
Characterization of Polyphenolics in Grape Pomace Extracts Using ESI Q-TOF MS/MS
*Corresponding Author(s):
Zhu MJSchool Of Food Science, Washington State University, Pullman, Washington, United States
Tel:+1 5093354815,
Fax:+1 5093354016
Email:meijun.zhu@wsu.edu
Abstract
Background: Grape pomaces are rich sources of polyphenolics that are reportedly beneficial for health, but their content and quality between red and white pomaces have not been systemically compared.
Methods and findings: The current study compared polyphenolics from different grape extracts and further characterized A-type Proanthocyanidins (PAC), which have not been studied previously. The total contents of polyphenolics, flavonoids and PAC and total antioxidant activities of Red Grape Pomace Extract (RGPE) were higher than those in White Grape Pomace Extract (WGPE). Using direct-infusion electrospray ionization tandem mass spectrometry, glucosides of quercetin and peonidin were detected in both RGPE and WGPE, while quercetin, malvidin derivatives and petunidin 3-p-coumaroylgluside only found in RGPE. (epi)catechins, B-type PACs, A-type PAC dimers, and single A-type linked PAC trimers and tetramers were detected in RGPE, WGPE and Grape Seed Extract (GSE). Other singly and doubly charged A-type PACs only detected in GSE. Furthermore, monogalloylated A-type dimers with (epi)cat and (epi)afz were detected in both GSE and WGPE.
Conclusion: RGPE contains relatively greater amounts of polyphenolics than WGPE, and more A-type PAC was detected in GSE. The total antioxidant content was higher in RGPE than WGPE.
Keywords
INTRODUCTION
Grape pomace is the major byproduct of the wine and juice industry, which is rich in polyphenols including flavonoids (anthocyanins, flavanols, flavonols, and flavanones) and non-flavonoids (phenolic acids and their derivatives, stilbenes, and lignans) [1]. Grape Seed Extracts (GSE) are known for their anti-oxidative and anti-inflammatory effects, and exert various physiological benefits including anti-carcinogenic, anti-aging, anti-diabetic, and cardioprotective effects [2]. Recent studies also show their roles in regulation of intestinal barrier and prevention of intestinal inflammatory diseases [3,4]. However, polyphenolic content and composition of GSE, red and white grape pomace have not been systemically compared, and these contents also differ due to grape cultivar varieties, environmental conditions and geological locations where grapes are produced; as a result, the efficacy of extracts in preventing diseases and protecting health varies. Characterization of polyphenols is not only critical for the quality control of extracts, but also mechanistic studies exploring their biological efficacy.
Flavan-3-ols are the main polyphenolics in grapes, which in general are monomeric catechin (cat) and epicatechin (epicat) and their oligomeric and polymeric (epi)cat known as Proanthocyanidins (PACs) (n ≤ 5, oligomers and n> 5, polymers). Proanthocyanidins have two types of linkages: B-type has only single linkage of C4-C6’ or C4-C8’ between (epi)cat units while A-type has double linkages between C4-C8' and O7'-C2. In general, B-type PACs consist of only B-type linkages, while A-type PACs have A-type linkages in addition to the B-type bonds [5]. Currently, the A-type PACs and their derivatives have not been well-characterized in grapes and their products [6,7], which leads to an important knowledge gap, considering A-type and B-type PACs may have different bioactivity. Indeed, an A-type PAC dimer from cranberry was more effective than those enriched in B-types in inhibiting in vitro bacterial adherence [8]. Furthermore, PACs rich in A-type linkages were more effective in the inhibition of pancreatic lipase activity than that in B-types [9]. In this study, we characterized polyphenols in Red and White Grape Pomace Extracts (RGPE and WGPE), further characterized and compared the main compounds among RGPE, WGPE and commercial GSE especially A-type PACs using direct infusion Electrospray Ionization (ESI) tandem mass spectrometry.
MATERIALS AND METHODS
Grape pomaces and chemicals
Ethanol, catechin, rutin, gallic acid, formic acid, glacial acetic acid, vanillin, aluminum chloride, DPPH• (2,2-diphenyl-1-picrylhydrazyl) radical, and Folin-Ciocalteu’s reagent were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Sodium carbonate and sodium acetate were from JT Baker (Center Valley, PA, USA).
Sample preparation
Chemical composition and antioxidant activity of Grape Pomace Extracts (GPEs)
Total phenolic content: Total phenolic content was determined using the modified Folin-Ciocalteu procedure [10]. Gallic acid (1.0 to 50.0 μg/ml) was used to generate the standard curve. In brief, 200 µL of diluted GPEs and standard solutions were added into each well, followed by 12.5 µL of Folin-Ciocalteu’s reagent and then 37.5 µL of 20% Na2CO3. The absorbance at 760 nm was read after 2 hour incubation. The results were expressed as milligrams of gallic acid equivalent per gram of dried pomace weight (mg GAE/g DW).
Total flavonoid content: The modified AlCl3-acetate method [11] was used to measure total flavonoid content. Briefly, 50 µL of diluted GPEs and standard solutions were mixed with 150 µL of 5% AlCl3 and 50 µL of acetate buffer (pH 5.0) sequentially. The absorbance at 420 nm was measured after one hour incubation at room temperature. Rutin was used as the standard with a range of 4.0 to 148.0 μg/ml and total flavonoid content was expressed as milligrams of rutin equivalent per gram of dried pomace weight (mg RE/g DW).
Total PAC content: Total PAC content was analyzed by the modified Vanillin-HCl procedure [12]. In brief, 50 µL of diluted GPEs and standard solutions were mixed with 150 µL of 4% vanillin and 50 µL of concentrated hydrochloride. The absorbance was measured at 500 nm after incubation for 30 min. The total PAC content was determined using catechin as a standard ranging between 5.0 and 250.0 μg/ml. Data were expressed as milligrams of catechin equivalent per gram of dried pomace weight (mg CE/g DW).
