
Combining Folate and Zn Biofortification in Fennel
*Corresponding Author(s):
Antonella FuriniDepartment Of Biotechnology, University Of Verona, Strada Le Grazie 15, 37134 Verona, Italy
Tel:+39 0458027950,
Email:antonella.furini@univr.it
Abstract
Improving nutritional health is one of the major socio-economic challenges of the 21st century. Therefore, research approaches toward crop biofortification are encouraged. Among nutritional deficiencies zinc and folates are of concern for their impact on public health. In this work a hydroponic culture was applied for combining zinc and folate biofortification in sweet and wild fennel. Zinc was highly accumulated in both cultivars, whereas folates were present only in leaves of wild fennel possibly due to their role in plant metabolism. The enriched plant tissues obtained can be employed for the production of herbal-derived dietary supplements.
Keywords
Biofortification; Dietary supplements; Fennel; Folates; Zinc
INTRODUCTION
Due to the continuous growth and ageing of world population, improving nutritional health could be considered as one of the major socio-economic challenges of the current century. According to recent studies, more that 52% of the USA population uses dietary supplements [1], and their consumption is increasing in many European countries, with Italy leading in terms of market for dietary supplements with a value of1.3 billion euros in 2008, increased to 2.8 billion euros in 2017 (https://www.statista.com/statistics/783654/sales-value-of-dietary-supplements-in-italy/). These food supplements include vitamins, mineral elements, herbal and botanical preparations, amino acids, enzymes and are present on the market in a variety of forms such as tablets, capsules, liquids or powders. In particular, the research on dietary supplements has been further driven in the direction of natural, plant-derived products, in consideration of the fact that vitamins and mineral nutrients in plant tissues are characterized by a higher bio-availability to absorption by the human body compared to chemically-synthesized supplements.
In a worldwide perspective, nutritional deficiencies have a strong impact on public health. In particular, it is estimated that about a third of the world population is at risk of zinc (Zn) deficiency, with prevalence in children under 5years of age for their higher Zn requirements to support growth and development [2]. While Zn deficiency in poorer countries is generally associated with malnutrition, in developed countries lack of dietary diversity seems to be the main reason for Zn deficiency. Indeed, the concentrations of Zn and of other mineral elements in edible products have declined in developed countries over the last half century [3]; this was mainly due, in particular for cereal crops, to the acquisition of modern practices and high-yielding genotypes, resulting in reduced concentrations of mineral elements in plant tissues [4]. Therefore, investing in research on efficient application of Zn fertilizers to cereal crops as well as plant breeding programs for Zn biofortification are of high priority [5].
Among vitamin deficiencies, that of vitamin B9 (including folic acid and folates) is one of the most impacting on public health. Folate deficiency is associated with a variety of pathological manifestations, such as anemia, cardiovascular diseases, neurological alterations and impairments in reproductive health [6], as well as severe alteration in fetal development, including neural-tube defects and congenital heart defects [7]. Folate deficiency is generally a consequence of poorly diversified diets and high consumption of starch-rich staple food, such as cereals, that contains low folate levels. Several approaches have been pursued to increase folate in crops. The identification of germplasm variations and quantitative trait loci affecting folate content in grains has been achieved in various crops [8-11]; however, conventional breeding strategies employing this natural variation require long time to reach the final goal. Metabolic engineering offers an alternative to increase folate in food crops by targeting folate biosynthesis and stability (reviewed by [12,13]), but this line of research can encounter limitations associated with GMO legislation.
In this work we tested the possibility to increase both the Zn and folate levels in sweet and wild fennels by co-administering them in hydroponic culture, with the aim of producing biofortified foods and ingredients for herbal-derived dietary supplements.
MATERIALS AND METHODS
Plant material, growth conditions and treatment
Sweet fennel cultivar 'Wadenromen' (Foeniculum vulgare var. dulce (Mill.) Batt. &Trab.) and wild fennel (Foeniculum vulgare Mill.) were used in this work. Seeds of both plant genotypes were germinated on filter paper saturated with water until the emergence of the first true leaves; uniform-looking plantlets were then transferred in hydroponic culture. Plants were grown in pots containing 2L quarter strength Hoagland’s solution [14,15], for two months, then either treated with 200µM folic acid and 10µM ZnSO4 or grown in control conditions for a further two-month period; hydroponic solution was oxygenated by bubbling. Plant growth was carried out in a growth chamber upon controlled light, temperature and humidity conditions (16-h photoperiod, light intensity 350µE m−2s−1, temperature 24-26ºC, relative humidity 50%). Both control and treatment were performed in triplicate, with each replicate containing six plants.
At the end of the experiment, the organs commonly used for human consumption (bulbs for sweet fennel and leaves for wild fennel) were collected, frozen in liquid N2, and reduced to powder.
