
Cannabinoids Terpenes and Inflammation
*Corresponding Author(s):
Erhan YararCo-Chairman Of The Nutrigenetic And Epigenetic Society, Istanbul, Turkey
Tel:+90 5313066556,
Email:eyarar7@gmail.com
Abstract
Inflammation and oxidative stress are involved in many diseases. Chronic inflammation may be caused by autoimmune disorders, untreated infections, or illnesses, and often plays a role in conditions such as asthma, cancer, and diabetes. Factors such as smoking, obesity, or stress may also contribute to chronic inflammation. Emerging research now demonstrates that Cannabidiol (CBD) has significant potential in terms of limiting inflammation and downstream effects in terms of free radicals as well.
Introduction
Inflammation happens as a common defensive reaction when the body is harmed. There are two sorts of inflammation: acute and chronic.
Acute inflammation occurs following an injury, infection, or illness. The immune system unleashes immune cells to the affected area to protect it, causing redness and swelling.
Chronic inflammation refers to a prolonged inflammatory response in the body. When inflammation lingers, it can detrimentally impact tissues and organs due to the increased production of free radicals, which results in oxidative stress, an imbalance between antioxidants and free radicals.
It is certainly clear that our most pervasive chronic conditions share a common feature in terms of their underlying cause. Whether coronary artery disease, hypertension, diabetes, depression, rheumatoid arthritis, or even Alzheimer’s disease, what current medical literature reveals is the powerful role that inflammation plays in these and other common conditions.
Eventually, the main issue with higher levels of irritation that shows as harm to tissue is the fact that when irritation has been turned on, it increments the generation of damaging free radicals, a circumstance called oxidative stress. When oxidative stress is running uncontrolled, harm happens to our proteins, and fat, and indeed our DNA.
Scientists described not only the complexities and challenges posed by trying to specifically target oxidative stress in a variety of disease states, but also the potential benefits of using CBD to accomplish this goal.
Unlike THC, CBD is a non-psychotropic subsidiary of the plant. As of late, research has illustrated that CBD has wide extending activity in terms of diminishing inflammation and the harming impacts of free radicals. Particularly, CBD tweaks the work of the immune system. CBD, for example, has been illustrated to be particularly successful in managing with various sorts of pain. This action is additionally thought to represent a sign of CBD working as an anti-inflammatory much as over the counter anti-inflammatory solutions are utilized for ordinary aches and pains.
Inflammation and oxidative stress are intimately involved in the genesis of many human diseases. Unraveling that relationship therapeutically has proven challenging, in part because inflammation and oxidative stress “feed off” each other. However, CBD would seem to be a promising starting point for further drug development given its anti-oxidant and anti-inflammatory actions on immune cells.
The research in terms of medical application of CBD is expanding dramatically, and with good reason. Moving forward, it is almost certain that CBD research will continue to expand, and likely validate it’s efficacy across a wide spectrum of health issues [1].
Cannabis Has Antiinflammatory Capacity
Cannabis sativa, commonly known as marijuana, has been used for several years for its medicinal effects, including antipyretic and analgesic properties. Approximately 80 cannabis constituents, termed cannabinoids, naturally occur as 21 carbon atom compounds of cannabis and analogues of such compounds and their metabolites [2]. Cannabidiol (CBD) is present in most cannabis preparations (hashish, marijuana, ganja) in higher concentrations than THC. Cannabidiol. Was, first isolated in 1940 by Todd and Adams. Its structure was elucidated by Mechoulam [3]. Its absolute stereochemistry was determined in 1967. The synthesis of cannabidiol in its racemic form and its natural form were reported in the 1960's.
