Characterization and Evaluation of Antimicrobial Activity of Bacteriocins from Lactobacillus Curvatus and Pediococcus Pentosaceus
*Corresponding Author(s):
Mona E ElyassNational Council For Research, Khartoum, Sudan
Tel:+249 9614415365,
Email:monadaliaya@gmail.com
Ahmed A Mahdi
Department Of Microbiology, Omar Al-Mukhtar University, Al-Bayda, Libyan Arab Jamahiriya
Tel:+218 911453987,
Email:mahdi.ahmed34@gmail.com
Idress Hamad Attitalla
Department Of Microbiology, Omar Al-Mukhtar University, Al-Bayda, Libyan Arab Jamahiriya
Tel:+218 913998351,
Email:idressattitalla2004@yahoo.com
Abstract
Lactobacillus Curvatus M3 and Pediococcus Pentosaceus N2, isolated from fermented beef and infant faeces, respectively, exhibited antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Enterococcus faecalis, Escherichia coli ATCC25922, Klebsiella pneumonia, Proteus vulgaris, Pseudomonas aeruginosa and Salmonella typhi. The activity was not due to H2O2 as similar inhibition was obtained by supernatants produced under aerobic or anaerobic growth conditions. The active ingredients were identified as bacteriocins of molecular mass of 3-4 kDa. The two bacteriocins were sensitive to proteinase-k and pepsin but were not affected by α-amylase, and experienced little reduction in activity on heating to 100?C for 30min but were destroyed by autoclaving. Both bacteriocins exhibited highest inhibitory activity at pH 5.0. The bacteriocin from Lb. curvatus M3 was bactericidal while that from N2 was bacteriostatic to Staph. aureus. Kinetics of growth and bacteriocin production showed that maximal inhibitory activity coincided with the phase of optimal growth and with a drop in the pH of the growth medium to 4.2-4.5.
Keywords
INTRODUCTION
Food consumers are nowadays concerned about the synthetic chemicals used as preservatives in food, with a noticeable inclination toward less processed foods, but it has to be remembered that untreated foods can harbor dangerous pathogens [1].
One of the oldest food processing techniques known to man is food fermentation. Since the dawn of civilization, methods for the fermentation of milk, meat and vegetables have been known [2]. Fermentations resulted in the development of foods with good keeping qualities and organoleptically desirable characteristics. The fermentation processes were generally artisan in nature, unaware of the role of microorganisms [3].
By the middle of the 19th century, the roles of the main microbial groups responsible for these fermentations, such as the Lactic Acid Bacteria (LAB), were being understood, and their activities started to be controlled and manipulated. Although fermentations originally aimed at preserving the fermented food items, other attributes of the process, such as imparting of unique flavors, textures and aromas, and enhancement of the keeping quality and microbiological safety, have gained prominence [3].
LAB have an important role in the inhibition of food-borne pathogenic and spoilage microorganisms such as Listeria monocytogenes, Clostridium sp and Staphylococcus sp [4]. LAB display a wide range of antimicrobial activities, important among which is the production of lactic and acetic acids. Moreover, certain strains of LAB are further known to produce bioactive molecules such as ethanol, formic acid, fatty acids, hydrogen peroxide, diacetyl, reuterin and reutericyclin [5]. In addition, many strains also produce bacteriocins and bacteriocin-like molecules that display antibacterial activity [6].
Screening for bacteriocins during the last few decades has yielded a myriad of bacteriocins with different properties, indicator species and producer organisms [7]. This has prompted researchers worldwide to pursue exploration in this area of natural and safe antimicrobial compounds. The aim of this study was to: (i) screen for the presence of bacteriocion-producing LAB in fermented beef and newborn infant faeces, (ii) characterize the bacteriocins for their heat and pH stability, enzyme action and their inhibitory spectrum against a wide range of bacteria.
MATERIALS AND METHODS
Isolation and characterization of the bacteriocinogenic bacteria
Gas production from glucose was determined in modified MRS broth containing inverted Durham tubes with diammonium citrate replaced by ammonium sulphate [13]. Glucose was sterilized separately (115ºC for 10min) and was aseptically added to the medium. Hydrolysis of arginine was tested in MRS broth without glucose or meat extract but containing 0.3% arginine and 0.2% sodium citrate instead of ammonium citrate. Ammonia was detected using Nessler’s reagent [14]. Growth at pH 4.4 and 9.6 was tested in MRS broth adjusted to these pH values using 1N HCl. Salt tolerance was tested in MRS broth supplemented with 6.5% NaCl. Growth at different temperatures was observed in MRS broth after incubation for 3 days at 15ºC and 45ºC.
