
Breastfeeding Duration Is Associated With Growth among Children Aged 0 To 23 Months; Analysis of the Zambia 2018 Demographic and Healthy Survey Data
*Corresponding Author(s):
Chiza KumwendaDepartment Of Food Science, School Of Agricultural Sciences, University Of Zambia, P.O. Box 32379, Lusaka, Zambia
Tel:(260) 974150967,
Email:chiza.kumwenda@unza.zm
Abstract
Background
The World Health Organization recommends exclusive breastfeeding for the first 6 months and continued breastfeeding up to 24 months and beyond. Breastfeeding is beneficial for infant growth, development, and survival. Evidence on the association between breastfeeding duration and growth is not conclusive. The aim of the present study was to establish the association between breastfeeding duration and growth among infants and young children.
Methods
The study is based on data from the 2018 Zambia Demographic and Health Survey (ZDHS). The primary outcome for the study was height-for-age Z-score (HAZ). Association between HAZ and breastfeeding duration was assessed by using regression analysis.
Results
The overall mean ± SD for HAZ was -1.24 ± 1.46 and the median (interquartile range) breastfeeding duration was 9 (4-14 months). After controlling for potential confounders, breastfeeding duration was inversely associated with HAZ (β=-0.07, p < 0.001) and WHZ (β=-0.04, p < 0.001). Path analysis demonstrated that the effect of breastfeeding duration is independent of child’s birth weight and maternal perceived birth size. Other determinants of HAZ were child’s weight and sex, while WHZ was predicted by maternal age, diarrhea episode and child’s birth weight.
Conclusion
Breastfeeding duration is negatively associated with both HAZ and WHZ among Zambian children. However, the magnitudes of the association are quite small to be of clinical significance. Thus, breastfeeding should continue to be promoted, protected and supported from birth up to two years and beyond.
Keywords
Breastfeeding duration; Growth; Infants; Zambia
Introduction
Breastfeeding infants and young children up to 24 months and beyond is beneficial to the mother-infant dyad and the entire society at large [1]. Breastfeeding is associated with health, nutritional and developmental benefits for infants [2], and optimal breastfeeding is one of the most cost- effective interventions for prevention of malnutrition in children [3]. Conversely, suboptimal breastfeeding practices increase the risk of malnutrition, morbidity, and mortality in infant and young children both in developed [4] and developing countries [5]. Scaling up optimal breastfeeding to a near universal level has the potential to prevent up to 823 000 deaths among children under five years and 20 000 maternal deaths from breast cancer per year [1]. According to the United Nations Children's Fund (UNICEF), optimal breastfeeding is described as initiating breastfeeding within the first hour of birth without discarding colostrum, exclusive breastfeeding for the first 6 months from birth and continued breastfeeding for 2 years or beyond, with the introduction of appropriate complementary feeding.
In Zambia, breastfeeding is almost universal. The most recent nationally representative data shows that almost 98% of children were breastfed in the last 2 years preceding the survey [6]. On the other hand, only about 16% of children aged between 6-23 months receive the minimum acceptable diet (meeting age-appropriate dietary diversity and frequency). At country level, there are substantial variations by province with respect to breastfeeding indicators and age-appropriate complementary feeding. For instance, timely initiation of breastfeeding is lowest in the northern province at 65.2% and highest in Northwestern province, at 86.3% [6]. Coincidentally, in Zambia stunting among children under the age of five is a serious public health problem.
Breast milk forms an important source of energy and high-quality nutrients for growth and development of infants and young children. Furthermore, breast milk contains bioactive components essential for the immune system development of the infants [7]. Some of the bioactive compounds found in breast milk have been linked to promotion of linear growth in infants and young children [8]. Despite the potential for breastfeeding to improve growth, there is still no consensus on whether continued breastfeeding from six months of age is associated with growth among infants and young children. Studies which demonstrated a positive association between breastfeeding and growth are characterized by methodological errors [9,10], evidence of reverse causality has been reported in several epidemiological analyses linking breastfeeding and growth outcomes among infants and young children [11-13]. In recent publications, breastfeeding duration has also been shown to be associated with higher adiposity both in young infants [14] and older children [15]. To address these concerns, we explore the relationship between breastfeeding and nutritional status among children under the age of five years using a national representative sample.