Marijuana-derived cannabinoids and related compounds have been tested for the treatment of various diseases, ranging from cancer to glaucoma. Marijuana-derived cannabinoids function by binding several subtypes of cannabinoid receptor in the brain and other organs. In addition, the body produces endocannabinoids that also function through binding these receptors. Compounds that are chemically related to cannabinoids have also been shown to function by binding other types of receptor, such as the NMDA (N-methyl-D-aspartate) receptor and the peroxisome-proliferative-activated receptor-γ (PPAR-γ), or by influencing other cellular components, such as lipid rafts. Cannabinoids and endocannabinoids regulate some of the inflammatory aspects of brain injury, through both cannabinoid-receptor-mediated and non-cannabinoid-receptor-mediated mechanisms. It is possible that these drugs reduce brain oedema and other aspects of neuroinflammation by inhibiting NMDA receptors, by functioning as antioxidants and by reducing the levels of pro-inflammatory cytokines in the brain. Cannabinoids regulate the tissue response to inflammation in the colon, and it is possible that this regulation occurs on two levels: the first, involving the smooth-muscle response to pro-inflammatory mediators, thereby affecting gastrointestinal transit time; and the second, involving the direct suppression of pro-inflammatory-mediator production. Plant-derived cannabinoids and synthetic derivatives are anti-inflammatory and immunosuppressive in animal models of arthritis. The mechanisms of action seem to be independent of cannabinoid receptors and cause suppression of pro-inflammatory cytokines that are produced by lymphocytes and macrophages. Endocannabinoids and Cannabinoid Receptor 1 (CB1) might function as regulators of inflammation-induced hypotension, whereas cannabinoids that bind CB2 might attenuate vascular inflammation. Cannabinoid-based drugs that do not function by interacting with cannabinoid receptors decrease the symptoms of septic shock, which might result from the ability of these drugs to inhibit pro-inflammatory-cytokine production. Immune activation causes lymphocytes and macrophages to produce endocannabinoids and to alter their expression of cannabinoid receptors. These effects and endocannabinoid-mediated effects on immune-cell migration and cytokine production indicate that the endocannabinoid system is involved in the host inflammatory response. Cannabinoids and related compounds have been shown to either suppress or increase the production of pro-inflammatory cytokines- such as tumour-necrosis factor, interleukin-1β (IL-1β) and IL-6- in both patients and animal models, indicating that these drugs can modulate pro-inflammatory mediators. Depending on the model system, the effects of these drugs do not always depend on their interaction with cannabinoid receptors. Cannabinoids bias the immune response away from T helper 1 (TH1)-cell responses, by mechanisms that involve cannabinoid receptors. It is possible that signalling through these receptors, expressed by T cells, B cells or antigen-presenting cells, suppresses the expression of TH1-cell-promoting cytokines and increases the expression of TH2-cell-promoting cytokines [4].
CBD has a wide spectrum of biological activity, including antioxidant and anti-inflammatory activity, which is why its activity in the prevention and treatment of diseases whose development is associated with redox imbalance and inflammation has been tested [5].
Based on the current research results, the possibility of using CBD for the treatment of diabetes, diabetes-related cardiomyopathy, cardiovascular diseases (including stroke, arrhythmia, atherosclerosis, and hypertension), cancer, arthritis, anxiety, psychosis, epilepsy, neurodegenerative disease (i.e., Alzheimer’s) and skin disease is being considered [6].
CBD also reduces Reactive Oxygen Species (ROS) production by chelating transition metal ions involved in the Fenton reaction to form extremely reactive hydroxyl radicals. In addition to the direct reduction of oxidant levels, CBD also modifies the redox balance by changing the level and activity of antioxidants. CBD antioxidant activity begins at the level of protein transcription by activating the redox-sensitive transcription factor referred to as the nuclear erythroid 2-related factor (Nrf2), which is responsible for the transcription of cytoprotective genes, including antioxidant genes [7]. It is known that under oxidative conditions, alterations in enzymatic activity may be caused by oxidative modifications of proteins, mainly aromatic and sulfur amino acids.
CBD also supports the action of antioxidant enzymes by preventing a reduction in the levels of microelements (e.g., Zink or Selenium), which are usually lowered in pathological conditions. These elements are necessary for the biological activity of some proteins, especially enzymes such as superoxide dismutase or glutathione peroxidase [8].
By lowering ROS levels, CBD also protects non-enzymatic antioxidants, preventing their oxidation, as in the case of GSH in the myocardial tissue of mice with diabetic cardiomyopathy [9]. An increase in GSH levels after CBD treatment was also observed in mouse microglia cells [10]. This is of great practical importance because GSH cooperates with other low molecular weight compounds in antioxidant action, mainly with vitamins such as A, E, and C [11]. CBD exhibits much more antioxidant activity (30-50%) than alpha-tocopherol or vitamin C [5].
The result of an imbalance between oxidants and antioxidants is oxidative stress, the consequences of which are oxidative modifications of lipids, nucleic acids, and proteins. This results in changes in the structure of the above molecules and, as a result, disrupts their molecular interactions and signal transduction pathways [12] (Figure 1).