Ultimately, the two isolates were identified to specific level through biochemical profiling using the KB009 HiCarbohydrate identification kit (HiMedia Laboratories, Mumbai, India), which makes use of the patterns of utilization of 35 sugars.
Screening for antagonistic activity
In the spot-on-lawn method, aliquots of about 20μL of 48-h old MRS broth cultures of each isolate were spotted on the surface of MRS agar plates (1.5% agar) and were aerobically incubated at 35 ºC for 24h. Twenty-four-h old physiological saline-washed cells of indicator bacteria [17] to be tested for sensitivity were inoculated (0.2mL of a 105CFU/mL bacterial suspension) into 7.0mL of soft Nutrient Agar medium (0.8% agar). The seeded soft agar was poured as an overlay onto the MRS plates on which the test bacteria had grown for 24h (deferred antagonism). The antagonism was detected by the formation of a growth inhibition halo of the indicator microorganism around bacteriocinogenic test isolates.
In the agar-well diffusion method, cell-free supernatants of M3 and N2 were used to inhibit growth of the indicator bacteria. The cell-free supernatants were prepared following the method of Çadirci and Çitak (2005) [17] in which 24-h old cultures were used to inoculate MRS broth (2%) in test tubes. The inoculated tubes were incubated at 30ºC for 24, 48 and 72h without shaking. At the end of the incubation period the broth cultures were centrifuged at 6000g for 15min in a centrifuge (EBA20, Zentrifugen, Tuttlingen, Germany). The cell-free supernatants were carefully decanted, filter-sterilized (pore size 0.22μm, Millipore, Bedford, Mass., USA) and were kept at 4ºC for use in the determination of their antagonistic activities against the four indicator bacteria, and for their characterization.
The test for spectrum of inhibition was later extended to cover another six bacteria, namely, Staph. aureus ATCC25923, E. coli (local isolate), Pseudomonas aeruginosa ATCC27853, Klebsiella pneumoniae ATCC10031, Proteus vulgaris ATCC6380 and Salmonella typhi ATCC1319106. To test for antagonism, molten Nutrient Agar (45-48ºC) (Oxoid,) medium was first seeded with washed cells of the indicator bacteria, and the inoculated medium was immediately poured into sterile Petri dishes. After solidification, the medium was allowed to dry for at least 30minutes at room temperature. Four wells of uniform diameter (about 5mm) were aseptically bored in the agar using a sterile Pasteur pipette. Fifty μL of the cell-free supernatants of each test isolate were dispensed into each of three wells (replicates), while sterile MRS broth was poured into the fourth well to serve as a control treatment. Plates were left to stand for at least five h at room temperature to allow diffusion of the cell-free extracts. The plates were then incubated at 30ºC for 24h. At the end of the incubation period, diameters of the resulting inhibition zones, if any, were measured and the results recorded in mm.
Characterization of the cell-free supernatants
Sensitivity of the partially purified supernatants to enzymes
Heat and pH stability
Production of the inhibitory factor during anaerobic growth
Molecular mass determination
Mode of action of the partially-purified supernatants
Kinetics of growth and bacteriocin production
RESULTS AND DISCUSSION
Spectrum of antibacterial activity
| Test strain | Staph. aureus | B. subtilis | Ent. faecalis | E. coli | ||||||||
| 24h | 48h | 72h | 24h | 48h | 72h | 24h | 48h | 72h | 24h | 48h | 72h | |
| L.curvatus M3 | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | + | ++ | ++ | ++ |
| P. pentosaceus N2 | +++ | +++ | +++ | ++ | ++ | ++ | + | + | + | + | + | + |
Table 1: Inhibition zones produced against four indicator bacteria by cell-free extracts obtained after 24, 48 and 72h of growth.