DPPH radical scavenging assay: The total antioxidant activity in GPEs was determined by the ability to scavenge DPPH• [13]. 200 µL of DPPH• solution (6 x 10-5 M) were added into microplate wells containing 50 µL of diluted GPEs or standards. The DPPH•scavenging activity was measured at 515 nm after 90 min incubation at room temperature. Data were expressed as mg CE/g DW that was calculated by the standard curve of catechin in the range of 0.3 to 6.0 μg/mL. The percent inhibition of DPPH• radical scavenging activity was calculated by the following equation: inhibition (%) =
Characterization of grape extracts using ESI Q-TOF-MS/MS
The relative percentages of (epi)cat and PACs were calculated from their respective peak intensities divided by total peak intensities of (epi)cat and PACs including galloylated PACs.
Statistical analysis
RESULTS AND DISCUSSION
Physical properties of grape pomace
Polyphenolics in grape pomace extracts
| RGPE | WGPE | |
| Total phenolics (mg GAE/g DW) | 69.83 ± 4.53 | 58.15 ± 5.21* |
| Total flavonoids (mg RE/g DW) | 43.89 ± 1.22 | 14.32 ± 1.67* |
| Total Proanthocyanidins (mg CE/g DW) | 133.79 ± 6.74 | 92.10 ± 6.00* |
| Antioxidant activity (mg CE/g DW) | 74.48 ± 1.12 | 58.66 ± 1.92* |
| DPPH• inhibition (%) | 68.28 ± 0.52 | 62.74 ± 1.34* |
Total antioxidant activities
TOF-MS profiles and main components of RGPE, WGPE and GSE
| Compound | Precursor ion | Product Ions (MS/MS) | GSE | RGPE | WGPE | |
| Measured | Calculated | |||||
| Organic acids and flavonols | ||||||
| Malic acid | 133.0157 | 133.0137 | + | + | ||
| Tartaric acid | 149.0080 | 149.0086 | + | + | ||
| Gallic acid | 169.0109 | 169.0137 | 125 | + | + | + |
| Caffeic acid | 179.0451 | 179.0344 | 135 | + | ||
| Citric acid | 191.0220 | 191.0192 | + | + | ||
| Quercetin | 301.0332 | 301.0348 | 273, 257, 229,179, 151, 137 | + | ||
| Quercetin 3-glucoside | 463.0952 | 463.0877 | 300, 133 | + | + | + |
| Quercetin 3-glucuronide | 477.0767 | 477.0669 | 301, 133 | + | + | + |
| Anthocyanines | ||||||
| Peonidin 3-glucoside | 463.0934* | 463.1240 | 300, 301 | + | + | |
| Petunidin 3-glucoside | 479.0968* | 479.1190 | 303, 317 | + | + | |
| Malvidin 3-glucoside | 491.1352 | 491.1190 | 329, 149 | + | + | |
| 493.1323* | 493.1346 | 331, 315, 287, 270, 242 | + | |||
| Peonidin 3-acetylglucoside | 505.1450* | 505.1346 | 301, 219, 145, 127 | + | + | |
| Malvidin 3-acetylglucoside | 535.1390* | 535.1454 | 331, 315, 287, 270, 242 | + | ||
| Petunidin 3-p-coumaroylglucoside | 625.1512* | 625.1557 | 463, 354, 317 | + | ||
| Mavindin 3-p-coumaroylglucoside | 639.1714* | 639.1714 | 463, 331, 315, 287, 270, 242 | + | ||
| Malvidin 3-(6-O-caffeoyl) monoglucoside | 655.1688* | 655.1663 | 381, 331, 301 | + | ||
| Monogalloylated (epi)cat oligomers | ||||||
| Monogalloylated A-type dimers of (epi)cat and (epi)afz | 711.1318 | 711.1350 | 693, 559, 423, 407, 289, 285, 137 | + | + | |
| 713.1506* | 713.1506 | 695, 561, 425, 409, 289, 287, 139 | + | |||
| Monogalloylated B-type PAC dimmers | 729.1400 | 729.1456 | 603, 577, 575, 559, 441, 407, 289, 169 | + | + | + |
| 731.1501* | 731.1612 | + | + | + | ||
| Monogalloylated B-type PAC trimers | 1017.2156 | 1017.2089 | 891, 865, 729, 695, 577, 575, 407, 289, 287 | + | + | + |
| 1019.1840* | 1019.2246 | 867, 731, 579, 577, 441, 381, 291, 289, 219 | + | + | ||
| Monogallyolated B-type PAC tetramers | 1305.2811 | 1305.2723 | 1179, 1153, 1017, 1015, 865, 863, 729, 727, 577, 575, 289, 287 | + | + | + |
| 1307.2263* | 1307.2880 | 1155, 1019, 1017, 867, 731, 729, 579, 577, 493, 381, 291, 289, 219 | + | + | ||
| (epi)catechins and PACs | ||||||
| (epi)catechin | 289.0698 | 289.0712 | 271, 245, 205, 179, 151, 137 | + | + | + |
| 291.0896* | 291.0869 | 273, 249, 207, 169, 165, 151, 147, 139, 123 | + | + | + | |