Methods
Study design and subjects
The present study is based on data from a nationally representative data from the 2018 Zambia Demographic and Health Survey (ZDHS). The 2018 ZDHS was conducted by the Zambia Statistics Agency (ZSA) and the Ministry of Healthy in conjunction with ICF. The survey design and sampling has been described elsewhere [6]. Briefly, the primary sampling unit was a household which was obtained using a stratified two stage sample design. The initial stage was aimed at sampling clusters from which eligible households were systematically sampled to obtain a total sample size of 13,625 households. Both men and women aged 15-49 from selected households whether permanent residents or visitors who stayed in the households the night before the survey were eligible to be interviewed. For the present study included living children aged between 0-23 months that had complete anthropometric data and infant and young child feeding variables. Permission to use the 2018 dataset was sought and obtained from the Demographic and Health Surveys (DHS) Program. The DHS protocols were all approved by relevant ethics institutional review boards in Zambia (Tropical Diseases Research Centre) as well as at ICF, thus for the current analysis no further ethical review was warranted.
Variables
Outcome variables
The primary outcome variable used to assess the relationship between breastfeeding and growth was height-for-age Z (HAZ) considering that it is the main nutritional problem in Zambia. Weight-for-age Z (WHZ) was used as a secondary outcome. Both HAZ and WHZ were calculated using the WHO child growth standards. Stunting and wasting were generated from the HAZ and WHZ scores as binary variables based on World Health Organization cut off points for malnutrition using anthropometric indices (
Predictor variables (main independent)
To determine the association between continued breastfeeding and growth, the main predictor variable used was duration of breastfeeding in months. Other independent variables included were; 1) child characteristics such as age, sex, birthweight, “size at birth”, morbidity in the last two weeks-diarrhea, fever and cough; 2) maternal characteristics such as age, education level (No formal education, primary, secondary and higher); infant and young child feeding-dietary diversity based on eight food groups which included breast milk; grains, roots, and tubers; flesh foods (meat, fish, poultry, and liver/organ meat); eggs; legumes and nuts; dairy products (milk, yogurt, and cheese); vitamin A-rich fruits and vegetables; and other fruits and vegetables; 3) household variables including sex of the household head, marital status of the respondent, number of children under the age of five, literacy (ability to read or write), household size and wealth index. The ZDHS calculated household wealth index based on consumer goods owned by the household such as radio, television. refrigerator, bicycle, motorcycle, and car; housing construction materials used for making the house main floor, walls and the roof and types of water access and sanitation facilities. The household wealth scores were generated using the principal component analysis.
Statistical Analysis
All statistical analyses were performed using Stata version 13 (version 13; STATA Corp, College Station, TX, USA). Before conducting any statistical analyses, sample weights were applied to address effects of two stage cluster sampling design to ensure that the results reflect representativeness of the children at national level [16]. Furthermore, the analysis was stratified by age (all children 0-59 months and children 0-23 months) to eliminate what has been termed as partial exposure bias [17]. All continuous variables were assessed for normality using Shapiro-Wilk test before running any statistical test. Associations were assessed using bivariate and multivariate analyses, the former was used to identify variables significantly associated with growth outcomes which were to be included in the latter analysis to establish the independent association between growth outcomes and each of the included variables. Pearson and multiple linear regression were used to assess correlation and independent association between growth and independent variables, respectively. Path analysis was conducted to assess hypothetical causal relationship between variables and their relationship with growth outcomes. For this purpose, three regression analyses were run to establish whether or not breastfeeding duration was a mediator or directly was associated with nutritional status. In one model breastfeeding duration was regressed on child’s birth weight, maternal age and education. The level of statistical significance was set at p < 0.05.
Results
Sample characteristics
Demographic characteristics of the sample are shown in Table 1. The proportion of girls in the sample was comparable to that of boys. The median age (IQR) for children was 9 (5-15) months. The children’s median birthweight (IQR) was 3.1 (2.8-3.5) kg. Incidence of diarrhea was low among children. The median maternal age was 26 years and most mothers had attended formal education up to primary school. Mothers were more likely to be rural resident and coming from households mostly headed by males. About two thirds of the households had access to clean water.
