
Comparison of One Injection to Three Monthly Injections of Anti-Vascular Endothelial Growth Factor Agents for Macular Oedema Associated with Branch Retinal Vein Occlusion
*Corresponding Author(s):
Toshiyuki OshitariDepartment Of Ophthalmology, International University Of Health And Welfare, Narita, Japan
Tel:432262124,
Fax:+81 432244162
Email:Tarii@aol.com
Abstract
Purpose: To compare the outcomes of one initial injection of an anti-vascular endothelial growth factor (VEGF) antibody followed by a pro re nata (PRN) (1+PRN) to that of three monthly injections followed by PRN (3+PRN) for Macular Oedema (ME) associated with a Branch Retinal Vein Occlusion (BRVO).
Methods: This was a review of the medical records of 35 eyes that had been treated with anti-VEGF agents for ME associated with BRVO. The results of 17 eyes that had received anti-VEGF injection of ranibizumab or aflibercept with the 1+PRN were compared to the results of 18 eyes that had received ranibizumab or aflibercept injections with the 3+PRN. The Central Macular Thickness (CMT) and Best-Corrected Visual Acuity (BCVA) were measured at the baseline, and at 1, 3, 6, and 12 months after the injections.
Results: After 12 months, the BCVAs and the CMTs were significantly improved in both the 1+PRN and the 3+PRN groups. The degree of improvement of the BCVAs and the CMTs were not significantly different in the two groups. However, the total number of injections after 12 months was significant fewer in the 1+PRN group.
Conclusions: We recommend the 1+PRN protocol for patients with ME associated with BRVO.
Keywords
Anti-vascular endothelial growth factor agent; Branch retinal vein occlusion; Macular oedema; 1+pro re nata; 3+pro re nata
ABBREVIATIONS
RVO: Retinal Vein Occlusion
CRVO: Central Retinal Vein Occlusion
BRVO: Branch Retinal Vein Occlusion
ME: Macular Oedema
VEGF: Vascular Endothelial Growth Factor
PRN: pro re nata
DME: Diabetic Macular Oedema
IVR: Intravitreal Ranibizumab
IVA: Intravitreal Aflibercept
CMT: Central Macular Thickness
SD-OCT: Spectral Domain-Optical Coherence Tomography
BCVA: Best-Corrected Visual Acuity
IVB: Intravitreal Bevacizumab
NPA: Non Perfusion Area
INTRODUCTION
A Retinal Vein Occlusion (RVO) is the second most common cause of vision decrease following diabetic retinopathy [1-3]. In Japan, the prevalence of Central Retinal Vein Occlusion (CRVO) is 0.2% and that for a BRVO is 2.0% in patients of age ≥40 years [4]. In a previous systematic review, 5 to 15% of eyes with a branch retinal vein occlusion (BRVO) developed Macular Oedema (ME) [5], and the ME is the major cause of the vision reduction in patients with a RVO [6,7].
Vascular Endothelial Growth Factor (VEGF) is associated with the development of the ME secondary to an RVO [8,9]. Noma et al., reported that the vitreous level of VEGF was significantly increased and correlated with the severity of the ME associated with a BRVO [9]. Thus, it was reasonable that anti-VEGF therapies became the gold standard therapies for the ME associated with an RVO because of their efficacy and safety [10,11]. Corticosteroids and laser photocoagulation are also effective for the treatment of ME secondary to RVO but corticosteroids have several ocular side effects including elevated intraocular pressure and cataracts [12]. In addition, it has been shown that laser photocoagulation is less effective in improving the visual acuities than anti-VEGF therapies [12].
In Japan, ranibizumab was approved for the indication of ME associated with a BRVO in 2013, and aflibercept was approved for the indication of ME associated with a BRVO in 2015. However, the frequency and number of injections have varied in clinical trials. For example in the BRAVO study, monthly ranibizumab injections have been performed for 6 months followed by pro re nata (PRN) dosing in patients with ME secondary to a BRVO [13,14]. In the VIBRANT study, monthly aflibercept injections have been used for 6 months followed by every 8 weeks of a fixed dose in patients with an ME associated with BRVO [15].