+ = 6-12 mm zone diameter; ++ = 13-19 mm; +++ = > 20 mm
Screening for the spectrum of inhibitory activity showed that seven of the 10 test bacteria were inhibited by both bacteriocins, but they could not inhibit Pseudomonas aeruginosa, Salmonella typhi or the local E. coli isolate (Table 2). Production by Lb. curvatus of different bacteriocins has been reported by many authors. For instance, Lb. curvatus LTH1174 produced curvaticin A [25,26], Lb. curvatus ACU-1 produced sakacin Q [27], Lb. curvatus FS47 produced curvaticin FS47 [28], Lb. curvatus L442 produced curvaticin L442 [29], and Lb. curvatus CRL705 produced lactocin 705 [30,31]. It has been shown that curvaticin LB65 produced by Lb. curvatus was inhibitory to Listeria monocytogenes, Staph. aureus, Enterococcus faecalis, Micrococcus leuteus, Klebsiella pnumoniae, E. coli and many LAB [32].
| Test strain | Ent.faecalis | B. subtilis | Staph. aureus | Staph. aureus | P. aeruginosa | K. pneumoniae | Proteus vulgaris | S. typhi |
E. coli ATCC 25922 |
E. coli (local) |
| L.curvatus M3 | 13 | 16 | 10 | 15 | 0.0 | 9 | 10 | 0.0 | 6 | 0.0 |
| P. pentosaceus N2 | 12 | 14 | 15 | 18 | 0.0 | 6 | 11 | 0.0 | 10 | 0.0 |
Table 2: Spectrum of inhibitory activity against ten indicator tbacteria (zone diameters, mm).
Sensitivity to enzymes
|
Test strain |
Proteinase-K | Pepsin | α -amylase | Enzyme-free supernatants | Medium with no enzyme or supernatant |
| L.curvatus M3 | 0.0 | 0.0 | 13 | 13 | 0.0 |
| P. pentosaceus N2 | 0.0 | 0.0 | 13 | 14 | 0.0 |
Table 3: Effect of enzymes on activity of cell-free supernatants obtained from two LAB isolates against Staph. aureus ATCC 43306 (Inhibition zone diameters in mm).
Heat and pH stability
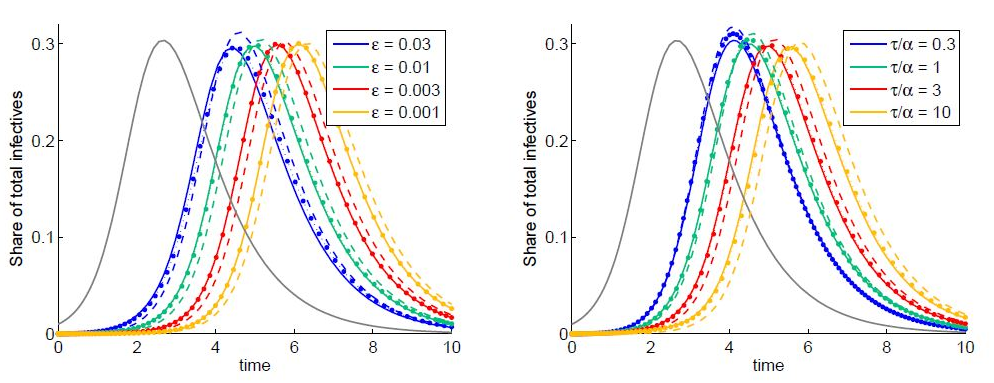
As for the effect of pH on stability, L. curvatus bacteriocin showed highest activity at pH 5.0 and a decline at pH 3.0, 7.0 and 9.0. However, the reduction in inhibitory activity was not great, amounting only to 16.7% at pH 9.0 (Figure 2). The highest inhibitory activity by the P. pentosaceus bacteriocin was recorded at pH 5.0, decreasing slightly at pH 7.0 and pH 3.0, but dropping sharply at pH 9.0 (a decrease of 41% from that at pH 5.0) as shown at figure 2. Temperature and pH have been shown to have a significant effect on bacteriocin [40]. A bacteriocin from L. acidophilus could withstand heating at 75ºC for 15 min [41]. Moreover, lactocin RN 78 produced by L. casei RN 78 withstood heating up to 121ºC for ºC 15min [42]. The bacteriocin of L. acidophilus NCIM5426 was found to be heat-stable (12°C for 15min) and active over a wide pH range of 4.0-10.0. It showed stability (60%) for 30 days at room temperature. Heat and pH stability are important characteristics that would allow bacteriocins to act over a wide range of environmental conditions.