| A-type PAC dimers | 575.1196 | 575.1189 | 539, 449, 423, 407, 289, 285 | + | + | + |
| 577.1323* | 577.1345 | 559, 437, 451, 409, 425, 299, 289, 287 | + | + | + | |
| B-type PAC dimers | 577.1385 | 577.1345 | 559, 451, 425, 407, 289, 287 | + | + | + |
| 579.1466* | 579.1502 | 561, 453, 427, 409, 397, 301, 291, 289, 287, 275, 163 | + | + | + | |
| B-type PAC trimers | 865.1990 | 865.1979 | 739, 713, 695, 577, 575, 449, 451, 425, 407, 289, 287 | + | + | + |
| 867.2170* | 867.2136 | 715, 697, 579, 577, 559, 535, 495, 427, 381, 291, 289, 287 | + | + | + | |
| B-type PAC tetramers | 1153.2675 | 1153.2613 | 1027, 1001, 983, 865, 863, 693, 577, 575, 425, 407, 289, 287 | + | + | + |
| 1155.2339* | 1155.2769 | 1003, 867, 865, 579, 577, 493, 381, 291, 289, 219 | + | + | + | |
| B-type PAC pentamers | 1441.3232 | 1441.3247 | 1153, 1151, 865, 863, 577, 575, 289, 287 | + | + | + |
| 1443.2625* | 1443.3403 | 1266, 1155, 1153, 867, 865, 579, 577, 493, 381, 291, 289, 219 | + | + | + | |
| B-type PAC hexamers | 1729.3572 | 1729.3882 | + | + | + | |
| 1731.2931* | 1731.4037 | + | + | + | ||
| Double A-type linked PAC trimers | 861.1669 | 861.1666 | 843, 735, 709, 693, 691, 575, 573, 571, 449, 421, 411, 289, 287, 285 | + | ||
| 863.1572* | 863.1823 | + | ||||
| Single A-type linked PAC trimers | 863.1818 | 863.1823 | 737, 711, 693, 575, 573, 559, 449, 451, 423, 411, 407, 289, 285 | + | + | + |
| 865.1859* | 865.1979 | 847, 713, 695, 577, 533, 467, 453, 289, 287, 247 | + | |||
| Triple A-type linked PAC tetramers | 1147.2241 | 1147.2143 | 979, 735, 575, 573, 447, 411, 287, 285, 245 | + | ||
| Double A-type linked PAC tetramers | 1149.2367 | 1149.2300 | 997, 979, 863, 861, 859, 575, 573, 449, 411, 289, 287, 285 | + | ||
| 1151.2112* | 1151.2456 | + | ||||
| Single A-type linked PAC tetramers | 1151.2512 | 1151.2456 | 999, 997, 981, 979, 863, 861, 693, 691, 575, 573, 411, 289, 287, 285 | + | + | + |
| 1153.2280* | 1153.2613 | 999, 865, 863, 713, 577, 575, 533, 287, 289, 247, 127 | + | |||
| Triple A-type linked PAC pentamers | 1435.2791 | 1435.2777 | 1283, 1147, 861, 709, 575, 411, 285, 125 | + | ||
| Double A-type linked PAC pentamers | 1437.2924 | 1437.2935 | 1285, 1267, 1149, 863, 861, 573, 575, 411, 289, 287, 285 | + | ||
| 1439.2556* | 1439.3090 | + | ||||
| Single A-type linked PAC pentamers | 1439.3209 | 1439.3090 | + | |||
| 1441.2583* | 1441.3247 | + | ||||
| Triple A-type linked PAC hexamers | 1723.3336 | 1723.3366 | + | |||
| 1725.2617* | 1725.3522 | + | ||||
| Double A-type linked PAC hexamers |
1725.3405
|
1725.3522 | 1437, 1435, 1151, 1149, 863, 861, 575, 573, 411, 287, 285 | + | ||
| 1727.3010* | 1727.3679 | + | ||||
| Single A-type linked PAC hexamers | 1727.3635 | 1727.3679 | + | |||
| 1729.2954* | 1729.3882 | + | ||||
| Doubly charged A-type PACs | ||||||
| 1-3 A-type linked PAC pentamers | 717.1359 | 717.1351 | ||||
| 718.1342 | 718.1428 | Double charged, see insert in figure 2A | + | |||
| 719.1429 | 719.1507 | |||||
| 1-3 A-type linked PAC heptamers | 1005.2025 | 1005.1984 | ||||
| 1006.1969 | 1006.2062 | Double charged, see insert in figure 2A | + | |||
| 1007.2153 | 1007.2140 | |||||
| 1007.1904* | 1007.2140 | |||||
| 1008.1979* | 1008.2219 | Double charged, see insert in figure 2B | + | |||
| 1009.1942* | 1009.2297 | |||||
| 1-4 A-type linked PAC nonamers | 1292.2518 | 1292.2539 | ||||
| 1293.2599 | 1293.2618 | Double charged, see insert in figure 2A | + | |||
| 1294.2683 | 1294.2696 | |||||
| 1295.2773 | 1295.2774 | |||||
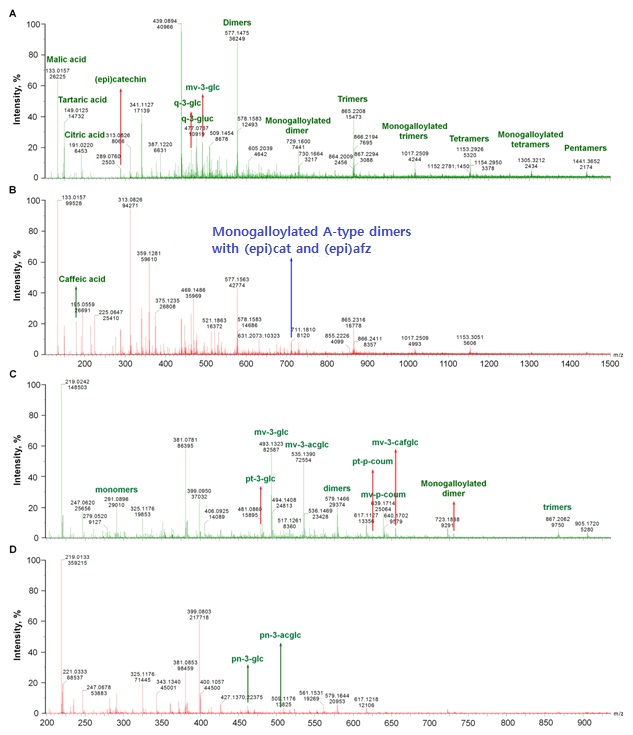
Organic acids, (epi)catechins and anthocyanins
The [M-H]- ion at m/z 301 were detected only in RGPE, which likely is quercetin. Its fragment ions mainly are m/z 273 [M-H-28(CO)]-, 257 [M-H-44(CO2)]-, 229 [M-H-44(CO2)-28(CO)]- (Table 2), which was similar to a previous report [24].