However in the real world, the price of a single injection of ranibizumab and aflibercept in Japan is approximately $1500 [16]. Because most patients must pay 30% of the medical costs before each injection, many refuse frequent injections of the anti-VEGF agents [16]. In fact, our previous real-world study showed that the mean number of aflibercept injection was 3.8 ± 2.4 in one year in patients with Diabetic Macular Oedema (DME) [17]. On the other hand, in the VISTA and VIVID clinical trials, the total number of aflibercept injections was 9 to 12 times/year in patients with DME [18]. Thus, a reduction of the total number of anti-VEGF agent injections is practical for the treatment of the ME associated with a BRVO.
Several studies have compared one initial injection (1+PRN) to three initial monthly injections (3+PRN) of an anti-VEGF antibody in patients with ME associated with BRVO [19-22]. Two groups have compared 1+PRN to 3+PRN dosing of bevacizumab [19,20], and two other groups have compared this regimen of ranibizumab [21,22]. All studies have concluded that there was no significant difference in the visual acuities or central macular thickness between the 1+PRN and 3+PRN regimens [19-22]. However, no studies have been performed that compared 1+PRN to 3+PRN dosing of aflibercept for the treatment of ME associated with a BRVO.
Thus, the purpose of this study was compared to the Best-Corrected Visual Acuity (BCVA) and Central Macular Thickness (CMT) after 1+PRN to that after 3+PRN dosing of ranibizumab or aflibercept injections for a ME associated with a BRVO.
PATIENTS AND METHODS
The medical records of 35 eyes of 35 patients who had an ME associated with a BRVO and were examined in the Chiba University Hospital from January in 2014 to June in 2016 were reviewed. Eighteen eyes of 18 patients had received 0.5 mg/0.05 ml of Intravitreal Ranibizumab (IVR) from January in 2014 to March in 2015, and 17 eyes of 17 patients had received 2 mg/0.05 ml of Intravitreal Aflibercept (IVA) from March in 2015 to August in 2015. Before March in 2015, IVR was the first choice of therapy for an ME secondary to BRVO in our hospital, and after March in 2015, aflibercept was approved for the indication of the treatment of ME associated with a BRVO and IVA became the first choice of therapy for the ME secondary to BRVO at the Chiba University Hospital, because IVA is relatively cheaper than IVR in Japan. In addition, aflibercept can block both cascades of VEGF1 and 2 receptors. Thus, we expected IVA may show longer lasting effects than IVR for the treatment of ME associated with BRVO. In fact, our recent study regarding comparisons of efficacy of IVA and IVR in eyes with DME indicates that the effects of IVA persist longer than that of IVR [16]. Before August in 2014, patients underwent one initial IVR injection followed by a PRN dosing (1+PRN), however after around August in 2014, three monthly IVR injection followed by PRN dosing (3+PRN) gradually became the major treatment protocol in our hospital partly because we reconsidered the treatment regimen to be consistent with the regimen of age-related macular degeneration. After March 2015, IVR was switched to IVA in all cases in our hospital and the treatment regimen has remained 3+PRN. However, around August in 2015, the treatment regimen was reviewed again and 1+PRN became the standard treatment regimen for an ME associated with a BRVO in our hospital partly because of increasing requirements of the patients.
For this study, we examined the medical records of 17 eyes of 17 patients that had received IVR or IVA with the 1+PRN dosing regimen, and18 eyes of 18 patients that had received IVR or IVA with the 3+PRN dosing regimen. Patients who had a CMT exceeding 300 µm in the spectral domain optical coherence tomographic (SD-OCT, Heidelberg Engineering, Heidelberg, Germany) images were examined. The center was 1 mm diameter circle in the ETDRS grid. Scan analysis was performed using version 5.3 software. The version was not changed during the study. In case of segmentation errors, the CMT was measured manually. Briefly, the examiner measured macular thickness at the fixation point and 0.5 mm from the fixation point 2 times and the average was used for the data analyses. The duration from the onset to the beginning of the treatment had to be <12 months. The patients received another anti-VEGF agent injection when there was an increase in the CMT of ≥100 µm. However, if the patients did not agree to the injection, other therapies including sub-Tenon triamcinolone acetonide injection and vitrectomy were performed. Eyes with diabetic retinopathy, uveitis, glaucoma, epiretinal membrane, and age-related macular degeneration were excluded. Patients with prior brain ischemia or ischemic heart diseases were also excluded.