Molecular weight determination
Interestingly, a very wide variation is seen in the molecular weight of the inhibitory substances produced by pediococci. For instance, pediocin A, produced by P. pentosaceus FBB61 had a molecular weight of 80 kDa [36], while P. pentosaceus K34 produced a bacteriocin (bacPPK34) of molecular weight of 2.5- 6.2 kDa [45], and P. pentosaceus IE-3 produced a non-bacteriocin chemical peptide of only 1.7 kDa [46]. Moreover, P. pentosaceus T1 produced an anti-listerial substance with an active fraction of molecular weight of 23 kDa [15]. The latter are more likely Bacteriocin-Like Substances (BLIS).
Mode of action of the two bacteriocins
Production kinetics
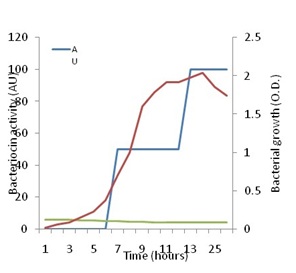
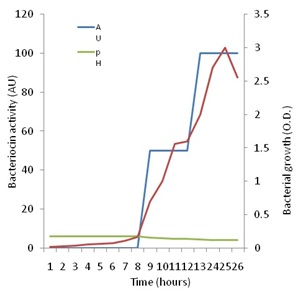
Figure 5 shows that increase in growth of P. pentosaceus N2 was detectable since the start of the investigation, but the active growth phase seemed to start after 8h from inoculation and continued up to the 25th hour after which it started to decline. On the other hand, no antagonistic activity could be detected against Staph. aureus up to eight h of growth. Thenceforth, antagonistic activity began to be detected, and showed a steady increase up to 26h of growth when the investigation was terminated. Maximal activity appeared to coincide with optimal growth, but also coincided with the drop in pH to the range of 4.5 to 4.2 cell growth and bacteriocin production are influenced by temperature and pH as has been shown by many authors [49]. These authors showed that the highest bacteriocin activity occurred at a low constant temperature of 28ºC and a constant pH of 5.4. The present results are in line with the finding that bacteriocin production is stimulated under unfavorable growth conditions such as low pH [13].
CONCLUSION
This study determined the bacteriocinogenic capabilities of Lactobacillus Curvatus M3 and Pediococcus Pentosaceus N2 isolated from traditional fermented Sudanese beef (sharmoot) and newborn infant’s faeces, respectively. Results of the study indicated the possible food preservation (M3) and clinical (N2) benefits of the two bacteria, and their possible utilization in these two aspect.
REFERENCES
- Ismail BB, Yusuf HL (2014) Consumer concerns about the use of additives in processed foods. Int J Cur Res 6: 65- 72.
- Soomoro AH, Masud T, Anwaar K (2002) Role of Lactic Acid Bacteria (LAB) in food preservation and human health - a review. Pak J Nutr 1: 20-24.
- Alexandraki V, Tsakalidou E, Papadimitriou K, Holzapfel W (2013) Status and trends of the conservation and sustainable use of microorganisms in food processes. FAO Background Study Paper No. 65. FOOD and Agriculture Organization, Rome.
- Galvez A, Lopez RL, Abriouel H, Valdivia E, Omar NB (2008) Application of bacteriocins in the control of foodborne pathogenic and spoilage bacteria. Crit Rev Biotechnol 28: 125-152.
- De Vuyst L, Leroy F (2007) Bacteriocins from lactic acid bacteria: production, purification, and food applications. J Mol Microbiol Biotechnol 13: 194-199.
- De Vuyst L, Vandamme EJ (1994) Antimicrobial potential of lactic acid bacteria. In: De Vuyst L, Vandamme EJ (eds.). Bacteriocins of Lactic Acid bacteria: Microbiology, Genetics and Applications. Springer US, USA. Pg no: 91-142.
- Ennahar S, Sonomoto K, Ishizaki A (1999) Class IIa bacteriocins from lactic acid bacteria: antibacterial activity and food preservation. J Biosci Bioeng 87: 705-716.
- de Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Appl Bacteriol 23: 130-135.
- Axelsson L (2004) Lactic acid bacteria: Classification and physiology. In: Salminen S, Wright AV, Ouwehand A (eds.). Lactic Acid Bacteria: Microbiological and Functional Aspects (3rd edn). Marcel Dekker, New York.