At ESI-, the [M-H]- ions at m/z 463 and 477 were observed in RGPE, WGPE and GSE, which might be quercetin 3-glucoside and quercetin 3-glucuronide with the fragment at m/z 300 (loss of a glucosyl unit) and 301 (loss of a glucuronate group), respectively (Table 2). Both of them were reported previously in grape skin at ESI- [25].
ESI+ signals attributable to anthocyanins were observed in grape pomace extracts. The [M+H]+ ion at m/z 479*(stands precursor ions generated from ESI+ mode) was detected in both RGPE and WGPE (Figure 1 C,D), which was assigned to petunidin-3-glucoside confirmed by its fragments at m/z 303 and 317 (Table 2) [26]. The [M+H]+ ions at m/z 463*, 493*, 505*, 535*, 625*, 639*, and 655* could be assigned to peonidin 3-glucoside, malvidin 3-glucoside, peonidin 3-acetylglucoside, malvidin 3-acetylglucoside, petunidin 3-p-coumaroylglucoside, and malvidin 3-p-coumaroylglucoside,and malvidin 3-(6-O-caffeoyl) monoglucoside (Figure 1 C,D) confirmed by their fragments of 301 (loss of 162 Da, a glucosyl unit), 331 (loss of 162 Da), 301 (loss of 204 Da, an acetyl glucosyl unit), 331 (loss of 204 Da), 317 (loss of 308 Da, a coumaroylglucosyl unit), 331 (loss of 308 Da), and 331 (lose of 224 Da, a caffeoylglucosyl unit) (Table 2), respectively [26-28]. Of which, peonidin 3-glucoside and peonidin 3-acetylglucoside were detected in both RGPE and WGPE, while malvidin derivatives and petunidin 3-p-coumaroylgluside only found in RGPE (Table 2).
The [M-H]- ion at m/z 491 could be assigned to malvidin 3-glucoside with the fragment at m/z 329 (loss of a glucosyl unit), which was only detected in RGPE and WGPE and was reported previously in grape skin as well [25].
Catechin and epicatechin were found in all grape extracts at both ESI+ and ESI- with the precursor ion at m/z 291* and 289, respectively (Figure 1) and backed by their characteristic fragmentations (Table 2) mainly via loss of one water for both of them, RDA (loss of 152 Da), HRF (loss of 126 Da) and BFF for the m/z 291* precursor ion, and loss of a -CH2-CHOH group or CO2, loss of C4H4O2 from the A-ring and C6H6O2 from B-ring for the m/z 289 to generate corresponding fragments [20,29,30].
Under ESI+ the monogalloylated B-type dimers at m/z 731* were detected in RGPE, WGPE and GSE; while the monogalloylated B-type trimers (m/z 1019*) and tetramers (m/z 1307*)were presented only in RGPE and GSE (Table 2). They all have similar fragmentation pattern as those at ESI-.
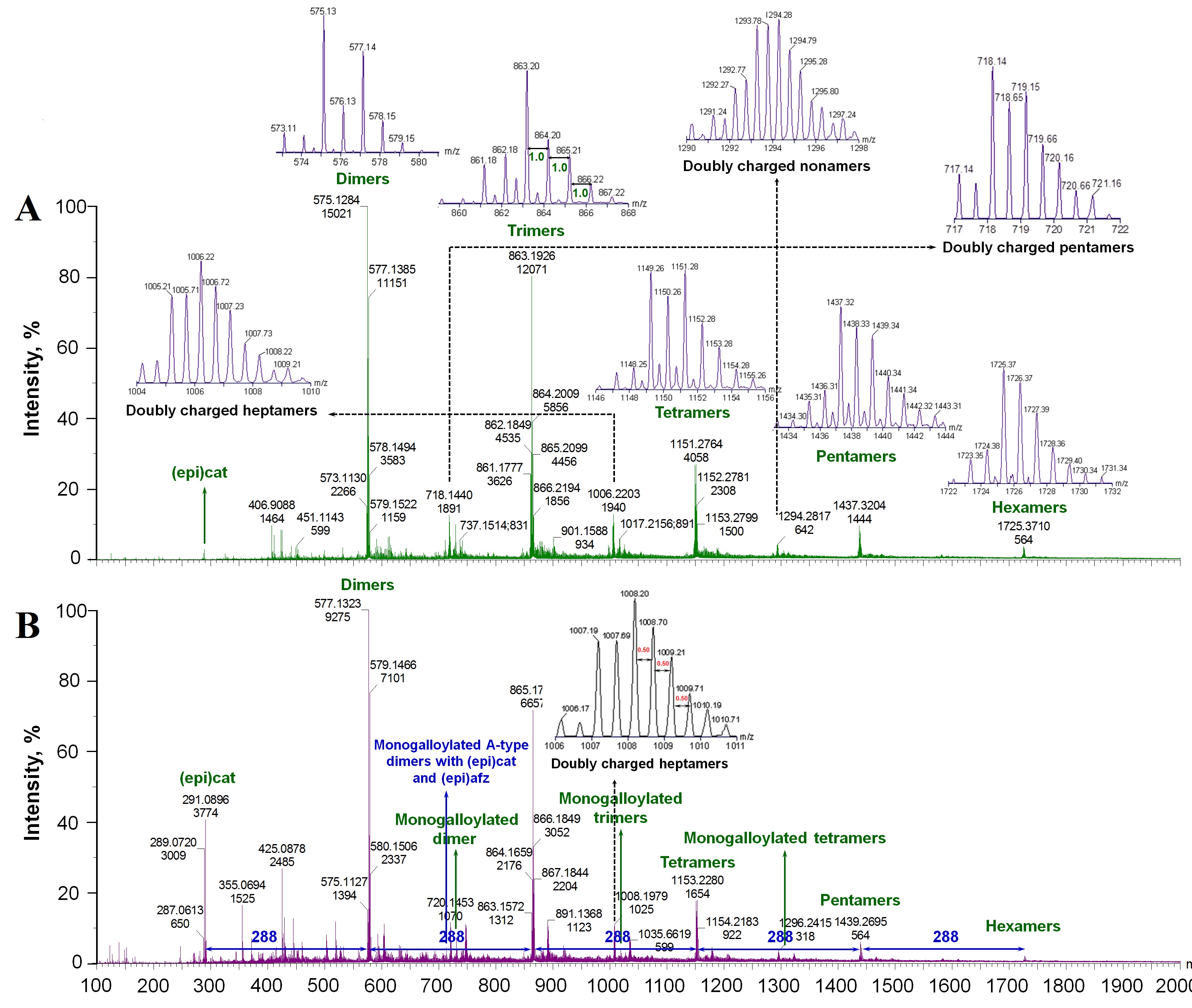
A-type PACs: A-type PACs were previously reported in other foods such as peanut skins, hops, and raspberry. However, they have been barelyreported in grapes and their products [6,34], which were characterized in this study.