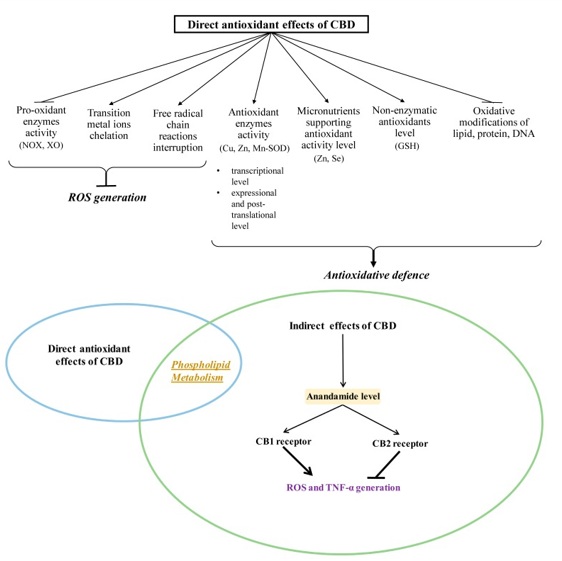 Figure 1: Direct antioxidant effects of CBD.
Figure 1: Direct antioxidant effects of CBD.
Oxidative modifications play an important role in the functioning of redox-sensitive transcription factors (including Nrf2 and the nuclear factor kappa B (NFkappaB). As a consequence, oxidative modifications play a role in the regulation of pathological conditions characterized by redox imbalances and inflammation, such as cancer, inflammatory diseases, and neurodegenerative diseases. In this situation, one of the most important processes is lipid peroxidation, which results in the oxidation of Polyunsaturated Fatty Acids (PUFA), such as arachidonic, linoleic, linolenic, eicosapentaenoic, and docosahexaenoic acids [13].
One of the most noticeable CBD antioxidant effects is the reduction in lipid and protein Modifications. CBD supplementation has been found to reduce lipid peroxidation, as measuredby MDA levels, in mouse Hippocampal (HT22) neuronal cells depleted of oxygen and glucose under reperfusion conditions [14].
Additionally, CBD caused a reduction in the level of PUFA cyclization products, such as isoprostanes, in the cortex of transgenic mice with Alzheimer’s disease [15]. Thus, CBD protects lipids and proteins against oxidative damage by modulating the level of oxidative stress, which participates in cell signaling pathways. Several interactions with relevance to the immune system and oxidative stress exist. Despite having low affinity for CB1 and CB2 receptors, CBD has been shown to antagonize the actions of cannabinoid CB1/CB2 receptor agonists in the low nanomolar range, consistent with non-competitive inhibition. CBD acts as an inhibitor of Fatty Acid Amide Hydrolase (FAAH), the major enzyme for endocannabinoid breakdown. Because FAAH activity correlates with gastrointestinal mobility, CBD may have utility in treating intestinal hypermotility associated with certain inflammatory diseases of the bowel. CBD may thus be of benefit in treating neueodegenerative diseases associated with hyperactivation of microglial, as well as retinal neuroinflammation seen in such conditions as uveitis, diabetic retinopathy, age-related macular degeneration, and glaucoma. CBD may have therapeutic utility in treating diabetic complications and atherosclerosis. CBD might be useful therapeutically to counter the increased risk of depression in diseases associated with immune activation and inflammation, which often lead to decreased tryptophan, the precursor of serotonin [1].
Cannabinoids have been shown to exert anti-inflammatory activities in various in vivo and in vitro experimental models as well as ameliorate various inflammatory degenerative diseases. However, the mechanisms of these effects are not completely understood. Using the BV-2 mouse microglial cell line and Lipopolysaccharide (LPS) to induce an inflammatory response a study investigated the signaling pathways engaged in the anti-inflammatory effects of cannabinoids as well as their influence on the expression of several genes known to be involved in inflammation. It has been found that the two major cannabinoids present in Marijuana (THC) and Cannabidiol (CBD), decrease the production and release of proinflammatory cytokines, including interleukin- 1beta, interleukin-6, and Interferon (IFN) beta, from LPS-activated microglial cells. The cannabinoid anti-inflammatory action does not seem to involve the CB1 and CB2 cannabinoid receptors or the abn-CBD-sensitive receptors. In addition, it is found that THC and CBD act through different, although partially overlapping, mechanisms. CBD, but not THC, reduces the activity of the NF-kappaB pathway, a primary pathway regulating the expression of proinflammatory genes. Moreover, CBD, but not THC, up-regulates the activation of the STAT3 transcription factor, an element of homeostatic mechanism(s) inducing anti-inflammatory events. Following CBD treatment, but less so with THC, it is observed a decreased level of mRNA for the Socs3 gene, a main negative regulator of STATs and particularly of STAT3. However, both CBD and THC decreased the activation of the LPS-induced STAT1 transcription factor, a key player in IFN beta-dependent proinflammatory processes. In summary, these observations show that CBD and THC vary in their effects on the anti-inflammatory pathways, including the NF-kappaB and IFNbeta-dependent pathways [16].
Preliminary studies showed that cannabidiol inhibited PBQ-induced writhing in mice when given orally at doses up to 10 mg/kg. Cannabidiol was also shown to reduce TPA-induced erythema, which is dependent upon prostaglandin release, in mice when applied topically [17].
In an in vitro study it is demonstrated that THC and cannabidiol inhibited Nitric Oxide (NO) produced by mouse peritoneal macrophages activated by LPS and IFNγ [18]. Studies in vitro the effects of THC and cannabidiol on secretions of IL-1, IL-2, IL-6, TNFα and IFNγ by human leukocytes following activation by mitrogen, found that both cannabinoids in low concentrations increase IFNγ production, whereas in high concentrations (5-24μg/ml) completely blocked IFNγ synthesis, and cannabidiol decreased both IL-1 and TNFα production and did not affect IL-2 secretion. Cannabinoids may be used to treat inflammatory diseases, such as rheumatoid arthritis and Crohn's disease. Inflammatory diseases involve the complex interaction between several components such as Interleukins (IL-1, IL-6 and IL-8), TNF-α and various mediators such as nitric oxide, ROI and PGE2.
Preferably the cannabinoid is used as an anti-inflammatory agent against inflammatory diseases, especially rheumatoid arthritis or Crohn's Disease, sarcoidosis, asthma, Alzheimer’s disease, multiple sclerosis, Psoriasis, ulcerative colitis, osteoarthritis or spondyloarthropathy (erg. ankylosing spondylitis). Anti-inflammatory action of Cannabis sativa may be due in part to the non-psychotropic constituent cannabidiol (and presumably also to its acidic metabolite). Cannabinoids may be used separately or as mixtures of two or more cannabinoids. They may be combined with one or more pharmaceutically acceptable compounds such as carriers. As a general proposition, the total pharmaceutically effective amount of cannabinoid administered will be in the range of 1μg/kg/day to 50mg/kg/day of patient body weight, preferably 2.5 to 10mg/kg/day especially 5mg/kg/day. (https://patents.google.com/patent/US6410588B1/en)
Terpenes Play Immense Role in Entourage Effect
Surely one of the most important elements of cannabis are terpenes. Terpenes are fragrant oils produced alongside CBD, THC, and other cannabinoids. They account for the distinctive smell and flavors of your cannabis. Some include pine, mint, berry, and citrus. Without terpenes cannabis would have very little taste or odor. Cannabis terpenes evolved for the cannabis plant to draw pollinators and repel predators. Weather, climate, maturation, age, soil type, and fertilizer use all help to determine which terpenes will develop. Thanks to the wide variety of factors, over 200 terpenes have been noted to date.
Structurally there is little in common between THC and the endocannabinoids. The plant cannabinoids are terpenophenols, while the endocannabinoids are fatty acid derivatives. Yet, pharmacologically they have much in common. Both THC and anandamide cause a typical tetrad of physiological effects: hypothermia, hypomotility, antinociception and catalepsy [19].
Terpenes bind to receptors in the brain. By doing so, they work to either activate or inhibit the effects of other compounds found in the cannabis plant. They also reduce the side effects of chemotherapy [20], provide antiparasitic benefits, and are powerful anti-inflammatories. The different Cannabis chemotypes showed distinct compositions of terpenoids. The terpenoid-rich essential oils exert anti-inflammatory and antinociceptive activities in vitro and in vivo, which vary according to their composition. Their effects seem to act independent of TNFα. None of the essential oils was as effective as purified CBD. In contrast to CBD that exerts prolonged immunosuppression and might be used in chronic inflammation, the terpenoids showed only a transient immunosuppression and might thus be used to relieve acute inflammation [21].
Some terpenes balance the less-desirable psychoactive and physiological effects of cannabis and provide therapeutic qualities not found in products that only contain CBD. One such terpenoid (a terpene that has been dried and cured and therefore undergone chemical modification) is beta-caryophyllene, or BCP. Cannabis contains a large amount of BCP, as do some food plants, legal herbs, and spices such as black pepper. It exists in some leafy green vegetables as well and acts essentially like a non-psychoactive anti-inflammatory [22].
The FDA has recognized terpenes and terpenoids as safe, though more research is necessary before professionals can adequately predict how cannabis terpenes can be used to treat various health conditions. Cannabinoid terpenoid interactions have been shown to be effective treatment for inflammation, addiction, depression, anxiety, epilepsy, bacterial and fungal infections, and general pain.