- Schillinger U, Lüke FK (1987) Identification of lactobacilli from meat and meat products. Food Microbiol 4: 199-208.
- Stiles ME, Holzapfel WH (1997) Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol 36: 1-29.
- Wood BJB, Holzapfel WH (1995) The Genera of Lactic Acid Bacteria. Springer US, USA.
- Samelis J, Roller S, Metaxopuolos J (1994) Sakacin B, a bacteriocin produced by Lactobacillus sake isolated from Greek dry fermented sausages. J Appl Bacteriol 76: 475- 486.
- Schillinger U, Lücke FK (1989) Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol 55: 1901-1906.
- Jang S, Lee J, Jung U, Choi H-S, Suh HJ (2014) Identification of an anti-listerial domain from Pediococcus pentosaceus derived from Kimchi, a traditional fermented vegetable. Food Control 43: 42-48.
- Barefoot SF, Klaenhammer TR (1983) Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl Environ Microbiol 45: 1808-1815.
- Çadirci BH, Çitak S (2005) A comparison of two methods used for measuring antagonistic activity of lactic acid bacteria. Pak J Nutr 4: 237-241.
- ten Brink B, Minekus M, van der Vossen JM, Leer RJ, Huis in't Veld JH (1994) Antimicrobial activity of lactobacilli: preliminary characterization and optimization of production of acidocin B, a novel bacteriocin produced by Lactobacillus acidophilus M46. J Appl Bacteriol 77: 140-148.
- Ivanova I, Miteva V, Stefanova Ts, Pantev A, Budakov I, et al. (1998) Characterization of a bacteriocin produced by Streptococcus thermophilus 81. Int J Food Microbiol 42: 147-158.
- Lewus CB, Montville TJ (1991) Detection of bacteriocins produced by lactic acid bacteria. J Microbiol Meth 13: 145-150.
- Faye T, Langsrud T, Nes IF, Holo H (2000) Biochemical and genetic characterization of propionicin T1, a new bacteriocin from Propionibacterium thoenii. Appl Environ Microbiol 66: 4230-4236.
- Nilsen T, Nes IF, Holo H (2003) Enterolysin A, a cell wall-degrading bacteriocin from Enterococcus faecalis LMG 2333. Appl Environ Microbiol 69: 2975-2984.
- Ghrairi T, Frere J, Berjeaud JM, Manai M (2008) Purification and characterisation of bacteriocins produced by Enterococcus faecium from Tunisian rigouta cheese. Food Control 19: 162 -169.
- van Reenen CA, Dicks LM, Chikindas ML (1998) Isolation, purification and partial characterization of plantaricin 423, a bacteriocin produced by Lactobacillus plantarum. J Appl Microbiol 84: 1131-1137.
- Mataragas M, Drosinos EH, Tsakalidou E, Metaxopoulos J (2004) Influence of nutrients on growth and bacteriocin production by Leuconostoc mesenteroides L124 and Lactobacillus Curvatus L442. Antonie Van Leeuwenhoek 85: 191-198.
- Rivas FP, Castro MP, Vallejo M, Marguet E, Campos CA (2014) Sakacin Q produced by Lactobacillus Curvatus ACU-1: functionality characterization and antilisterial activity on cooked meat surface. Meat Sci 97: 475-479.
- Garver KI, Muriana PM (1994) Purification and partial amino acid sequence of curvaticin FS47, a heat-stable bacteriocin produced by Lactobacillus Curvatus FS47. Appl Environ Microbiol 60: 2191-2195.
- Mataragas M, Metaxopoulos J, Drosinos E (2002) Characterization of two bacteriocins produced by Leuconostoc mesenteroides L124 and Lactobacillus Curvatus L442, isolated from dry fermented sausages. World J Microbiol Biotechnol 18: 847- 856.
- Castellano P, Holzapfel W, Vignolo G (2004) The control of Listeria innocua and Lactobacillus sakei in broth and meat slurry with the bacteriocinogenic strain Lactobacillus casei CRL705. Food Microbiol 21: 291- 298.
- Vignolo G, Palacios J, Farías ME, Sesma F, Schillinger U, et al. (2000) Combined effect of bacteriocins on the survival of various Listeria species in broth and meat system. Curr Microbiol 41: 410-416.