The observed [M-H]- ions at m/z 575, 863, 1151, 1439, and 1727 revealed a series of compounds with a mass difference of 288 Da that can be attributed to A-type PAC dimers, trimers, tetramers, pentamers and hexamers, respectively. They displayed 2 amu difference from the corresponding B-type PACs at m/z 577, 865, 1153, 1441 and 1729 [5,7,24,31,35,36]. Further, the observed [M+H]+ ions for A-type PAC dimers to hexamers at m/z 577*, 865*, 1153*, 1441* and 1729* also present 2 amu difference from the corresponding B-types at m/z 579*, 867*, 1155*, 1443* and 1731* [6,20,21] (Figure 1,2 and Table 2).
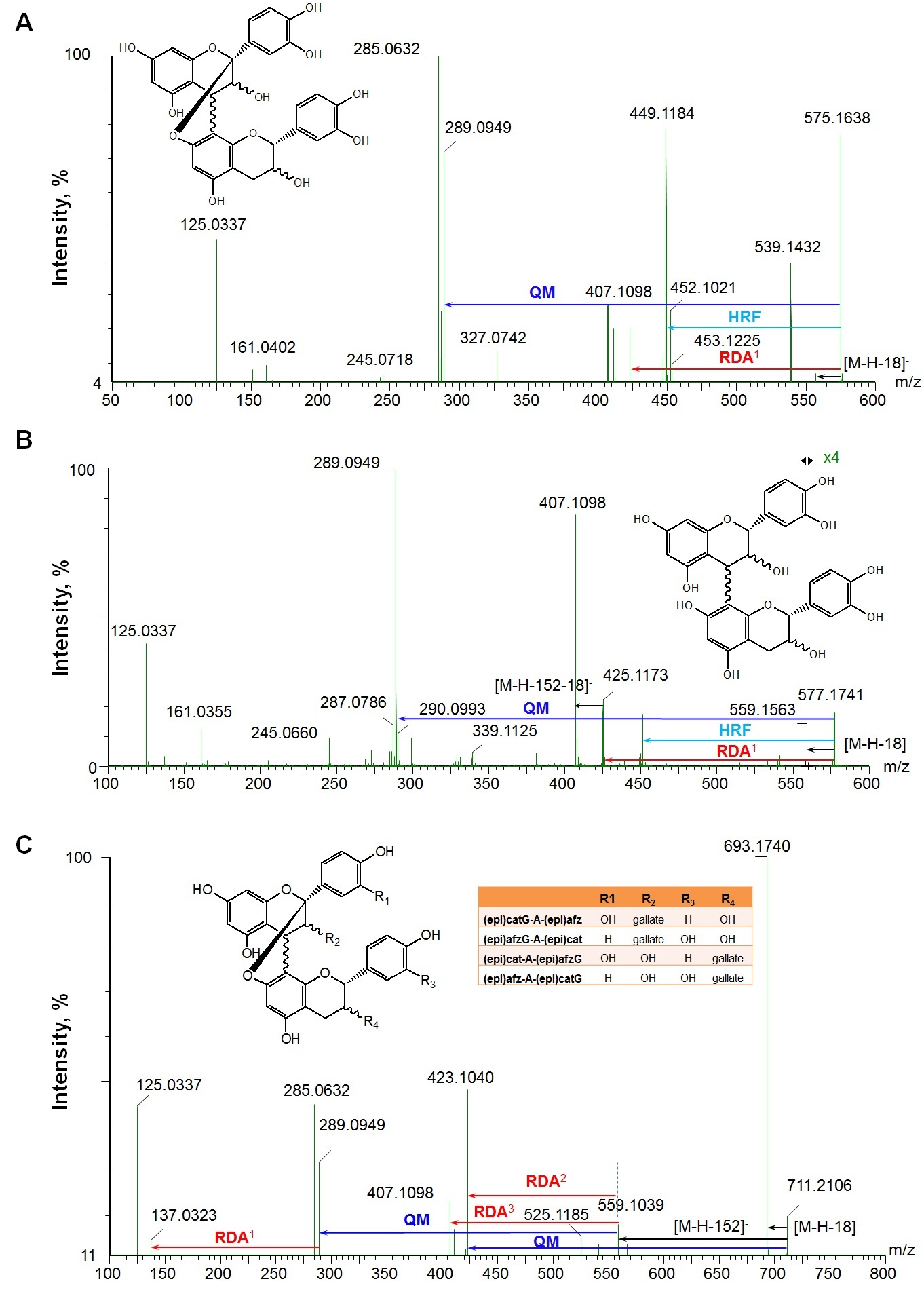
Figure 3, showed the fragmentation patterns for three selected precursor ions at ESI- mode. Figure 3A and 3B, showed the fragment pathways of A-type (m/z 575) and B-type (m/z 577) dimers. Their characteristic fragmentations are mainly via HRF (loss of 126 Da), RDA (loss of 152 Da) and QM cleavages at the top and bottom units (Table 2), which are consistent with previous reports at ESI- for A-type [5,24,37-39] and for B-type [5,7,29,33,35,39]. At ESI+, the main fragments for both A-type (m/z 577*) and B-type (m/z 579*) dimers (Table 2) are also similar to previous reports [20,21].