The Best-Corrected Visual Acuity (BCVA) and the CMT were measured before and at 1, 3, 6, and 12 months after IVR or IVA in both groups. The total numbers of injections in both groups were also compared.
The clinical data and demographics of the patients before treatment are presented in Table 1. All parameters including age, sex, baseline BCVAs, baseline CMTs, interval from onset to treatment, incidence of systemic diseases including hypertension and diabetes mellitus, ratio of ischemic and non-ischemic, numbers of patients who underwent scattered photocoagulation, and ratio of IVR and IVA were not significantly different in the two groups.
|
1+PRN |
3+PRN |
P value |
|
|
Number (persons/eyes) |
17/17 |
18/18 |
|
|
Gender (men/women) |
7 / 10 |
6 / 12 |
0.73 #1 |
|
Age (years) |
69.7 ± 11.0 |
68.4 ± 7.3 |
0.73 #2 |
|
Baseline BCVA (logMAR) |
0.51 ± 0.33 |
0.59 ± 0.36 |
0.25 #2 |
|
CMT(µm) |
605 ± 175 |
627 ± 156 |
0.52 #2 |
|
Durationof symptoms(day) |
5.3 ± 4.1 |
3.7 ± 3.6 |
0.14 #2 |
|
Systemic hypertension (persons) |
10 |
6 |
0.18 #1 |
|
Diabetes mellitus (persons) |
0 |
2 |
0.49 #1 |
|
NPA(+) / NPA(-) (eyes) |
8 / 9 |
8 / 10 |
1.0 #1 |
|
Photocoagulation (eyes) |
8 |
6 |
0.50 #1 |
|
IVR / IVA (eyes) |
7 / 10 |
11 / 7 |
0.32 #1 |
Table 1: Baseline characteristic of the patients.
BCVA: Best-Corrected Visual Acuity; CMT: Central Macular Thickness; NPA: Non Perfusion Area; IVR: Intravitreal Ranibizumab; IVA: Intravitreal Aflibercept; PRN: pro re nata; #1: Fisher’s exact test; #2: Mann-Whitney U test.
Statistical analyses
The data are expressed as the means ± standard deviations or standard error of the means. The significance of the difference in the values was determined by Mann-Whitney U test, Fisher’s exact test, Wilcoxon rank-sum test, or repeated measures ANOVA. A P value <0.05 was considered significant.
RESULTS
For the groups receiving the anti-VEGF antibodies, ranibizumab or aflibercept, the baseline BCVAs in the 1+PRN group was 0.51 ± 0.34 logMAR unit, and it improved significantly to 0.39 ± 0.24 logMAR units (P= 0.019) at 1 month, to 0.32 ± 0.17 logMAR units (P= 0.039) at 3 months, to 0.3 ±0.20 (P= 0.005) at 6 months, and to 0.26 ± 0.24 logMAR units (P= 0.001) at 12 months after the initial injection (Wilcoxon rank-sum test; Figure 1). The BCVAs of the eyes receiving anti-VEGF antibodies, ranibizumab or aflibercept, in the 3+PRN group was 0.59 ± 0.36 logMAR units at the baseline, and it improved significantly to 0.40 ± 0.38 logMAR units (P= 0.002) at 1 month, to 0.33 ± 0.42 logMAR units (P= 0.0018) at 3 months, to 0.34 ± 0.43 logMAR units (P= 0.0037) at 6 months, and to 0.28 ± 0.38 logMAR units (P= 0.001) at 12 months after the initial injection (Wilcoxon rank-sum test; Figure 1). In both groups, the mean BCVAs were not significantly different at the baseline and at all post-injections times (P>0.05; repeated measures ANOVA and Mann-Whitney U test; Figure 1).