- Abdelbasset M, Manel D, Djamila K (2014) Purification and characterization of a novel bacteriocin produced by Lactobacillus Curvatus LB65 isolated from Algerian traditional fresh cheese (Jben). Advances in Experimental Biology 8: 1222- 1232.
- Strasser de Saad AM, Pasteris SE, Manca de Nadra MC (1995) Production and stability of pediocin N5p in grape juice medium. J Appl Bacteriol 78: 473-476.
- Todorov SD, Dicks LMT (2005) Pediocin ST18, an antilisterial bacteriocin produced by Pediococcus Pentosaceus ST18 isolated from boza, a traditional cereal beverage from Bulgaria. Process Biochem 40: 365- 370.
- Swetwiwathana A (2005) Microbiological quality enhancement of Thai fermented meat product (Nham) using Nham-associated pediocin-producing lactic acid bacteria (Pediococcus Pentosaceus TISTR 536). Ph.D. Thesis, Department of Bioscience and Biotechnology, Kyushu University, Japan.
- Todorov SD, Dicks LM (2009) Bacteriocin production by Pediococcus Pentosaceus isolated from marula (Scerocarya birrea). Int J Food Microbiol 132: 117-126.
- Heng NCK, Wescombe PA, Burton JP, Jack RW, Tagg JR (2007) The Diversity of bacteriocins in Gram-positive bacteria. In: Riley MA, Chavan MA (eds.). Bacteriocins: Ecology and Evolution. Springer-Verlag, Berlin, Heidelberg.
- Nghe D, Nguyen T (2014) Characterization of antimicrobial activities of Pediococcus Pentosaceus Vtcc-B-601. J Appl Pharm Sci 4: 61- 64.
- Schillinger U, Lücke FK (1989) Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol 55: 1901-1906.
- Elamathy S, Kanchana D (2013) Characterization of heat stable and inhibitory activity of bacteriocin produced by Lactobacillus acidophilus. J Chem Tech 5: 1281-1283.
- Mojgani N, Amirinia C (2007) Kinetics of growth and bacteriocin production in L. casei RN 78 isolated from a dairy sample in Iran. Int J Diary Sci 2: 1- 12.
- Messens W, Verluyten J, Leroy F, De Vuyst L (2003) Modelling growth and bacteriocin production by Lactobacillus Curvatus LTH 1174 in response to temperature and pH values used for European sausage fermentation processes. Int J Food Microbiol 81: 41-52.
- Strasser de Saad AM, Pasteris SE, Manca de Nadra MC (1995) Production and stability of pediocin N5p in grape juice medium. J Appl Bacteriol 78: 473-476.
- Abrams D, Barbosa J, Albano H, Silva J, Gibbs PA et al. (2011) Characterization of bacPPK34, a bacteriocin produced by Pediococcus Pentosaceus strain K34 isolated from “Alheira”. Food Control 22: 940- 946.
- Singh PK, Sharma S, Kumari A, Korpole S (2014) A non-pediocin low molecular weight antimicrobial peptide produced by Pediococcus Pentosaceus strain IE-3 shows increased activity under reducing environment. BMC Microbiol 14: 226-234.
- Bouttefroy A, Millière JB (2000) Nisin-curvaticin 13 combinations for avoiding the regrowth of bacteriocin resistant cells of Listeria monocytogenes ATCC 15313. Int J Food Microbiol 62: 65-75.
- Díez L, Rojo-Bezares B, Zarazaga M, Rodríguez JM, Torres C, et al. (2012) Antimicrobial activity of pediocin PA-1 against Oenococcus oeni and other wine bacteria. Food Microbiol 31: 167-172.
- Neysens P, Messens W, Gevers D, Swings J, De Vuyst L (2003) Biphasic kinetics of growth and bacteriocin production with Lactobacillus amylovorus DCE 471 occur under stress conditions. Microbiology 149: 1073-1082.
- De Vuyst L, Callewaert R, Crabbe K (1996) Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology 142: 817- 827.
Citation: Elyass ME, Altayar MA, Mahdi AA, Abdelrawaf SS, Shigidi MT, et al. (2015) Characterization and Evaluation of Antimicrobial Activity of Bacteriocins from Lactobacillus Curvatus and Pediococcus Pentosaceus. J Infect Non Infect Dis 1: 001.
Copyright: © 2015 Mona E Elyass, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.