Quantification of zinc and iron
Samples for element quantification were oven-dried at 60ºC. Zn and iron (Fe) quantification was performed by inductively coupled plasma atomic emission spectrometry (ICP-AES) according to the specifications of the AOAC Official Method 2013.06 [16].
Quantification of folates
Vitamin B9, in the forms of folic acid, 5-methyl-tetrahydrofolate and 5-formyl-tetrahydrofolate, was quantified from frozen powdered tissues by liquid chromatography associated with mass spectrometry (LC-MS/MS), according to the specifications of the AOAC Official Method 2011.06 [16].
Statistical analysis
The statistical significance of accumulation data was estimated by Student’s t test. In the figures, data are represented as mean ± Standard Error (SE) and statistically significant differences (p<0.05) are marked with asterisks [17].
RESULTS AND DISCUSSION
Throughout the experiment, plants of both cultivars showed normal growth in hydroponic culture in both control conditions and under Zn and folic acid treatment. At the end of the experiment, about five times more Zn was accumulated in bulbs and leaves of treated sweet and wild fennel, respectively, in comparison to control conditions (Figure 1). Regarding folates, vitamin B9 was accumulated at higher level in the leaves of treated wild fennel in comparison to control conditions. In particular, although folic acid content was not significantly higher under Zn and folic acid supplementation, the folate forms 5-methyl-tetrahydrofolate and 5-formyl-tetrahydrofolate were detectable only in treated leaves (Figure 2); it is interesting to notice that wild fennel was able to both absorb and translocate folic acid to the green tissues and to convert it to different folate forms. On the other hand, folates were not detected in bulbs of sweet fennel (data not shown). This result could be interpreted considering that in plants folates are generally accumulated mainly in meristematic and photosynthetic tissues, where they are required at higher levels due to their role in the biosynthesis of nucleotides and in the photo respiratory cycle, respectively [18-20]. Therefore, it is reasonable that the accumulation of exogenous folates is consistent in fennel leaves, while virtually absent in bulbs, which are mainly constituted of non-photo synthetically active tissues.
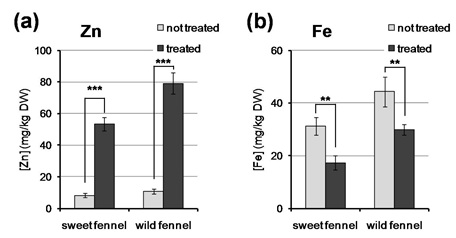 Figure 1: Analysis of Zn and Fe content. Concentration of Zn (a) and Fe (b) in bulbs of sweet fennel and leaves of wild fennel grown in hydroponic culture under non-treated and treated (200µM folic acid + 10µM ZnSO4) conditions. Asterisks above the histograms indicate statistical significance, evaluated by Student’s t test: ** p<0.01; *** p<0.001.
Figure 1: Analysis of Zn and Fe content. Concentration of Zn (a) and Fe (b) in bulbs of sweet fennel and leaves of wild fennel grown in hydroponic culture under non-treated and treated (200µM folic acid + 10µM ZnSO4) conditions. Asterisks above the histograms indicate statistical significance, evaluated by Student’s t test: ** p<0.01; *** p<0.001.
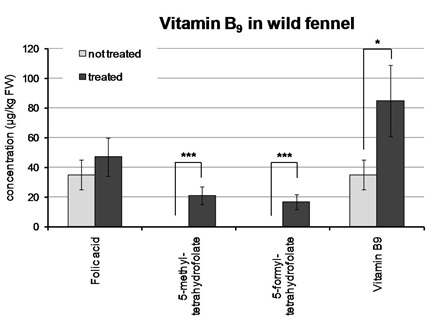 Figure 2: Analysis of folate content. Concentration of different folates in leaves of wild fennel grown in hydroponic culture under non-treated and treated (200µM folic acid + 10µM ZnSO4) conditions. Asterisks above the histograms indicate statistical significance, evaluated by Student’s t test: * p<0.05; *** p<0.001.
Figure 2: Analysis of folate content. Concentration of different folates in leaves of wild fennel grown in hydroponic culture under non-treated and treated (200µM folic acid + 10µM ZnSO4) conditions. Asterisks above the histograms indicate statistical significance, evaluated by Student’s t test: * p<0.05; *** p<0.001.
Since Zn and Fe are possibly interconnected in plant mineral nutrition [21], we chose to consider also Fe accumulation in the tissues. Indeed, Fe levels were significantly lower under Zn and folic acid treatment than in control conditions in both sweet fennel bulbs and wild fennel leaves (Figure 1). The antagonistic action of Zn supplementation on Fe uptake and homeostasis in plants has been documented in some cases [22,23] and derives from the fact that Zn and Fe often pass through the same transporters for their uptake, translocation and compartmentalization [24,25]. On the other hand, no interaction has been previously reported between folic acid and micronutrient uptake: Given this evidence, the reduced Fe accumulation is most likely due to the competition with Zn uptake.