|
Characteristic |
n |
% or Median (IQR)* |
|||||
|
Child characteristics |
|
|
|||||
|
Proportion of females |
1611 |
51.1 |
|||||
|
Age in months |
3116 |
9 (5-15) |
|||||
|
Age categorized |
|
|
|||||
| < 6 months |
971 |
32.4 |
|||||
|
|
891 |
29.7 |
|||||
|
6 -11 months |
|||||||
|
12-18 months |
816 |
27.2 |
|||||
|
>18 months |
324 |
10.8 |
|||||
|
Perceived size at birth |
|
|
|||||
|
Very large |
143 |
4.6 |
|||||
|
Larger than average |
618 |
19.8 |
|||||
|
Average |
1888 |
60.6 |
|||||
|
Smaller than average |
337 |
10.8 |
|||||
|
Very small |
72 |
2.8 |
|||||
|
Birth weight |
4150 |
3.1 (2.8-3.5) |
|||||
|
Diarrhea episode |
|
|
|||||
|
Yes |
702 |
24.2 |
|||||
|
No |
2194 |
75.8 |
|||||
|
Fever episode |
|
|
|||||
|
Yes |
576 |
19.9 |
|||||
|
No |
2320 |
80.1 |
|||||
|
Maternal characteristics |
|
|
|||||
|
Median age |
2896 |
26 (21-32) |
|||||
|
Median age at first child |
2896 |
18 (17-20) |
|||||
|
Age at first childbirth |
|
|
|||||
|
<19 |
1242 |
42.9 |
|||||
|
19-49 |
1654 |
57.1 |
|||||
|
Education level |
|
|
|||||
|
No education |
287 |
9.9 |
|||||
|
Primary |
1531 |
52.9 |
|||||
|
Secondary |
976 |
33.7 |
|||||
|
Tertiary |
102 |
3.5 |
|||||
|
Literacy level |
|
|
|||||
|
Yes |
1583 |
54.7 |
|||||
|
No |
1313 |
45.3 |
|||||
|
Household characteristics |
|
|
|||||
|
Province |
|
|
|||||
|
Central |
283 |
9.77 |
|||||
|
Copperbelt |
227 |
7.84 |
|||||
|
Eastern |
382 |
13.19 |
|||||
|
Luapula |
342 |
11.81 |
|||||
|
Lusaka |
263 |
9.08 |
|||||
|
Muchinga |
257 |
8.87 |
|||||
|
Northern |
324 |
11.19 |
|||||
|
North Western |
230 |
7.94 |
|||||
|
Southern |
303 |
10.46 |
|||||
|
Western
|
285 |
9.84 |
|||||
|
Residence
|
|
|
|||||
|
Rural |
2098 |
72.4 |
|||||
|
Urban |
798 |
27.6 |
|||||
|
Wealth quintile |
|||||||
|
Poorest |
881 |
30.42 |
|||||
|
Poorer |
723 |
24.97 |
|||||
|
Middle |
578 |
19.96 |
|||||
|
Richer |
391 |
13.5 |
|||||
|
Richest |
323 |
11.15 |
|||||
|
Source of drinking water |
|
|
|||||
|
Protected |
1779 |
63 |
|||||
|
Unprotected |
1045 |
37 |
|||||
|
Household size |
2896 |
6 (4-8) |
|||||
|
Number of children < 5 years |
2896 |
2 (1-2) |
|||||
|
Sex of household head |
|
|
|||||
|
Female |
583 |
20.1 |
|||||
|
Male |
2313 |
79.9 |
|||||
|
Age of household head |
2896 |
36 (29-45) |
|||||
Table 1: Sample characteristics.
*IQR, Interquartile range
Bivariate analysis
Results for correlational analysis are presented in Table 2. Breastfeeding duration was negatively correlated with both HAZ (r=-0.284, p < 0.000) and WHZ (r=-0178, p < 0.000). Other variables associated with both HAZ and WHZ were dietary diversity, wealth index, water source, residence, maternal education, birthweight, perceived birth size, maternal literacy, diarrhea, and fever. Some variables were only associated with HAZ but not WHZ, such as child sex (r=0.114, p < 0.000) and bottle feeding (r=0.041, p=0.029). Variables only correlated with WHZ but not HAZ were maternal marital status, age when the mother had her first child.
|
Variable |
|
Anthropometric index |
||
|
|
HAZ |
|
WHZ |
|
|
Dietary diversity |
|
-0.138* |
|
-0.112* |
|
|
|
|
|
|
|
Breastfeeding initiation |
|
-0.002 |
|
-0.030 |
|
|
|
|
|
|
|
Age of the Household head |
|
0.031 |
|
-0.010 |
|
Maternal age at first child |
|
-0.030 |
|
-0.046* |
|
Maternal age |
|
0.014 |
|
-0.051* |
|
Breastfeed duration |
|
-0.284* |
|
-0.178* |
|
|
|
|
|
|
|
Female sex of child |
|
0.114* |
|
0.027 |
|
|
|
|
|
|
|
Maternal marital status |
|
-0.009 |
|
-0.042* |
|
|
|
|
|
|
|
Household size |
|
0.014 |
|
-0.023 |
|
|
|
|
|
|
|
Bottle feeding |
|
0.041*** |
|
-0.002 |
|
Number of children U5 |
|
0.017 |
|
-0.035 |
|
|
|
|
|
|
|
Wealth index |
|
0.118* |
|
0.071* |
|
|
|
|
|
|
|
Water source (1=safe, 0=unsafe) |
|
0.050** |
|
0.053* |
|
|
|
|
|
|
|
Residence (Urban/rural) |
|
-0.068* |
|
-0.051* |
|
|
|
|
|
|
|
Maternal education |
|
0.101* |
|
0.072* |
|
|
|
|
|
|
|
Birthweight |
|
0.243* |
|
0.119* |
|
|
|
|
|
|
|
Perceived birth size |
|
-0.152* |
|
-0.116* |
|
|
|
|
|
|
|
Maternal Literacy |
|
0.078* |
|
0.042* |
|
Type of toilet facility |
|
0.047*** |
|
0.055* |
|
|
|
|
|
|
|
Fever |
|
-0.081* |
|
-0.056* |
|
Diarrhea |
|
-0.040* |
|
-0.098* |
Table 2: Bivariate analysis for HAZ and WHZ.
*P < 0.001, **P < 0.01, *** < 0.05
Multivariate analysis
After controlling for maternal, child and household characteristics, breastfeeding duration was negatively associated with HAZ (β=-0.07, p<0.001) (Table 3). Female sex and birthweight positively predicted HAZ, the latter was the strongest determinant of HAZ among Zambian children under the age of 2 years. The rest of the other variables ceased to be significantly associated with HAZ (Table 3). In secondary analysis, when HAZ was dichotomized to classify children as either stunted or not, breastfeeding duration was independently associated with stunting (Odds ratio=1.10, p < 0.001) after controlling for potential confounders (Supplementary Table 1). On the other hand, an increase in birth weight by one kilogram was associated with 65% decrease in the odds of stunting (Odds ratio 0.35, p < 0.001).
|
HAZ |
|
Coefficient. |
P>t |
|
95% CI |
||
|
Dietary diversity |
|
0.03 |
0.090 |
|
-0.01 |
|
0.07 |
|
|
|
|
|
|
|
|
|
|
Breastfeeding duration |
|
-0.07 |
<0.001 |
|
-0.08 |
|
-0.06 |
|
Female sex |
|
0.41 |
<0.001 |
|
0.31 |
|
0.52 |
|
Bottle feeding |
|
0.02 |
0.90 |
|
-0.25 |
|
0.29 |
|
Wealth |
|
0.06 |
0.06 |
|
0.00 |
|
0.13 |
|
|
|
|
|
|
|
|
|
|
Water source |
|
0.08 |
0.21 |
|
-0.05 |
|
0.21 |
|
|
|
|
|
|
|
|
|
|
Residence |
|
0.02 |
0.82 |
|
-0.14 |
|
0.18 |
|
|
|
|
|
|
|
|
|
|
Maternal education in years |
|
0.08 |
0.13 |
|
-0.02 |
|
0.19 |
|
|
|
|
|
|
|
|
|
|
Birthweight |
|
0.59 |
<0.001 |
|
0.47 |
|
0.71 |
|
|
|
|
|
|
|
|
|
|
Perceived birth size |
|
-0.08 |
0.07 |
|
-0.17 |
|
0.01 |
|
Literacy |
|
0.01 |
0.83 |
|
-0.12 |
|
0.15 |
|
Toilet facility |
|
-0.03 |
0.66 |
|
-0.15 |
|
0.10 |
|
|
|
|
|
|
|
|
|
|
Fever in the previous two weeks |
|
-0.13 |
0.08 |
|
-0.27 |
|
0.02 |
|
|
|
|
|
|
|
|
|
|
Diarrhea in the previous two weeks |
|
0.10 |
0.16 |
|
-0.04 |
|
0.24 |
Table 3: Predictors for HAZ for children aged 0 to 23 months from linear regression model.
|
Stunting |
|
Odds Ratio |
|
P-value |
|
95% Confidence Interval |
||
|
Dietary diversity |
|
0.95 |
|
0.131 |
|
0.89 |
|
1.02 |
|
Breastfeeding duration |
|
1.10 |
|
0.000 |
|
1.08 |
|
1.12 |
|
Male sex |
|
0.49 |
|
0.000 |
|
0.40 |
|
0.60 |
|
Bottle-feeding |
|
1.17 |
|
0.531 |
|
0.71 |
|
1.92 |
|
Number of children < 5 years |
|
1.01 |
|
0.851 |
|
0.90 |
|
1.14 |
|
Wealth |
|
0.86 |
|
0.005 |
|
0.77 |
|
0.96 |
|
Residence |
|
0.84 |
|
0.240 |
|
0.63 |
|
1.12 |
|
Maternal education in years |
|
0.81 |
|
0.026 |
|
0.67 |
|
0.97 |
|
Birthweight |
|
0.35 |
|
0.000 |
|
0.28 |
|
0.42 |
|
Literacy |
|
1.06 |
|
0.616 |
|
0.84 |
|
1.35 |
|
Fever in the previous two weeks |
|
1.02 |
|
0.882 |
|
0.80 |
|
1.29 |
Supplementary Table 1: Determinants of stunting among Zambian children aged 0-23 months.
Results of the path analysis models are presented in Figure 1; breastfeeding duration mediated the effects of maternal age (β=0.11, p < 0.001) and education (β=-0.05, p=0.018). On the other hand, breastfeeding duration continued to directly affect HAZ scores. The direct, indirect and total effects of variables on HAZ scores are presented in Table 4. Birthweight had a non-significant effect on breastfeeding duration, indicative of absence of reverse causality, but had the strongest direct and indirect effects on HAZ-scores than all the other covariates.
Predictors for weight-for-height. Controlling for potential confounding factors, breastfeeding duration (β=-0.04, p < 0.001), maternal age, children under the age of five, and diarrhea were all negatively associated with WHZ while birth weight was a positive determinant of WHZ among children under the age of five (Supplementary Table 2).
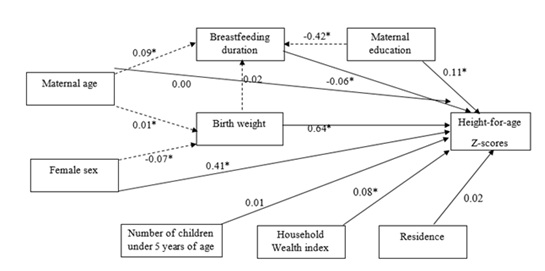 Figure 1: Path analysis for HAZ-Scores and independent variables.
Figure 1: Path analysis for HAZ-Scores and independent variables.
|
Variable |
Effects |
||||
|
|
Direct |
|
Indirect |
|
Total |
|
Breastfeeding duration |
-0.06* |
|
|
-0.06* |
|
|
Birth weight |
0.64* |
|
-0.001 |
|
0.64* |
|
|
|
|
|
|
|
|
Maternal age |
0.00 |
|
0.002 |
|
0.007 |
|
|
|
|
|
|
|
|
Maternal education |
0.11* |
|
0.027 |
|
0.134* |
|
Female sex |
0.41* |
|
-0.04* |
|
0.368* |
|
Residence |
0.02 |
|
|
0.023 |
|
|
|
|
|
|
|
|
|
Household wealth index |
0.08* |
|
|
0.08* |
|
|
Number of children under the age of five years |
0.01 |
|
|
0.01 |
|
Table 4: Standardized effects of the variables on HAZ-Scores from the path analysis.
|
Variables |
|
Coef. |
|
P>t |
|
95% CI |
||
|
Dietary diversity |
|
0.00 |
|
0.82 |
|
-0.040 |
|
0.032 |
|
|
|
|
|
|
|
|
|
|
|
Maternal age at birth of first child |
|
-0.08 |
|
0.14 |
|
-0.195 |
|
0.028 |
|
|
|
|
|
|
|
|
|
|
|
Maternal age |
|
-0.01 |
|
0.01 |
|
-0.019 |
|
-0.002 |
|
|
|
|
|
|
|
|
|
|
|
Breastfeeding duration |
|
-0.04 |
|
<0.001 |
|
-0.047 |
|
-0.024 |
|
Maternal marital status |
|
-0.03 |
|
0.25 |
|
-0.087 |
|
0.022 |
|
|
|
|
|
|
|
|
|
|
|
Wealth |
|
0.00 |
|
0.99 |
|
-0.064 |
|
0.063 |
|
Water source |
|
0.03 |
|
0.63 |
|
-0.093 |
|
0.154 |
|
Residence |
|
-0.05 |
|
0.56 |
|
-0.200 |
|
0.109 |
|
|
|
|
|
|
|
|
|
|
|
Maternal education |
|
0.03 |
|
0.53 |
|
-0.072 |
|
0.139 |
|
|
|
|
|
|
|
|
|
|
|
Birth weight |
|
0.23 |
|
<0.001 |
|
0.117 |
|
0.352 |
|
|
|
|
|
|
|
|
|
|
|
Perceived birth size |
|
-0.09 |
|
0.06 |
|
-0.173 |
|
0.002 |
|
Literacy |
|
-0.01 |
|
0.83 |
|
-0.147 |
|
0.119 |
|
|
|
|
|
|
|
|
|
|
|
Toilet facility |
|
0.04 |
|
0.53 |
|
-0.082 |
|
0.160 |
|
Fever in the previous two weeks |
|
-0.04 |
|
0.61 |
|
-0.175 |
|
0.104 |
|
Diarrhea in the previous two weeks |
|
-0.14 |
|
0.04 |
|
-0.276 |
|
-0.009 |
Supplementary Table 2: Predictors of WHZ among children under the age of five.