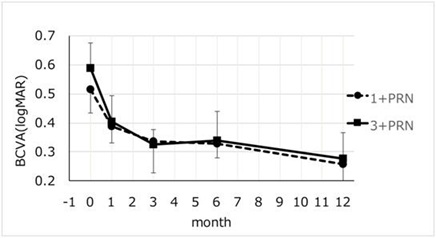
Figure 1: Changes in the mean Best-Corrected Visual Acuity (BCVA) from the baseline to 12 months after the intravitreal injection of anti-Vascular Endothelial Growth Factor (anti-VEGF) injections. The one initial injection of an Anti-Vascular Endothelial Growth Factor (VEGF) antibody followed by a pro re nata (1+PRN) regimen to that of three-monthly injections followed by PRN (3+PRN) regimen for Macular Oedema (ME) associated with a Branch Retinal Vein Occlusion (BRVO) were compared. In the 1+PRN group, the mean BCVA significantly improved from 0.51 to 0.26 logarithm of the minimum angle of resolution (logMAR) units at month 12 (P= 0.0015, Wilcoxon rank-sum test). In the 3+PRN group, the mean BCVA significantly improved from0.59 to 0.28 logMAR units at month 12 (P= 0.001, Wolcoxon rank-sum test). There was no significant difference between the two treatment groups in the mean BCVA (P> 0.05, repeated measures ANOVA).
After receiving the anti-VEGF antibodies, ranibizumab or aflibercept, the CMTs in the 1+PRN group was 605 ± 175 µm at the baseline, and it decreased significantly to 283 ± 45 µm (P= 0.0003) at 1 month, to 37 ± 146 µm (P= 0.001) at 3 months, to 294 ± 38 µm (P= 0.0004) at 6 months, and to 282 ± 33 µm (P= 0.0004) at 12 months after the initial injection (Wilcoxon rank-sum test; Figure 2). After receiving anti-VEGF antibodies, ranibizumab or aflibercept, the CMTs in the 3+PRN group was 627 ± 156 µm at the baseline and it decreased significantly to 321 ± 83 µm (P= 0.0002) at 1 month, to 272 ± 47 µm (P= 0.0002) at 3 months, to 302 ± 68 µm (P= 0.0002) at 6 months, and to 285 ± 57 µm (P= 0.0002) at 12 months after the initial injection (Wilcoxon rank-sum test; Figure 2). In both groups, the mean CMTs were not significantly different at the baseline and at all post-injections times (P> 0.05, repeated measures ANOVA and Mann-Whitney U test) except at 3 months (P=0.0048; Mann-Whitney U test; Figure 2).
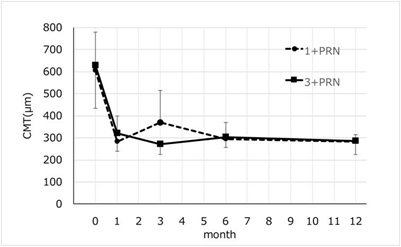
Figure 2: Changes in the mean Central Macular Thickness (CMT) from baseline to 12 months after the anti-VEGF injections. In the 1+PRN group, the mean CMT significantly improved from 604.8 µm to 282.0 µm at month 12 (P= 0.0004, Wilcoxon rank-sum test). In the 3+PRN group, the mean CMT improved significantly from 627.1 µm to 285.0 µm at 12 months after the anti-VEGF injections (P= 0.0002, Wilcoxon rank-sum test). There was no significant difference in the mean CMT between the two groups at any time (P>0.05, repeated measures ANOVA).
The geometric mean number of anti-VEGF, IVR or IVA, injections was 3.1 ± 1.6 in the 1+PRN group and 5.1 ± 1.7 in the 3+PRN group (P=0.006, Mann-Whitney U test; Figure 3). The range numbers were 1 to 6 in the 1+PRN group and 3 to 9 in the 3+PRN group, respectively.