RDA: Retro-Diels-Alder fission; QM: Quinone-Methide fission; HFR: Heterocyclic Ring Fission; [M-H-152]-in C: this fragment comes from two possible ways: 1) loss of one galloyl group, 2) via RDA if the terminal unit is (epi)cat. RDA1 means loss of 152 Da from precursor ions (A,B) or the fragment of m/z 289 (C); RDA2: this fragment generates from the fragment of m/z 559 (loss one galloyl group) through RDA if the terminal unit is or to become (epi)afz (lose 136 Da) (C); RDA3: this is from the fragment of m/z 559 (loss one galloyl group) through RDA if the terminal unit is or to become (epi)cat (lose 152 Da) (C), HRF, [M-H-126]-; QM, lose one (epi)catechin; “x 4” means the fragment of m/z 559 is zoomed in by 4 times
One monogalloylated A-type PAC dimer with the [M-H]- at m/z 711 was observed in WGPE and GSE, which gave the MS/MS fragments at m/z 693 ([M-H-18]-, loss of one water), 559 ([M-H-152]-, loss of a galloyl group or via RDA if the terminal unit is (epi)cat), 423 (QM of m/z 711 or from the fragment ion at m/z 559 via RDA if the terminal unit is or to be (epi)afz), 407 (from the ion at m/z 559 via RDA if the terminal unit is or to be (epi)cat), 289 (QM cleavage from terminal unit of (epi)cat while the top unit is (epi)afz after loss of galloyl group and 285 (QM cleavage from terminal unit of (epi)afz while the top unit is (epi)cat after loss of galloyl group), and 137 (RDA of fragment at m/z 289) (Figure 3C and Table 2). It could be (epi)catG-A-(epi)afz, (epi)afzG-A-(epi)cat, (epi)cat-A-(epi)afzG, or (epi)afz-A-(epi)catG [(epi)afz G, (epi)afzelechin 3-O-gallate].
A-type dimers and B-type PAC dimers to hexamers were detected in all grape extracts under both ESI- and ESI+. In addition, single A-type linked PAC trimers and tetramers can be detected in GSE, RGPE and WGPE under ESI- (Table 2). Otherwise, all A-type PACs described below only detected in GSE under ESI+ and/or ESI- (Table 2 and Figure 2 A,B).
Under ESI- mode, precursor ions at m/z 863 and 861 could be assigned to PAC trimers with 1 and 2 A-type linkages, respectively (insert in Figure 2A). Their main fragments are aligned with previous reports [24,35,36,40]. Trimers with one A-type linkage could be assigned as (epi)cat-A-(epi)cat-(epi)cat or (epi)cat-(epi)cat-A-(epi)cat depending on the fragments: (epi)cat-A-(epi)cat-(epi)cat has fragement ions at m/z 573 and 289 via QM cleavages between middle and terminal units while (epi)cat-(epi)cat-A-(epi)cat has m/z 575 and 287 fragments via QM cleavages between top and middle units. In addition, both of them generate fragments at m/z 737 (loss of 126 Da through HRF) and 711 (loss 152 Da by RDA). Under ESI+ mode,the precursorions at m/z 865* and 863* are PAC trimers with 1 and 2 A-type linkages. Based on their fragments in table 2, the [M+H]+ at m/z 865* might be (epi)cat-(epi)cat-A-(epi)cat [6].
PAC tetramers, pentamers and hexamers with 1 to 3 A-type linkages were detected under ESI- with the corresponding precusor ions at m/z 1151, 1149 and 1147; 1439, 1437 and 1435; 1727, 1725 and 1723 (insert in Figure 2A). The main fragments of [M-H]- ions at m/z 1147, 1149, 1435, 1437 and 1725 are listed in table 2, which are generally produced from [M-H-152]- (RDA), [M-H-(288)n]- (progressively loss (epi)cat units) or loss water molecules. A portion of these precursor ions and possible isomers of tetramers and pentamers with one and two A-type linkages are mentioned previously in other foods such as peanuts and cranberry [5,24,35,36].
Under ESI+ mode, tetramers, pentamers and hexamers with 1 and 2 A-type linkages were also detected in GSE with precursor ions at m/z 1153* and 1151*; 1441* and 1439*; 1729* and 1727*, respectively (Table 2). In addition, hexamers with three A-type linkages were detected in GSE at m/z 1725* under ESI+. Overall, with increasing the degree of polymerization, the detected amount of A-type PACs decreased.