Discussion
The present study was designed to assess the relationship between breastfeeding duration and nutritional status among children aged 0 to 23 months. Among Zambian infants and young children, breastfeeding duration was inversely associated with HAZ after controlling for potential child, maternal and household confounders. Other than breastfeeding duration, birth weight was the strongest predictor of HAZ among children under the age of 24 months. Additionally, being female was positively associated with HAZ. Using stunting as an outcome variable, breastfeeding duration increased the odds of stunting among children. Similar findings were observed between breastfeeding duration and WHZ. Apart from breastfeeding duration, birth weight and child sex were the other variables significantly associated with both HAZ and WHZ. Diarrhea episode in the previous two weeks was only associated with WHZ.
Our finding that duration of breastfeeding is negatively associated with nutritional status among children is in line with findings from other low- and middle-income countries. In a prospective cohort study conducted by Fall, Sachdev [18], breastfeeding duration was negatively correlated with HAZ among South African and Filipino infants and young children. Similarly, among Pakistani [19] breastfeeding duration was negatively associated with lower HAZ and height respectively among children. In an earlier analysis, Caulfield, Bentley [11] also observed a positive association between prolonged breastfeeding and malnutrition among child less than three years old. Our results on breastfeeding duration and HAZ are not in agreement with findings among Nepalese [20] and Guatemalan [18] children, in whom breastfeeding duration was shown to be positively associated with HAZ. The differences in the results could be due to failure to account for confounding in some studies or mediating factors such as age of the children. It may as well be due to reverse causality, children perceived to be smaller and shorter are likely to be breastfed longer than their taller counterparts [13,21]. In the present study we controlled for potential confounding and we explored mediation as well as possibility for reverse causality using path analysis. The latter was not evidence in our study.
Our study demonstrates that being female among Zambian children under the age of 24 months was associated with greater HAZ. Similar findings have been reported among African countries. In a meta-analysis on the determinants of stunting and obesity among children in sub-Saharan Africa, Keino, Plasqui [22] demonstrated higher prevalence of stunting among boys than girls, confirming what was reported by Wamani, Astrom [23]. The similar findings have recently been observed in Tanzania [24] and Ethiopia [25]. The differential sex growth may be related to differences in feeding and care practices for boys and girls or morbidity, the former was observed among Senegalese children younger than three years [26]. There is also some evidence that the differences may be linked to differences in the biology of growth between boys and girls [27]. Overall, there is currently no universally agreed plausible explanation for the differential sex growth outcomes for children under the age of five years. Further research is needed to unequivocally elucidate the mechanisms explaining sex differences with respect to malnutrition between boys and girls. Such evidence may prove quite beneficial in public health nutrition programming.
The strongest determinant of HAZ in our study was birth weight. Our results confirm results from earlier studies demonstrating the association between birth weight and HAZ among children [28]. The positive association between birth weight and height is also evident beyond childhood. A study among Brazilian adolescents demonstrated an increase of 0.28 cm in height corresponding to an increase of 100 g in birth weight [29]. Similarly, Jelenkovic, Yokoyama [30] demonstrated birth weight was significantly associated with height from infancy up to adulthood among twins. The mechanisms underlying the association between birth weight and height remain less elucidated. However, it is speculated that intrauterine conditioning may partly explain the associations observed postnatally [31].
Birth weight was the strongest and only positive determinant of WHZ in our sample. Similar association have previously been reported from nationally representative samples [32]. Apart from breastfeeding duration, the other factors negatively associated with WHZ were episode of diarrhea in the last two weeks and maternal age. Our results on diarrhea are in line with findings from other countries from sub-Saharan Africa. Masibo and Makoka [33] and Wasihun, Dejene [34] observed an inverse relationship between diarrhea episode and wasting among Kenyan and Ethiopian children under the age of five respectively. Diarrhea can lead to undernutrition through impaired appetite, malabsorption, acute phase catabolism [35]. The negative association between maternal age and WHZ is in contrast with evidence from other studies both in sub-Saharan Africa [36]. The difference between our results and those showing a positive association may be due to differential socioeconomic mechanisms operating in different environment [37].