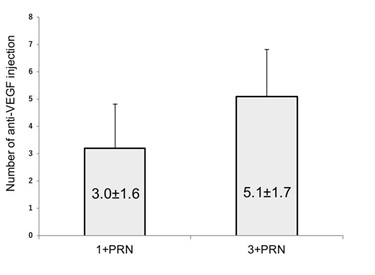 Figure 3: Comparison of total numbers of injection between the 1+PRN and the 3+PRN group at 12 months. The geometric mean total number of injections in the 1+PRN group was 3.0 ± 1.6, which was significantly fewer than the 5.1 ± 1.7 in the 3+PRN group (P= 0.006, Mann-Whitney U test). VEGF; Vascular Endothelial Growth Factor; PRN: pro re nata.
Figure 3: Comparison of total numbers of injection between the 1+PRN and the 3+PRN group at 12 months. The geometric mean total number of injections in the 1+PRN group was 3.0 ± 1.6, which was significantly fewer than the 5.1 ± 1.7 in the 3+PRN group (P= 0.006, Mann-Whitney U test). VEGF; Vascular Endothelial Growth Factor; PRN: pro re nata.
For the eyes that received only IVA, there were 10 eyes of 10 patients that had the 1+PRN and 7 eyes of 7 patients had the 3+PRN injection protocol. The background parameters of the eyes in these two groups were not significantly different. The mean BCVAs in the 1+PRN group at the baseline was 0.58 ± 0.36 logMAR units and it improved significantly to 0.43 ± 0.23 logMAR units (P= 0.052) at 1 month, to 0.35 ± 0.16 logMAR units (P= 0.035) at 3 months, to 0.36±0.24 logMAR units (P= 0.0069) at 6 months, and to 0.33 ± 0.27 logMAR units (P = 0.012) at 12 months after the initial IVA injection (Wilcoxon rank-sum test; Figure 4).
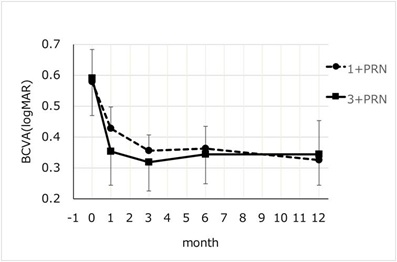
Figure 4: Changes in the mean BCVA from the baseline to month 12 after the IVA injection. In the 1+PRN group, the mean BCVA significantly improved from 0.58 to 0.33 logMAR units at 12 months (P= 0.012, Wilcoxon rank-sum test). In the 3+PRN group, the mean BCVA significantly improved from 0.59 to 0.34 logMAR units at 12 months (P= 0.03, Wolcoxon rank-sum test). There was no significant difference between two groups in the mean BCVA at any time (P>0.05, repeated measures ANOVA).
The mean BCVAs in the 3+PRN group was 0.59 ± 0.26 logMAR units at the baseline, and it improved significantly to 0.35 ± 0.31 logMAR units (P= 0.018) at 1 month, to 0.32 ± 0.26 logMAR units (P= 0.012) at 3 months, to 0.34 ± 0.27 logMAR units (P= 0.018) at 6 months, and to 0.34 ± 0.31 logMAR units (P= 0.030) at 12 months after the initial IVA injection (Wilcoxon rank-sum test; Figure 4). In both groups, the mean BCVAs were not significantly different at any time (P>0.05, repeated measures ANOVA and Mann-Whitney U test; Figure 4).
The mean CMTs in the 1+PRN group was 598 ± 197 µm at the baseline, and it decreased significantly to 307 ± 38 µm (P= 0.0033) at 1 month, to 309 ± 83 µm (P= 0.003) at 3 months, to 307 ± 42 µm (P= 0.004) at 6 months, and to 294 ± 34 µm (P= 0.004) at 12 months after the initial IVA injection (Wilcoxon rank-sum test; Figure 5).
