souza CA (2000) GVHD dry eyes treated with autologous serum tears. Bone Marrow Transplant 25: 1101-1103.
- Baudouin C, Haouat N, Brignole F, Bayle J, Gastaud P (1992) Immunopathological findings in conjunctival cells using immunofluorescence staining of impression cytology specimens. Br J Ophthalmol 76: 545-549.
- Versura P, Profazio V, Schiavi C, Campos EC (2011) Hyperosmolar stress upregulates HLA-DR expression in human conjunctival epithelium in dry eye patients and in vitro models. Invest Ophthalmol Vis Sci 52: 5488-5496.
- Rolando M, Barabino S, Mingari C, Moretti S, Giuffrida S, et al. (2005) Distribution of conjunctival HLA-DR expression and the pathogenesis of damage in early dry eyes. Cornea 24: 951-954.
- Matsumoto Y, Dogru M, Goto E, Ohashi Y, Kojima T, et al. (2004) Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology 111: 1115-1120.
- Guadilla AM, Balado P, Baeza A, Merino M (2013) [Effectiveness of topical autologous serum treatment in neurotrophic keratopathy]. Arch Soc Esp Oftalmol 88: 302-306.
- Alder J, Mertsch S, Menzel-Severing J, Geerling G (2019) [Current and experimental treatment approaches for neurotrophic keratopathy]. Ophthalmologe 116: 127-137.
- Ziakas NG, Boboridis KG, Terzidou C, Naoumidi TL, Mikropoulos D, et al. (2010) Long-term follow up of autologous serum treatment for recurrent corneal erosions. Clin Exp Ophthalmol 38: 683-687.
- del Castillo JMB, de la Casa JMM, Sardiña RC, Fernández RM, Feijoo JG, et al. (2002) Treatment of recurrent corneal erosions using autologous serum. Cornea 21: 781-783.
- Sharma N, Kaur M, Agarwal T, Sangwan VS, Vajpayee RB (2018) Treatment of acute ocular chemical burns. Surv Ophthalmol 63: 214-235.
- Goto E, Shimmura S, Shimazaki J, Tsubota K (2001) Treatment of superior limbic keratoconjunctivitis by application of autologous serum. Cornea 20: 807-810.
- Noda-Tsuruya T, Asano-Kato N, Toda I, Tsubota K (2006) Autologous serum eye drops for dry eye after LASIK. J Refract Surg 22: 61-66.
- Skalicky SE, Goldberg I, McCluskey P (2012) Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol 153: 1-9.
- Liu L, Hartwig D, Harloff S, Herminghaus P, Wedel T, et al. (2005) An optimised protocol for the production of autologous serum eyedrops. Graefes Arch Clin Exp Ophthalmol 243: 706-714.
- Pan Q, Angelina A, Marrone M, Stark WJ, Akpek EK (2017)Autologous serum eye drops for dry eye. Cochrane Database Syst Rev: 009327.
- Jeng BH, Dupps Jr WJ (2009) Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea 28: 1104-1108.
- Lopez-Garcia JS, García-Lozano I, Rivas L, Ramirez N, Mendez MT, et al. (2016) Stability of Growth Factors in Autologous Serum Eyedrops After Long-Term Storage. Curr Eye Res 41: 292-298.
- Stenwall PA, Bergström M, Seiron P, Sellberg F, Olsson T, et al. (2015) Improving the anti-inflammatory effect of serum eye drops using allogeneic serum permissive for regulatory T cell induction. ActaOphthalmol 93: 654-657.
- Soni NG, Jeng BH (2016) Blood-derived topical therapy for ocular surface diseases. Br J Ophthalmol 100: 22-27.
- Badami KG, McKellar M (2012) Allogeneic serum eye drops: time these became the norm? Br J Ophthalmol 96: 1151-1152.
- Yoon KC (2014) Use of umbilical cord serum in ophthalmology. Chonnam Med J 50: 82-85.
- Chiang CC, Lin JM, Chen WL, Tsai YY (2007) Allogeneic serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Cornea 26: 861-863.
- Harritshoj LH, Nielsen C, Ullum H, Hansen MB, Julian HO (2014) Ready-made allogeneic ABO-specific serum eye drops: production from regular male blood donors, clinical routine, safety and efficacy. Acta Ophthalmol 92: 783-786.
- Chiang CC, Chen WL, Lin JM, Tsai YY (2009)Allogeneic serum eye drops for the treatment of persistent corneal epithelial defect. Eye (Lond) 23: 290-293.
- Na KS, Kim MS (2012) Allogeneic serum eye drops for the treatment of dry eye patients with chronic graft-versus-host disease. J Ocul Pharmacol Ther 28: 479-483.
- Pleyer U, Schlickeiser S (2009) The taming of the shrew? The immunology of corneal transplantation. Acta Ophthalmol 87: 488-497.
- Choi BH, Im CJ, Huh JY, Suh JJ, Lee SH (2014) Effect of platelet-rich plasma on bone regeneration in autogenous bone graft. Int J Oral Maxillofac Surg 33: 56-59.
- Alio JL, Arnalich-Montiel F, Rodriguez AE (2012) The role of "eye platelet rich plasma" (E-PRP) for wound healing in ophthalmology. Curr Pharm Biotechnol 13: 1257-1265.
- Alio JL, Rodriguez AE, WrobelDudzinska D (2015) Eye platelet-rich plasma in the treatment of ocular surface disorders. Curr Opin Ophthalmol 26: 325-332.
- Alio JL,Colecha JR, Pastor S, Rodriguez A, Artola A (2017) Symptomatic dry eye treatment with autologous platelet-rich plasma. Ophthalmic Res 39: 124-129.
- Alio JL, Abad M, Artola A, Rodriguez-Prats JL, Pastor S, et al. (2007) Use of autologous platelet-rich plasma in the treatment of dormant corneal ulcers. Ophthalmology 114: 1286-1293.
- Alio JL, Pastor S, Ruiz-Colecha J, Rodriguez A, Artola A (2007) Treatment of ocular surface syndrome after LASIK with autologous platelet-rich plasma. J Refract Surg 23: 617-619.
- Javaloy J, Alio JL, Rodriguez AE, Vega A, Muñoz G (2013) Effect of platelet-rich plasma in nerve regeneration after LASIK. J Refract Surg 29: 213-219.
- Sanchez-Avila RM, Merayo-Lloves J,Riestra AC, Anitua A, Muruzabal F, et al. (2017) The Effect of Immunologically Safe Plasma Rich in Growth Factor Eye Drops in Patients with Sjogren Syndrome. J Ocul Pharmacol Ther 33: 391-399.
- Anitua E, Pardo R, Sanchez R, Orive G (2012) Platelet-Rich Plasma: Preparation and Formulation. Operative Techniques in Orthopaedics 22: 25-32.
- Anitua E, Prado R, Troya M, Zalduendo M, de la Fuente M, et al. (2016) Implementation of a more physiological plasma rich in growth factor (PRGF) protocol: Anticoagulant removal and reduction in activator concentration. Platelets 27: 459-466.
- Lopez-Plandolit S, Morales MC, Freire V, Grau AE, Durán JA (2011) Efficacy of plasma rich in growth factors for the treatment of dry eye. Cornea 30: 1312-1317.
- Merayo-Lloves J, Sanchez-Avila RM, Riestra AC, Anitua E, Begoña L, et al. (2016) Safety and Efficacy of Autologous Plasma Rich in Growth Factors Eye Drops for the Treatment of Evaporative Dry Eye. Ophthalmic Res 56: 68-73.
- Merayo-Lloves J, Sanchez RM, Riestra AC, Anitua E, Begoña L, et al. (2015) Autologous Plasma Rich in Growth Factors Eyedrops in Refractory Cases of Ocular Surface Disorders. Ophthalmic Res 55: 53-61.
- Lopez-Plandolit S, Morales MC, Freire V, Etxebarría J, Durán JA (2010) Plasma rich in growth factors as a therapeutic agent for persistent corneal epithelial defects. Cornea 29: 843-848.
- Anitua E, de la Fuente M, Muruzábal F, Merayo-Lloves J (2020) Stability of freeze-dried plasma rich in growth factors eye drops stored for 3 months at different temperature conditions. Eur J Ophthalmol 1120672120913035.
- Sandri G, Bonferoni MC, Rossi S, Ferrari F, Mori M, et al. (2011) Platelet lysate formulations based on mucoadhesive polymers for the treatment of corneal lesions. J Pharm Pharmacol 63: 189-198.
- Pezzotta S, Del Fante C, Scudeller L, Cervio M, Antoniazzi ER, et al. (2012) Autologous platelet lysate for treatment of refractory ocular GVHD. Bone Marrow Transplant 47: 1558-1563.
- Fea AM, Aragno V, Testa V, Machetta F, Parisi S, et al. (2016) The Effect of Autologous Platelet Lysate Eye Drops: An In Vivo Confocal Microscopy Study. Biomed Res Int 2016: 8406832.
- Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5: 163-178.
- Versura P, Profazio V, Buzzi M, Stancari A, Arpinati M, et al. (2013) Efficacy of standardized and quality-controlled cord blood serum eye drop therapy in the healing of severe corneal epithelial damage in dry eye. Cornea 32: 412-418.
- Ljubimov AV, Saghizadeh M (2015) Progress in corneal wound healing. ProgRetin Eye Res 49: 17-45.
- Nakamura Y, Sotozono C, Kinoshita S (2001) The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp Eye Res 72: 511-517.
- Ohashi Y, Motokura M, Kinoshita Y, Mano T, Watanabe H, et al. (1989) Presence of epidermal growth factor in human tears. Invest Ophthalmol Vis Sci30: 1879-1882.
- Sriwidodo S, Syah ISK, Maksum IP, Subroto T, Zasvia U, et al. (2020) Stabilization of eye drops containing autologous serum and recombinant human epidermal growth factor for dry eye syndrome. J Adv Pharm Technol Res 11: 184-188.
- Lopez-Garcia JS, García-Lozano I, Rivas L,Viso-Garrote M, Raposo R, et al. (2020) Lyophilized Autologous Serum Eyedrops: Experimental and Comparative Study. Am J Ophthalmol 213: 260-266.
- Choi CW, Kim BS, Seo JH, Shin SW, Kim YH, et al. (2001) Long-term engraftment stability of peripheral blood stem cells cryopreserved using the dump-freezing method in a -80 degrees C mechanical freezer with 10% dimethyl sulfoxide. Int J Hematol 73: 245-250.
- Li Q, Reed DA, Min L, Gopinathan G, Li S, et al. (2014) Lyophilized platelet-rich fibrin (PRF) promotes craniofacial bone regeneration through Runx2. Int J Mol Sci 15: 8509-8525.
- Bode AP, Fischer TH (2007) Lyophilized platelets: fifty years in the making. Artif Cells Blood SubstitImmobil Biotechnol 35: 125-133.
- Zhang J, Qi X, Luo X, Li D, Wang H, et al. (2017) Clinical and immunohistochemical performance of lyophilized platelet-rich fibrin (Ly-PRF) on tissue regeneration. Clin Implant Dent Relat Res 19: 466-477.
- Chen LW, Huang CJ, Tu WH, Lu CJ, Sun YC, et al. (2018) The corneal epitheliotrophic abilities of lyophilized powder form human platelet lysates. PLoS One 13: 0194345.
Citation: Tovar AA, Sabater AL (2021) Autologous Blood Products: When, Where, and How? J Ophthalmic Clin Res 8: 078.
Copyright: © 2021 Arianna A Tovar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