The present study has several of strengths including large sample size, robust statistical approach to account for confounding, effect modification, and mediation. The main weaknesses of the study were failure to control for maternal height, which is a positive predictor of HAZ for children; potential for recall bias for variables measured using maternal memory and cross-sectional design rendering it difficult to ascertain causality. Finally, the effect of residual confounding may not be ruled out. Overall, taken together the present study has demonstrated that breastfeeding duration is negatively associated with growth outcomes among children, however, the effect is unlikely to be of clinical importance.
Conclusion
Our study has demonstrated that breastfeeding duration is negatively associated with both HAZ and WHZ among Zambian children. However, the magnitudes of the association were quite small to be of clinical significance. Thus, breastfeeding should continue to be protected and supported from birth up to two years.
Acknowledgements
Not applicable.
Funding
Authors did not receive funding for the present work.
Ethical approval
Permission to use the 2018 dataset was sought and obtained from the Demographic and Health Surveys (DHS) Program. The DHS protocols were all approved by relevant ethics institutional review boards in Zambia (Tropical Diseases Research Centre) as well as at ICF, thus for the current analysis no further ethical review was warranted.
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Author's contribution
CK conceptualized the study, performed data analysis and drafted the manuscript. LMZ, MU, DN, HNS and KA contributed in writing the manuscript. All authors read and approved the final manuscript.
Availability of data and materials
The main data set used for the present analysis was obtained from the Demographic and Health Survey Program is available from the https://www.dhsprogram.com/data/dataset_admin/login_main.cfm. However, sharing of these data to a third party is not allowed. The weighted date used for preparing the current manuscript may be obtained from the corresponding author upon reasonable request and with permission of DHS Program.
References
- Victora CG, Bahl R, Barros AJ, França GV, Horton S, et al. (2016) Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect.Lancet 387: 475-490.
- Gartner LM (2005) Breastfeeding and the use of human milk.Pediatrics 115: 496-506.
- Bhutta ZA, Das JK, Rizvi A, Gaffey F, Walker N, et al. (2013) Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost?Lancet 382: 452-477.
- Roberts TJ, Carnahan E, Gakidou E (2013) Can breastfeeding promote child health equity? A comprehensive analysis of breastfeeding patterns across the developing world and what we can learn from them.BMC Med 11:
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, et al. (2008) Maternal and child undernutrition: Global and regional exposures and health consequences.Lancet 371: 243-260.
- Zambia Statistics Office , Ministry Of Health , and Icf, Zambia demographic and health survey 2018, 2019, Central Statistical Office, Ministry of Health, and ICF International, Lusaka.
- Ballard O, Morrow AL (2013) Morrow, Human milk composition: nutrients and bioactive factors.Pediatr Clin North Am 60: 49-74.
- Eriksen KG, Christensen SH, Lind MV, Michaelsen KF (2018) Human milk composition and infant growth.Curr Opin Clin Nutr Metab Care 21: 200-206.
- Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N (2007) Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: Evidence from a large randomized trial.Am J Clin Nutr 86: 1717-1721.
- Owen CG, Martin RM, Whincup PH, Davey-Smith G, Gillman MW (2005) The effect of breastfeeding on mean body mass index throughout life:A quantitative review of published and unpublished observational evidence.Am J Clin Nutr 82: 1298-1307.
- Caulfield LE, Bentley ME, Ahmed S (1996) Is prolonged breastfeeding associated with malnutrition? Evidence from nineteen demographic and health surveys.Int J Epidemiol 25: 693-703.
- Kramer MS (2010) Breastfeeding, complementary (solid) foods, and long-term risk of obesity.Am J Clin Nutr 91: 500-501.
- Marquis GS, Habicht JB, Lanata CF, Black RE, Rasmussen KM (1997) Association of breastfeeding and stunting in Peruvian toddlers: An example of reverse causality.Int J Epidemiol 26: 349-356.
- Ua-areechit T, Suteerojntrakool O, Pongcharoen T, Winichagoon P, Judprasong K, et al. (2022) Breastfeeding duration is associated with higher adiposity at 6–8 months of age.Maternal & Child Nutrition 19: e13438.
- Dang J (2022) Associations between Breastfeeding Duration and Obesity Phenotypes and the Offsetting Effect of a Healthy Lifestyle.Nutrients 14: 1999.
- Bell BA, Onwuegbuzie AJ, Ferron J, Jiao QG, Hibbard S, et al (2012) Use of design effects and sample weights in complex health survey data: a review of published articles using data from 3 commonly used adolescent health surveys.Am J Public Health 102: 1399-1405.
- Alderman H, Headey D (2018) The timing of growth faltering has important implications for observational analyses of the underlying determinants of nutrition outcomes.PLoS One 13: e0195904.