Doubly charged A-type PACs: Doubly charged ([M-2H]2-) A-type PACs were detected only in GSE (inserts in Figure 2). Under ESI-, [M-2H]2- PAC pentamers with 1-3 A-type linkages (m/z 1439, 1437 and 1435, respectively) were occurred at m/z 719, 718 and 717, respectively (insert in Figure 2A). [M-2H]2-heptamers with 1-3 A-type linkages (m/z 2015, 2013 and 2011, respectively) were detected at m/z 1007, 1006 and 1005, respectively under ESI- mode (insert in Figure 2A). Under ESI+, the double charged heptamers with 1-3 A-type linkages were at m/z 1009*, 1008* and 1007* (insert in Figure 2B). [M-2H]2-PAC nonamers with 1-4 A-type linkages (m/z 2591, 2589, 2587, and 2585, respectively) were also detected respectively at m/z 1295, 1294, 1293 and 1292 (insert in Figure 2A). Up to now, there was no detailed report about doubly charged precursor ions in grapes, especially doubly charged A-type PACs, though doubly charged A-type PAC tetramers (m/z 1149) and pentamers (m/z 1439) were recently reported in dry-blanched peanut skins [24].
Some singly charged precursor ions overlaped with the doubly charged ones in some cases along with some unkown precusor ions with high intensities such as at m/z 313, 325, 359, and 439 in grape pomaces warrant future characterization.
Relative content of PACs analyzed by ESI Q-TOF MS: The relative content of monomeric and polymeric (epi)catechins were different under different ionization mode (Table 3). The percentage of monomeric (epi)catechin calculated from ESI+ mode was much higher than that from ESI- mode, but the relative content of oligomers from ESI- was generally higher than that from ESI+ (Table 3). Overall, oligomers were the major PACs in all grape extracts, dominant by dimers and trimers.
CONCLUSION
RGPE had higher content of phenolics, flavonoids and proanthocyanidins, and antioxidant activities than WGPE. The oligomers of (epi)catechin were the major PACs in all grape extracts studied. Monogalloylated dimers, trimers and tetramers were detected in GSE, RGPE and WGPE, while anthocyanins were detected only in RGPE and WGPE. The B-type PACs could be found in all grape extracts under both ESI-/+ mode, while A-type PACs were more detectable in GSE. Under ESI-, A-type dimer, single A-type linked PAC trimers and tetramers can be also detected in RGPE and WGPE in addtion to GSE. Of which, monogalloylated A-type dimers with (epi)cat and (epi)afz were detected in both GSE and WGPE for the first time.
REFERENCES
- Guendez R, Kallithraka S, Makris DP, Kefalas P (2005) An analytical survey of the polyphenols of seeds of varieties of grape(Vitis vinifera) cultivated in Greece: implications for exploitation as a source of value-added phytochemicals. Phytochem Anal 16: 17-23.
- Lizarraga D, Vinardell MP, Noé V, van Delft JH, Alcarraz-Vizán G, et al. (2011) A lyophilized red grape pomace containing proanthocyanidin-rich dietary fiber induces genetic and metabolic alterations in colon mucosa of female C57BL/6J mice. J Nutr 141: 1597-1604.
- Wang H, Xue Y, Zhang H, Huang Y, Yang G, et al. (2013) Dietary grape seed extract ameliorates symptoms of inflammatory bowel disease in IL10-deficient mice. Mol Nutr Food Res 57: 2253-2257.
- Cheah KY, Bastian SE, Acott TM, Abimosleh SM, Lymn KA, et al. (2013) Grape seed extract reduces the severity of selected disease markers in the proximal colon of dextran sulphate sodium-induced colitis in rats. Dig Dis Sci 58: 970-977.
- Gu L, Kelm MA, Hammerstone JF, Zhang Z, Beecher G, et al. (2003) Liquid chromatographic/electrospray ionization mass spectrometric studies of proanthocyanidins in foods. J Mass Spectrom 38: 1272-1280.
- Passos CP, Cardoso SM, Domingues MRM, Domingues P, Silva CM, et al. (2007) Evidence for galloylated type-A procyanidins in grape seeds. Food Chemistry 105: 1457-1467.
- Rockenbach II, Jungfer E, Ritter C, Santiago-Schübel B, Thiele B, et al. (2012) Characterization of flavan-3-ols in seeds of grape pomace by CE, HPLC-DAD-MSn and LC-ESI-FTICR-MS. Food Research International 48: 848-855.
- Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, et al. (2005) A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 66: 2281-2291.
- Yokota K, Kimura H, Ogawa S, Akihiro T (2013) Analysis of A-Type and B-Type Highly Polymeric Proanthocyanidins and Their Biological Activities as Nutraceuticals. Journal of Chemistry: 1-7.
- Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16: 144-158.
- Dame CJ, Chichester CO, Marsh GL (1957) Studies of processed all-green asparagus: I. quantitative analysis of soluble compounds with respect to strain and harvest variables, and their distribution within the asparagus spear. Journal of Food Science 22: 658-672.
- Garcia-Parrilla MC, Heredia FJ, Troncoso AM, González GA (1997) Spectrophotometric determination of total procyanidins in wine vinegars. Talanta 44: 119-123.
- Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology 28: 25-30.
- González-Centeno MR, Jourdes M, Femenia A, Simal S, Rossello C, et al. (2013) Characterization of polyphenols and antioxidant potential of white grape pomace byproducts (Vitis vinifera L.). J Agric Food Chem 61: 11579-11587.
- Hogan S, Canning C, Sun S, Sun X, Kadouh H, et al. (2011) Dietary supplementation of grape skin extract improves glycemia and inflammation in diet-induced obese mice fed a Western high fat diet. J Agric Food Chem 59: 3035-3041.
- Rockenbach II, Rodrigues E, Gonzaga LV, Caliari V, Genovese MI, et al. (2011) Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chemistry 127: 174-179.
- Hogan S, Zhang L, Li J, Sun S, Canning C, et al. (2010) Antioxidant rich grape pomace extract suppresses postprandial hyperglycemia in diabetic mice by specifically inhibiting alpha-glucosidase. Nutr Metab (Lond) 7: 71.