In conclusion, Zn exogenous supplementation has proven especially effective for the enrichment of edible tissues in sweet and wild fennel, although it reduces Fe accumulation in both genotypes. On the other hand, folic acid is efficiently accumulated in wild fennel leaves, whereas it is undetectable in sweet fennel bulbs. The positive results obtained in wild fennel show the possibility for the combined biofortification of vitamins and mineral nutrients. Although similar strategies cannot be employed for the enrichment of staple crops to prevent nutritional deficiencies in poorer countries, these biofortified vegetal products can be successfully used for the production of nutritionally complete herbal-derived dietary supplements.
ACKNOWLEDGEMENT
Research was supported by a grant from the European Social Fund (project code 1695-6-2216-2016).
REFERENCES
- Kantor ED, Rehm CD, Du M, White E, Giovannucci EL (2016) Trends in dietary supplement use among US adults from 1999-2012. JAMA 316: 1464-1474.
- Wessells KR, Brown KH (2012) Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS One 7: 50565.
- Davis RD (2009) Declining fruit and vegetable nutrient composition: What is the evidence? HortScience 44: 15-19.
- Loladze I (2002) Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry? Trends Ecol Evol 17: 457-461.
- Cakmak I (2008) Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 302: 1-17.
- Green R, Miller JW (1999) Folate deficiency beyond megaloblastic anemia: Hyperhomocysteinemia and other manifestations of dysfunctional folate status. Semin Hematol 36: 47-64.
- Czeizel A, Dudás I, Vereczkey A, Bánhidy F (2013) Folate deficiency and folic acid supplementation: The prevention of neural-tube defects and congenital heart defects. Nutrients 5: 4760-4775.
- Piironen V, Edelmann M, Kariluoto S, Bedo Z (2008) Folate in wheat genotypes in the HEALTHGRAIN diversity screen. J Agric Food Chem 56: 9726-9731.
- Shohag MJ, Wei YY, Yu N, Zhang J, Wang K, et al. (2011) Natural variation of folate content and composition in spinach (Spinacia oleracea) germplasm. J Agric Food Chem 59: 12520-12526.
- Robinson BR, Sathuvalli V, Bamberg J, Goyer A (2015) Exploring folate diversity in wild and primitive potatoes for modern crop improvement. Genes (Basel) 6: 1300-1314.
- Dong W, Cheng ZJ, Xu JL, Zheng TQ, Wang XL, et al. (2014) Identification of QTLs underlying folate content in milled rice. J Integr Agric 13: 1827-1834.
- Blancquaert D, De Steur H, Gellynck X, Van Der Straeten D (2014) Present and future of folate biofortification of crop plants. J Exp Bot 65: 895-906.
- Strobbe S. Van Der Straeten D (2017) Folate biofortification in food crops. Curr Opin Biotechnol 44: 202-211.
- Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circular. California Agricultural Experiment Station, University of California, Berkeley, USA.
- AOAC International (2016) Official methods of analysis of AOAC International. (20th edn). Gaithersburg, USA.
- Statista: The Statistics Portal.
- Gambonnet B, Jabrin S, Ravanel S, Karan M, Douce R, et al. (2001) Folate distribution during higher plant development. J Sci Food Agric 81: 835-841.
- Jabrin S, Ravanel S, Gambonnet B, Douce R, Rébeillé F (2003) One-carbon metabolism in plants. Regulation of tetrahydrofolate synthesis during germination and seedling development. Plant Physiol 131: 1431-1439.
- Rébeillé F, Ravanel S, Jabrin S, Douce R, Storozhenko S, et al. (2006) Folates in plants: Biosynthesis, distribution, and enhancement. Physiol Plant 126: 330-342.
- Alloway BJ (2008) Zinc in Soils and Crop Nutrition. International Zinc Association, Brussels, Belgium.
- Ambler JE, Brown JC, Gauch HG (1970) Effect of zinc on translocation of iron in soybean plants. Plant Physiol 46: 320-323.
- Haldar M, Mandal LN (1981) Effect of phosphorus and zinc on the growth and phosphorus, zinc, copper, iron and manganese nutrition of rice. Plant Soil 59: 415-425.
- Schaaf G, Schikora A, Häberle J, Vert G, Ludewig U, et al. (2005) A putative function for the Arabidopsis Fe-phytosiderophore transporter homolog AtYSL2 in Fe and Zn homeostasis. Plant Cell Physiol 46: 762-774.
- Lee S, An G (2009) Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ 32: 408-416.
- Lin YF, Liang HM, Yang SY, Boch A, Clemens S, et al. (2009) Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytol 182: 392-404.
Citation: Fasani E, DalCorso G, Furini A (2019) Combining Folate and Zn Biofortification in Fennel. J Food Sci Nut 5: 047.
Copyright: © 2019 Elisa Fasani, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