- Fall CH, Sachdev HS, Osmond C, Restrepo-Mendez MC, Victora C, et al. (2015) Association between maternal age at childbirth and child and adult outcomes in the offspring: A prospective study in five low-income and middle-income countries (COHORTS collaboration).Lancet Glob Health 3: e366-77.
- Chaudhary SR, Govil S, Lala MK, Yagnik, HB (2018) Infant and Young Child Feeding Index and its association with nutritional status: A cross-sectional study of urban slums of Ahmedabad.J Family Community Med 25: 88-94.
- Hanley-Cook G, Argaw A, Dahal P, Chitekwe S, Kolsteren P (2020) Infant and young child feeding practices and child linear growth in Nepal Regression decomposition analysis of national survey data 1996-2016. Matern Child Nutr e12911.
- Simondon KB, Costes R, Delaunay V, Diallo A, Simondon F (2001) Children's height, health and appetite influence mothers' weaning decisions in rural Senegal.Int J Epidemiol 30: 476-481.
- Keino S, Plasqui G, Ettyang G, van den Borne B (2014) Determinants of stunting and overweight among young children and adolescents in sub-Saharan Africa. Food Nutr Bull 35: 167-178.
- Wamani H, Åstrøm AN, Peterson S, Tumwine J, Tylleskär T (2007) Boys are more stunted than girls in sub-Saharan Africa: a meta-analysis of 16 demographic and health surveys. BMC Pediatr 7: 17.
- Muhimbula H, Kinabo J, O'Sullivan A (2019) Determinants of infant nutrition status in rural farming households before and after harvest.Matern Child Nutr 15: e12811.
- Amare ZY, Ahmed ME, Mehari AB (2019) Determinants of nutritional status among children under age 5 in Ethiopia: Further analysis of the 2016 Ethiopia demographic and health survey. Global Health 15: 62.
- Bork KA, Diallo A (2017) Boys Are More Stunted than Girls from Early Infancy to 3 Years of Age in Rural Senegal.J Nutr 147: 940-947.
- Thurstans S, Opondo C, Seal A, Wells J, Khara T, et al. (2020) Boys are more likely to be undernourished than girls: A systematic review and meta-analysis of sex differences in undernutrition. BMJGlob Health 5: 12.
- Binkin NJ, Yip R, Fleshood L, Trowbridge L (1988) Birth weight and childhood growth.Pediatrics 82: 828-834.
- Ferreira VR, Jardim TV, Póvoa TR, Mendonça KL, Nascente FN, et al. (2018) Birth weight and its association with blood pressure and nutritional status in adolescents.J Pediatr (Rio J) 94: 184-191.
- Jelenkovic A, Yokoyama Y, Sund R, Hur YM, Harris JR, et al. (2018) Associations between birth size and later height from infancy through adulthood: An individual based pooled analysis of 28 twin cohorts participating in the CODATwins project.Early Hum Dev 120: 53-60.
- Strauss RS (1997) Effects of the intrauterine environment on childhood growth. Br Med Bull 53: 81-95.
- McGovern ME (2019) How much does birth weight matter for child health in developing countries? Estimates from siblings and twins. Health Econ 28: 3-22.
- Masibo PK, Makoka D (2012) Trends and determinants of undernutrition among young Kenyan children: Kenya Demographic and Health Survey; 1993, 1998, 2003 and 2008-2009. Public Health Nutr 15: 1715-1727.
- Wasihun AG, Dejene TA, Teferi M, Marugán J, Negash L, et al. (2018) Risk factors for diarrhoea and malnutrition among children under the age of 5 years in the Tigray Region of Northern Ethiopia. PLoS One 13: e0207743.
- Guerrant RL, Oriá RB, Moore SR, Oriá MO, Lima AA (2008) Malnutrition as an enteric infectious disease with long-term effects on child development. Nutr Rev 66: 487-505.
- Wemakor A, Garti H, Azongo T, Garti H, Atosona A (2018) Young maternal age is a risk factor for child undernutrition in Tamale Metropolis, Ghana. BMC Res Notes 11: 877.
- Finlay JE, Ozaltin E, Canning D (2011) The association of maternal age with infant mortality, child anthropometric failure, diarrhoea and anaemia for first births: Evidence from 55 low- and middle-income countries. BMJ Open 1: e000226.
Citation: Kumwenda C, Zgambo LM, Umugwaneza M, Nthani D, Nyambe-Silavwe H, et al. (2023) Breastfeeding Duration Is Associated With Growth among Children Aged 0 To 23 Months; Analysis of the Zambia 2018 Demographic and Healthy Survey Data. J Food Sci Nutr 9: 155
Copyright: © 2023 Chiza Kumwenda, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