Approximately 200 terpenes have been identified in the cannabis plant so far. Each plant strain is made up of a unique combination of these terpenes, which affects the different tastes, smells, and effects of the different strains.
A List Of Some Terpenes And Characteristics
While there are too many terpenes and their diverse effects to list here, a few of the most popular are as follows:
Limonene: Limonene has a citrusy smell. It has potential anti-carcinogenic properties, among many other benefits. Extremely beneficial for gastrointestinal issues and pulmonary system.
Myrcene: This is the most prevalent terpene in cannabis varieties and is thought to increase the psychoactive effects of THC. It can also be used as an antiseptic and anti-inflammatory.
Linalool: Linalool, a terpene with citrusy lavender smell, has tranquilizing effects and can help those with psychosis.
Beta-caryophyllene: This terpene has a smell reminiscent of black pepper and is being studied for potential benefits in diabetes reduction and autoimmune disorders.
Alpha bisabolol: This terpene is also found in chamomile, and also has a floral flavor and scent.
Borneol: Borneol smells similar to camphor and mint and can potentially help reduce fatigue and stress.
Delta-3 carene: This terpene has a piney scent and has been found in 80 different strains in 162 cannabis plants.
Eucalyptol: Eucalyptol, predictably, smells like eucalyptus. Only small levels of this terpene are found in cannabis.
Nerolidol: This terpene smells like tree bark and has potential as a sleep aid.
Pinene: Pinene, like delta-3 carene, smells like pine. It is mostly found in citrus fruits and pine woods and has medical potential as an expectorant [22].
References
- Booz GW (2011) Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic Biol Med 51(5): 1054-1061.
- Mechoulam R, Sumariwalla PF, Feldmann M, Gallily R (2005) Cannabinoids in Models of Chronic Inflammatory Conditions. Phytochem Rev 4: 11-18.
- Burstein SH (1973) Marijuana Chemistry, Metabolism and Clinical effects. Academic Press, New York, USA.
- Klein TW (2005) Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol 5: 400-411.
- Iffland K, Grotenhermen F (2017) An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res 2: 139-154.
- Hammell DC, Zhang LP, Ma F, Abshire SM, McIlwrath SL, et al. (2016) Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain 20: 936-948.
- Jastrzab A, Gegotek A, Skrzydlewska E (2019) Cannabidiol regulates the expression of keratinocyte proteins involved in the inflammation process through transcriptional regulation. Cells 8: 827.
- Fouad AA, Albuali WH, Al-Mulhim AS, Jresat I (2013) Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environ Toxicol Pharmacol 36: 347-357.
- Rajesh M, Mukhopadhyay P, Bátkai S, Patel V, Saito K, et al. (2010) Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol 56: 2115-2125.
- Wu HY, Goble K, Mecha M, Wang CC, Huang CH, et al. (2012) Cannabidiol-induced apoptosis in murine microglial cells through lipid raft. Glia 60: 1182-1190.
- Geiotek A, Ambroziewicz E, Jastrza A, Jarocka-Karpowicz I, Skrzydlewska E (2019) Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch Dermatol Res 311: 203-219.
- Atalay S, Jarocka-Karpowicz I, Skrzydlewska E (2019) Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 9: 21.
- Gaschler MM, Stockwell BR (2017) Lipid peroxidation in cell death. Biochem Biophys Res Commun 482: 419-425
- Sun S, Hu F, Wu J, Zhanga S (2017) Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol 11: 577-585.
- Cheng D, Low JK, Logge W, Garner B, Karl T (2014) Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe /PS1ΔE9 mice. Psychopharmacology 231: 3009-3017.
- Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, et al. (2010) Cannabinoids Delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J Biol Chem 285: 1616-1626.
- Formukong EA, Evans AT, Evans FJ (1988) Analgesic and antiinflammatory activity of constituents of Cannabis sativa L. Inflammation 12: 361-371.
- Friedman, Herman, Klein, Thomas W, Specter, et al, (1991) Drugs of Abuse, Immunity, and Immunodeficiency. Plenum Press, New York. Pg no: 63-70.
- Fride E, Mechoulam R (1993) Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur J Pharmacol 231: 313-314.
- Thoppil RJ, Bishayee A (2011) Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World Journal of Hepatology 3: 228-249.
- Gallily R, Yekhtin Z, Hanuš LO (2018) The anti-inflammatory properties of terpenoids from cannabis. Cannabis Cannabinoid Res 3: 282-290.
- Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, et al. (2008) Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A 105: 9099-9104.
Citation: Yarar E (2021) Cannabinoids Terpenes and Inflammation. J Food Sci Nutr 7: 097.
Copyright: © 2021 Erhan Yarar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