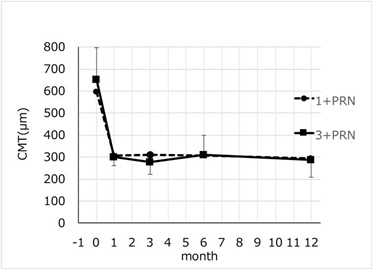
Figure 5: Changes in the mean CMT from baseline to month 12 after IVA injections of the thr. In the 1+PRN group, the mean CMT significantly improved from 597.9 µm to 294.2 µm at months 12 (P= 0.0044, Wilcoxon rank-sum test). In the 3+PRN group, the mean CMT significantly improved from 653.6 µm to 289.0 µm at month 12 (P= 0.012, Wilcoxon rank-sum test). There was no significant difference between two groups in the mean CMT (P>0.05, repeated measures ANOVA). CMT: Central Macular Thickness, PRN: pro re nata.
The mean CMTs in the 3+PRN group was 653 ± 143 µm at the baseline and it was significantly decreased to 302 ± 42 µm (P= 0.012) at 1 month, to 277 ± 57 µm (P= 0.012) at 3 months, and to 309 ± 90 µm (P= 0.012) at 6 months, and to 289 ± 79 µm (P= 0.012) at 12 months after the initial IVA injection (Wilcoxon rank-sum test; Figure 5).
The geometric mean total number of IVA injection was 2.3 ± 1.3 in the 1+PRN group and 4.5 ± 1.6 in the 3+PRN group (P= 0.0006, Mann-Whitney U test). The range numbers were 1 to 4 in the 1+PRN group and 3 to 8 in the 3+PRN group, respectively.
No serious complications including endophthalmitis, uveitis, retinal detachment, cataract, vitreous hemorrhage, stroke, and ischemic heart diseases developed in both groups.
DISCUSSION
Ito et al., [19] and Ahn et al., [20] reported that the changes in the BCVA and CMT of 1+PRN Intravitreal Bevacizumab (IVB) regimen were not significantly different from the 3+PRN IVB injection at 6 months [20] or 12 months [19] after performing these injection protocols. Miwa et al., [21] and Bayat et al., [22] also reported similar results at 12 months after IVR injections.
In this study, the BCVA and CMT after the 1+PRN of IVR or IVA injection regimen was not significantly different than that after the 3+PRN regimenat any time during the 12 month follow-up period. However, the total number of anti-VEGF injections in the 1+PRN group was significantly fewer than in the 3+PRN group. The percentage of patients who did not require additional anti-VEGF injection at the second year was 58.8% in the 1+PRN group and 55.6% in the 3+PRN group (P>0.05). The BCVA and the CMT in the 1+PRN group were significantly improved during the follow-up period after both IVR and IVA. Taken together, the 1+PRN protocol should be preferred for patients with ME associated with BRVO because a fewer number of injections was needed.
Because studies have not been performed comparing 1+PRN to 3+PRN dosing of aflibercept for the treatment of ME associated with BRVO, we analyzed the data after IVA for treatment of ME associated with BRVO separately. Ten eyes had received 1+PRN IVA injection dosing and 7 eyes had received 3+PRN IVA injection dosing for ME secondary to BRVO. There was no significant difference in BCVA and CMT at any time during the follow-up period. However, the total number of IVA injection in the 1+PRN IVA group was significantly fewer than that in the 3+PRN IVA group. The percentage of patients who did not require additional anti-VEGF injection at the second year was 72.7% in the 1+PRN group and 62.5%in the 3+PRN group. Thus, the 1+PRN IVA injection protocol is preferable than the 3+PRN IVA injection protocol for patients with ME associated with BRVO.
In the BRVAO study, the mean total number of 0.5mg ranibizumab injection was 8.4 times for 12 months [14]. In the VIBRANT study, the total number of 2mg aflibercept injection was 9 times for 52 weeks [10]. In our study, the mean number of anti-VEGF (IVR and IVA) injection was 3.1 times in the 1+PRN group and 5.1 times in the 3+PRN group. The mean number of only IVA injection was 2.3 times in the 1+PRN group and 4.5 times in the 3+PRN group. In addition, the mean BCVAs and CMTs were not significantly different in both the 1+PRN and the 3+PRN groups. The significantly fewer number of injections with the 1+PRN protocol is more cost-effective for the patient than the 3+PRN protocol. The total number of anti-VEGF injections in the clinical trials appears to be over dosing for the treatment of ME associated with BRVO.
There are limitations of this study. First, this was a retrospective study. Second, the patient number was small. Thus, larger prospective studies are required to compare the efficacy of the 1+PRN anti-VEGF injection regimens to the 3+PRN regimen for ME associated with BRVO.
In conclusion, the lack of significant differences in the BCVA and CMT and significantly fewer injection during the 12 months after the two treatment protocols suggests that the 1+PRN protocol should be preferred for patients with ME associated with BRVO. This will be more convenient for the patients and doctors and will be more cost effective for the patient.
ACKNOWLEDGEMENT
We thank Professor Emeritus Duco Hamasaki of the Bascom Palmer Eye Institute of the University of Miami for editing of the manuscript.
STATEMENT OF ETHICS
All of the procedures conformed to the tenets of the World Medical Association Declaration of Helsinki. A written Informed consent was obtained from all patients. Approval for this study was obtained from the Institutional Review Board of the Chiba University Graduates School of Medicine.
DISCLOSURE STATEMENT
The authors have no proprietary interest in the materials used in this study.
REFERENCES
- Cugati S, Wang JJ, Rochtchina E, Mitchell P (2006) Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol 124: 726-732.
- Hayreh SS, Podhajsky PA, Zimmerman MB (2009) Branch retinal artery occlusion: natural history of visual outcome. Ophthalmology 116: 1188-1194.
- Bikbova G, Oshitari T, Yamamoto S (2012) Diabetes Mellitus and retinal vein occlusion as risk factors for open angle glaucoma and neuroprotective therapies for retinal ganglion cell neuropathy. J Clin Experiment Ophthalmol 3: 002.
- Yasuda M, Kiyohara Y, Arakawa S, Hata Y, Yonemoto K, et al. (2010) Prevalence and systemic risk factors for retinal vein occlusion in a general Japanese population: the Hisayama study. Invest Ophthalmol Vis Sci 51: 3205-3209.
- Rogers SL, McIntosh RL, Lim L, Mitchell P, Cheung N, et al. (2010) Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology 117: 1094-1101.
- Wong TY, Scott IU (2010) Clinical practice. Retinal-vein occlusion. N Eng J Med 363: 2135-2144.
- Jaulim A, Ahmed B, Khanam T, Chatziralli IP (2013) Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina 33: 901-910.
- Funk M, Kriechbaum K, Prager F, Benesch T, Georgopoulos M, et al. (2009) Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Invest Ophthalmol Vis Sci 50: 1025-1032.
- Noma H, Funatsu H, Yamasaki M, Tsukamoto H, Mimura T, et al. (2008) Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye (Lond) 22: 42-48.
- Clark WL, Boyer DS, Heier JS, Brown DM, Haller JA, et al. (2016) Intravitreal Aflibercept for Macular Edema Following Branch Retinal Vein Occlusion: 52-Week Results of the VIBRANT Study. Ophthalmology 123: 330-336.
- Heier JS, Campochiaro PA, Yau L, Li Z, Saroj N, et al. (2012) Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology 119: 802-809.
- Ehlers JP, Kim SJ, Yeh S, Thorne JE, Mruthyunjaya P, et al. (2017) Therapies for Macular Edema Associated with Branch Retinal Vein Occlusion: A Report by the American Academy of Ophthalmology. Ophthalmology 124: 1412-1423.
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, et al. (2010) Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 117: 1102-1112.
- Brown DM, Campochiaro PA, Bhisitkul RB, Ho AC, Gray S, et al. (2011) Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 118: 1594-1602.
- Campochiaro PA, Clark WL, Boyer DS, Heier JS, Brown DM, et al. (2015) Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology 122: 538-544.
- Shimizu N, Oshitari T, Tatsumi T, Takatsuna Y, Arai M, et al. (2017) Comparisons of efficacy of intravitreal aflibercept and ranibizumab in eyes with diabetic macular edema. Biomed Res Int 2017: 1747108.
- Kaiho T, