
Biomarkers for Preclinical Diagnosis of Alzheimer’s Disease and Potential Strategies
*Corresponding Author(s):
Hong QingKey Laboratory Of Molecular Medicine And Biotherapy, School Of Life Science, Beijing Institute Of Technology, Beijing 100081, China
Tel:8610-68918409,
Email:hqing@bit.edu.cn
Abstract
Early diagnosis of Alzheimer's disease (AD) is important to select the best treatment for AD patients. In the past few decades, great progress has been made in the development of biomarkers, such as Aβ and phosphorylated tau, for AD diagnosis and the development of neuroimaging techniques. These biomarkers can reflect the pathophysiological features of AD and provide better diagnostic support for early onset of AD. In this review, we summarized the current biomarkers for AD and hope to provide new perspectives for the early diagnosis of AD.
Keywords
Alzheimer's disease; Pathophysiology; Diagnosis; Biomarker
INTRODUCTION
Alzheimer's disease (AD) is a slowly progressive neurodegenerative disease, which is characterized by gradual deterioration of episodic memory and continuous impairment of additional cognitive domains, then leading to general dementia syndrome [1,2]. The typical pathological of AD are senile plaques formed by Aβ deposition and neurofibrillary tangles (NFT) caused by phosphorylated tau [3]. Although many possible pathogenic associations that lead to the production and accumulation of amyloid β (Aβ) have been unveiled, and the mechanism of tau protein homeostasis has been deciphered, there is still few effective way to cure AD. In most circumstances, diagnosed AD patients in the clinical already showed cognition impairment, which indicated the significance of pre-diagnosis and prevention. Most studies investigate mild cognitive impairment (MCI), but the symptoms of MCI patients have already showed cognitive decline, which make it not suitable for preclinical diagnostic research. Subjective cognitive decline (SCD) is recently used to describe people that feel memory and other cognitive decline but is hardly diagnosed with cognition impairment [4]. Studying the changes between SCD and AD may accelerate research progress, and make preclinical diagnosis and prevention of AD as early as possible. To achieve such goal, studied of efficient biomarkers, convenient and accurate diagnosis strategies are crucial. Biomarkers are very important indicators, and many studies have dedicated to find biomarker for various diseases. The core proteins in different pathologies of AD can be used as potential Biomarkers for research. Aβ and tau are the landmark biomarkers of two classic pathologies. In addition to chemicals in the human circulation, neuroimaging technique also uses atrophy in related brain areas as a marker. This review summarizes the main biomarkers in blood and some imaging techniques currently used clinically, hoping to provide some ideas for the development of new biomarkers and new rapid and convenient diagnostic methods.
FLUID BIOMARKERS
Cerebrospinal fluid is in direct contact with the brain and is considered to be the best source of AD biomarkers. However, obtaining cerebrospinal fluid is an invasive surgical method, which has certain defects. Compared with cerebrospinal fluid, the detection of AD biomarkers through blood is a better choice, which is easier to obtain and more convenient. Current research shows that the mainly studied blood markers are Aβ, Tau, BACE1.
Aβ
Aβ is the production of amyloid precursor protein (APP), which will be formed only after a specific sequential cutting. There are different types of Aβ, only Aβ1-40 and Aβ1-42 are considered to have neural toxicity. Aβ40 and 42 tends to form aggregation and became, what we called, senile plaque and harms neurons. At present, decreased levels of Aβ1-42 in the cerebrospinal fluid (CSF) help distinguish healthy people from AD patients [5,6]. Recent studies have suggested that plasma Aβ has been considered as a simple and non-invasive diagnostic biomarker for Alzheimer's disease research. Randall Batman of the University of Washington School of Medicine has found that about 30 to 50 of Aβ in the brain Flow into the blood [7]. Similarly, Aβ42 was detected in blood exosomes. The concentration of Aβ42 in the AD group was higher than in the normal group and a MCI group [8]. Over the past two decades, Aβ42 have been identified as important biomarkers for neuropathogensis processes of AD and ratio of Aβ42/Aβ40 showed even better accuracy. Finding other biomarkers related to AD cognitive decline is essential to track mental status, advance diagnosis, and develop new strategy of treatment.
Tau
Microtubule-associated tau protein is the core of NTF pathology, which is another important hallmark of AD. Tau is microtubule binding protein, aberrant phosphorylation leads to disaggregation from cellular microtubules and finally causes neurofibrillary tangles. Phosphorylated tau protein (P-tau) and total tau protein (T-tau) are two important biomarkers. Studies have demonstrated an increasing T-tau in the CSF, and the concentrations of T-tau is about three times higher in AD patients than in normal aging individuals [9]. In blood plasma samples, P-tau181 protein levels in preclinical AD are elevated by 3.5 times and further increased at the MCI and dementia stages [10,11]. There are several phosphorylated epitopes of tau, including threonine 181 and 231, threonine 181, threonine 231 and serine 235, serine 199, threonine 231, and serine 396 and 404. Phosphorylation at threonine 231 have been proved to be correlated with neurofibrillary pathology in the CSF [12]. In addition, in blood exosomes T-tau, P-Ttau181 can also distinguish AD patients from the control group [8]. During further development of investigation, tau protein showed a specificity levels between 80 and 90 [13], which made it quite reliable for AD diagnosis (Figure 1).
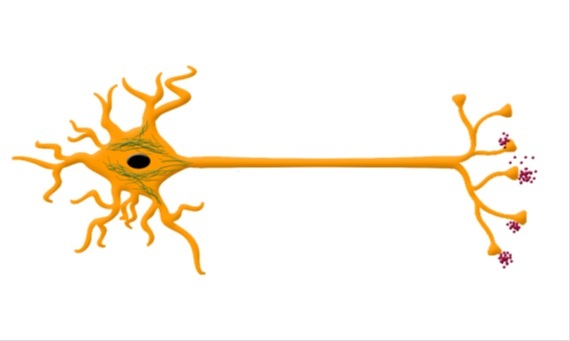
Figure 1: Pathological biomarkers of AD. APP sequentially cleaved by β-secretase and γ-secretase can produce Aβ42 and other shorter Aβ fragments. Aggregation of Aβ42 results in senile plaque as shown. Tau, which is microtubules binding protein, providing microtubule stability. After hyperphosphorylation, tau dissociates from microtubules and aggregates into NFT as shown in cell body.
β -SECRETASE (BACE1)
Degeneration of neurons in the brain of AD patients is closely related to the deposition of Aβ. BACE is the important enzyme that contribute to the formation of Aβ in neurons. BACE1 is the principal β-secretase in the cells. Studies have shown that BACE1 activity in human brain tissue with AD is much higher than that of normal cases, and the increasing in BACE1 activity is also positively correlated with the pathogenesis of AD [14,15], suggesting that BACE1 activity may reflect clinical stage of AD or potential risk. Shen and his colleagues measured the BACE1 enzyme activity and protein levels in the plasma of AD patients, MCI patients who converted to AD, MCI patients who did not convert to AD and healthy control subjects. They found that BACE1 enzyme activity is easily detected in human plasma and very high level of BACE1 activity in AD patients [16]. This may provide a new prospect for diagnosis of early onset of AD.
IMAGING BIOMARKERS
Magnetic resonance imaging (MRI)
MRI, a technique using magnetic resonance to obtain electromagnetic signals from the human body and reconstruct human information, and functional MRI (fMRI) have been widely applied in medical and research filed. Main usage of MRI is to observe volumetric changes that progressed along the different stages of AD. Several regions are damaged in AD, including hippocampus, amygdala, entorhinal cortex and so on. Among them, studies show that hippocampus, a region involved in learning and memory, is one of the earliest to be impaired. Atrophy of hippocampus can even be observed by MRI in preclinical stages of AD and can predict later conversion to AD [17]. Beside the hippocampus, evidences also suggesting that entorhinal cortex is also a very promising anatomic structure for the early diagnosis of AD, which lies near the hippocampus. Combined them together might improve prognostic efficiency by with hippocampal volume alone [18]. Other region like amygdala have showed volumetric changes consist with hippocampus atrophy, which may be a new biomarker for AD pre-diagnosis.
Positron emission tomography (PET)
Positron emission tomography (PET) is used to study cortical metabolism with injection of radionuclide labeled with a positron compound into the subject. For AD diagnosis, the main focus is regional cerebral metabolism, using 18F-2-deoxy-2-fluoro-D-glucose as a marker (18FDG-PET). Up to now, decreased metabolism in a lot of region have been observed, including temporoparietal, posterior cingulate, hippocampal complex and medial thalamic regions [19-21]. Studies, using 18FDG-PET, showed that in mild to moderate stages of AD, prefrontal association areas were affected as well. Using dynamic PET techniques, De Leon MJ et al. demonstrated that AD patients showed significant lower clearance of Aβ in CSF patients and that this correlates inversely with Aβ deposition [22]. Recent year, Pittsburgh Compound B (PIB) is gaining a lot of attention for it sensitivity [23].
Clinical diagnostic application
The mainly methods currently used for the diagnosis of AD are clinical scale testing, CSF marker identification, MRI and PET brain imaging. Clinical scale test is the most widely used detection method for its simplicity. The main scale used is the Mini-mental State Examination (MMSE). It involves time/place orientation, attention, delayed memory, language functions and etc. The total score is 30 and below 27 is considered to be cognitive impaired, 21-26 is mild impairment, 10-20 is moderate impairment and 0-9 is severe impairment. However, the scale test has certain disadvantages. It cannot diagnose potential AD patients timely, and the person who can be detected often has a greater risk of disease. Biomarkers of AD have the potential to support preclinical diagnosis and predict disease progression. Currently, the most commonly used biomarker detection method in clinical research and routine diagnosis is ELISA, which is used to measure the levels of Aβ42, T-tau, and P-tau in cerebrospinal fluid. AD cerebrospinal fluid T-tau and P-tau increased significantly [24,25]. Jia used ELISA to detect neuro-derived exosome in the plasma, the results showed that Aβ42, T-au and P-Tau181 were all increased, which is consist with the CSF result [8].
A meta-analysis showed that 231 articles containing 15,699 patients with Alzheimer‘s disease and 13,018 controls were included in the analysis. The changes in biomarkers were very consistent, and a mean fold change in the elderly control group was 2.54 for T-tau, 1.88 for P-tau, and 0.56 for Aβ42 [26]. Although PET and cerebrospinal fluid biomarker analysis are expected to improve the accuracy of clinical diagnosis, these methods show limitations precluding their use as first-line diagnostic tools [27].
Recent studies have shown that blood sample in clinical trials has advantages over cerebrospinal fluid. When measuring biomarkers of AD in fluid samples, blood is more easily to obtain than cerebrospinal fluid. Due to blood-brain barrier, only a portion of the proteins enter the blood, which also limits the detection potential blood biomarkers of AD. Nevertheless, the development of mass spectrometry technology brings bright for blood biomarkers detection [28].
Identifying potential blood-based AD biomarkers requires consideration of blood complexity, including plasma, exosomes, and cell compartments. Plasma contains a large amount of high- abundance proteins, which will cover a large amount of protein information transmitted from other parts. Exosomal biomarker research has received much attention in recent years. By collecting exosomes and specifically isolating nerve-related exosomes, it can effectively remove high-abundance proteins and secreted proteins from other parts The interference generated improves the ability to identify biomarkers. Winston et al. used antibody-enriched neural-specific exosome technique to identify p-Tau, Aβ, neurogranin and REST to predict the conversion of MCI to AD. The accuracy of multi-protein combined analysis could reach 99.3 [29]. Proteomics can be used to quantify the differential proteins associated with biomarkers in the blood of AD patients and normal patients, and find a group of proteins that are significantly associated with AD. A joint study by Seyfried and Levey's team found that blood protein proteomics differential protein cluster analysis could effectively distinguish between normal people, AD, and early AD patients [30].
DISCUSSION
Biomarkers are important tools for future diagnosis and monitoring of AD. Aβ and P-tau both are important biomarker in preclinical diagnosis of AD. PET imaging technology, serve as the gold standard for early diagnosis, is not widely used as a daily detection method for AD, due to highly cost and relatively complicated technology. CSF, as a system that directly exchanges substances with the brain, has apparent advantages, but such an invasive method for diagnosis and prevention hardly be used in clinical before the obvious symptoms appear. Hence therefore, Scientists and clinicians are focusing on blood tests, t hey try to develop blood testing methods. Proteomics has developed rapidly, which can be used to find specific disease-related proteins as biomarkers for early diagnosis. Using proteomics based screening of diagnostic biomarkers technologies applied in the diagnosis of neurodegenerative diseases can not only reveal the nature of the disease at the protein level, also help to comprehensively explore its pathological mechanisms, establish diagnostic criteria, discover drug treatment targets and identify multiple AD biomarkers [31]. The combined detection of multiple protein components can also be used as a new strategy. Finding markers in the blood and establishing fast and painless detection methods are our urgent pursuit. Finally, we hope that blood biomarkers can be used as a screening tool for preclinical evaluation of AD patients.
FUNDING SOURCE
This work was supported by the National Natural Science Foundation of China under Grant No. 81870844, 81671268 & 81701260.
REFERENCES
- Alzheimer's Association (2016)2016 Alzheimer's disease facts and figures. Alzheimer's & Dementia12: 459-509.
- Boja E, Hiltke T, Rivers R, Kinsinger C, Rahbar A, et al. (2011) Evolution of clinical proteomics and its role in medicine. J Proteome Res 10: 66-84.
- Selkoe DJ (1991) The molecular pathology of Alzheimer's disease. Neuron 6: 487-498.
- Sun Y, Dai Z, Li Y, Li H1, Wang X, et al. (2016) Subjective Cognitive Decline: Mapping Functional and Structural Brain Changes-A Combined Resting-State Functional and Structural MR Imaging Study. Radiology 281: 185-192.
- Rosen C, Hansson O, Blennow K, Zetterberg H (2013) Fluid biomarkers in Alzheimer's disease - current concepts. Mol Neurodegener 8: 20.
- Slaets S, Le Bastard N, Martin JJ, Sleegers K, Van Broeckhoven C, et al. (2013) Cerebrospinal fluid Abeta1-40 improves differential dementia diagnosis in patients with intermediate P-tau181P levels. J Alzheimers Dis 36: 759-67.
- Roberts KF, Elbert DL, Kasten TP, Patterson BW, Sigurdson WC, et al. (2014) Amyloid-beta efflux from the central nervous system into the plasma. Ann Neurol 76: 837-844.
- Jia L, Qiu Q, Zhang H, Chu L, Du Y, et al. (2019) Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimer’s Dement 15: 1071-1080.
- Mattsson N, Schöll M, Strandberg O, Smith R, Palmqvist S, et al. (2017) 18F-AV-1451 and CSF T-tau and P-tau as biomarkers in Alzheimer's disease. EMBO Mol Med 9: 1212-1223.
- Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach TG, et al. (2020) Plasma P-tau181 in Alzheimer's disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med 26: 379-386.
- Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, et al. (2020) Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med 26: 387-397.
- Buerger K, Ewers M, Pirttilä T, Zinkowski R, Alafuzoff I, et al. (2006) CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 129: 3035-3041.
- Blennow K, Hampel H (2003) CSF markers for incipient Alzheimer’s disease. Lancet Neurol 2: 605-613.
- Jia Q, Deng Y, Qing H (2014) Potential therapeutic strategies for Alzheimer's disease targeting or beyond β-amyloid: Insights from clinical trials. Biomed Res Int 2014: 837157.
- Zetterberg H, Andreasson U, Hansson O, Wu G, Sankaranarayanan S, et al. (2008) Elevated cerebrospinal fluid BACE1 activity in incipient Alzheimer disease. Arch Neurol 65: 1102-1107.
- Shen Y, Wang H, Sun Q, Yao H, Keegan AP, et al. (2017) Increased Plasma Beta-Secretase 1 May Predict Conversion to Alzheimer’s Disease Dementia in Individuals with Mild Cognitive Impairment. Biological Psychiatry 2017: S0006322317300987.
- Zhao K, Ding Y, Wang P, et al. (2017) Early classification of Alzheimer's disease using hippocampal texture from structural MRI. Proceedings 137:101372E.
- Maass A, Lockhart SN, Harrison TM, Bell RK, Mellinger T, et al. (2018) Entorhinal Tau Pathology, Episodic Memory Decline, and Neurodegeneration in Aging. J Neurosci 38: 530-543.
- Herholz K, Haense C, Gerhard A, Jones M, Anton-Rodriguez J, et al. (2017) Metabolic regional and network changes in Alzheimer's disease subtypes. J Cereb Blood Flow Metab 38: 1796-1806.
- Loskutova N, Honea RA, Brooks WM, Burns JM (2010) Reduced Limbic and Hypothalamic Volumes Correlate with Bone Density in Early Alzheimer's Disease. J Alzheimers Dis 20: 313-322.
- Scheltens P, Zwan M, Ossenkoppele R (2017) Amyloid PET imaging in patients with Alzheimer's disease. Naure 161: D808.
- De Leon M J, Li Y, Okamura N, Tsui WH, Saint-Louis LA, et al. (2017) CSF clearance in Alzheimer Disease measured with dynamic PET. Journal of Nuclear Medicine 58: 1471-1476.
- Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, et al. (2018) High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 554: 249-254.
- Vanmechelen E, Vanderstichele H, Davidsson P, Van Kerschaver E, Van Der Perre B, et al. (2000) Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: A sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett 285: 49 -52.
- Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, et al. (1995)Tau protein in cerebrospinal fluid: A biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol 26: 231-245.
- Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, et al. (2016) CSF and blood biomarkers for the diagnosis of Alzheimer's disease: A systematic review and meta-analysis. Lancet Neurol 15: 673-684.
- Hampel H, O'Bryant SE, Molinuevo JL, Zetterberg H, Masters CL, et al. (2018) Blood-based biomarkers for Alzheimer disease: Mapping the road to the clinic. Nat Rev Neurol 14: 639-652.
- Liu Y, Qing H, Deng Y (2014) Biomarkers in Alzheimer's disease analysis by mass spectrometry- based proteomics. Int J Mol Sci 15: 7865-7882.
- Winston C N, Goetzl E J, Akers J C, Carter BS, Rockenstein EM, et al. (2016) Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 3: 63-72.
- Seyfried NT, Dammer EB, Swarup V, Nandakumar D, Duong DM, et al. (2017) A Multi-Network Approach Identifies Protein- Specific Co-expression in Asymptomatic and Symptomatic Alzheimer's Disease. Cell Syst 4: 60-72 e4.
- Liao PC, Yu L, Kuo CC, Lin C, Kuo YM (2010) Proteomics analysis of plasma for potential biomarkers in the diagnosis of Alzheimer's disease. Proteomics Clinical Applications 1: 506-512.
Citation: Ailikemu A, Yan Y, Qing H (2020) Biomarkers for Preclinical Diagnosis of Alzheimer’s Disease and Potential Strategies. J Alzheimers Neurodegener Dis 6: 040.
Copyright: © 2020 Ailikemu Aierkenvv#, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