- Parry JW, Li H, Liu JR, Zhou K, Zhang L, et al. (2011) Antioxidant activity, antiproliferation of colon cancer cells, and chemical composition of grape pomace. Food and Nutrition Sciences 2: 530-540.
- Yemis O, Bakkalbasi E, Artik N (2008) Antioxidative activities of grape (Vitis vinifera) seed extracts obtained from different varieties grown in Turkey. International Journal of Food Science and Technology 43: 154-159.
- Li HJ, Deinzer ML (2007) Tandem mass spectrometry for sequencing proanthocyanidins. Anal Chem 79: 1739-1748.
- Li HJ, Deinzer ML (2008) The mass spectral analysis of isolated hops A-type proanthocyanidins by electrospray ionization tandem mass spectrometry. J Mass Spectrom 43: 1353-1363.
- Biasoto ACT, Catharino RR, Sanvido GB, Eberlin MN, da Silva MAAP (2010) Flavour characterization of red wines by descriptive analysis and ESI mass spectrometry. Food Quality and Preference 21: 755-762.
- Cottica SM, de Morais DR, Rotta EM, Sargi SC, Silva FLN, et al. (2013) Effects of grape processing on antioxidant capacity and ESI-MS fingerprints of grape products. Journal of Food Science and Engineering 3: 341-348.
- Ma Y, Kosi?ska-Cagnazzo A, Kerr WL, Amarowicz R, Swanson RB, et al. (2014) Separation and characterization of soluble esterified and glycoside-bound phenolic compounds in dry-blanched peanut skins by liquid chromatography-electrospray ionization mass spectrometry. J Agric Food Chem 62: 11488-11504.
- Cantos E, Espín JC, Tomás-Barberán FA (2002) Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC-DAD-MS-MS. J Agric Food Chem 50: 5691-5696.
- Kammerer D, Claus A, Carle R, Schieber A (2004) Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J Agric Food Chem 52: 4360-4367.
- Wang H, Race EJ, Shrikhande AJ (2003) Characterization of anthocyanins in grape juices by ion trap liquid chromatography-mass spectrometry. J Agric Food Chem 51: 1839-1844.
- Xu Y, Simon JE, Welch C, Wightman JD, Ferruzzi MG, et al. (2011) Survey of polyphenol constituents in grapes and grape-derived products. J Agric Food Chem 59: 10586-10593.
- Sandhu AK, Gu L (2010) Antioxidant capacity, phenolic content, and profiling of phenolic compounds in the seeds, skin, and pulp of Vitis rotundifolia (Muscadine Grapes) As determined by HPLC-DAD-ESI-MS(n). J Agric Food Chem 58: 4681-4692.
- Crupi P, Coletta A, Anna Milella R, Perniola R, Gasparro M, et al. (2012) HPLC-DAD-ESI-MS analysis of flavonoid compounds in 5 seedless table grapes grown in Apulian Region. J Food Sci 77: 174-181.
- Tala VR, Candida da Silva V, Rodrigues CM, Nkengfack AE, dos Santos LC, et al. (2013) Characterization of proanthocyanidins from Parkia biglobosa (Jacq.) G. Don. (Fabaceae) by flow injection analysis-electrospray ionization ion trap tandem mass spectrometry and liquid chromatography/electrospray ionization mass spectrometry. Molecules 18: 2803-2820.
- Montero L, Herrero M, Prodanov M, Ibáñez E, Cifuentes A (2013) Characterization of grape seed procyanidins by comprehensive two-dimensional hydrophilic interaction x reversed phase liquid chromatography coupled to diode array detection and tandem mass spectrometry. Anal Bioanal Chem 405: 4627-4638.
- Hayasaka Y, Waters EJ, Cheynier V, Herderich MJ, Vidal S (2003) Characterization of proanthocyanidins in grape seeds using electrospray mass spectrometry. Rapid Commun Mass Spectrom 17: 9-16.
- Oliveria J, da Silva MA, Parola AJ, Mateus N, Brás NF, et al. (2013) Structural characterization of a A-type linked trimeric anthocyanin derived pigment occurring in young Port wine. Food Chem 141: 1987-1996.
- Appeldoorn MM, Vincken JP, Sanders M, Hollman PC, Gruppen H (2009) Combined normal-phase and reversed-phase liquid chromatography/ESI-MS as a tool to determine the molecular diversity of A-type procyanidins in peanut skins. J Agric Food Chem 57: 6007-6013.
- Lin LZ, Sun J, Chen P, Monagas MJ, Harnly JM (2014) UHPLC-PDA-ESI/HRMSn profiling method to identify and quantify oligomeric proanthocyanidins in plant products. J Agric Food Chem 62: 9387-9400.
- Ogawa S, Kimura H, Niimi A, Katsube T, Jisaka M, et al. (2008) Fractionation and structural characterization of polyphenolic antioxidants from seed shells of Japanese horse chestnut (Aesculus turbinata BLUME). J Agric Food Chem 56: 12046-12051.
- Jaiswal R, Jayasinghe L, Kuhnert N (2012) Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS. J Mass Spectrom 47: 502-515.
- Zhu QY, Hammerstone JF, Lazarus SA, Schmitz HH, Keen CL (2003) Stabilizing effect of ascorbic acid on flavan-3-ols and dimeric procyanidins from cocoa. J Agric Food Chem 51: 828-833.
- Garrett R, Romanos MTV, Borges RM, Santos MG, Rocha L, et al. (2012) Antiherpetic activity of a flavonoid fraction from Ocotea notata leaves. Rev bras farmacogn 22: 306-313.
Citation: Zhang S, Zhu MJ (2015) Characterization of Polyphenolics in Grape Pomace Extracts Using ESI Q-TOF MS/MS. J Food Sci Nutr 1: 001.
Copyright: © 2015 Zhang S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

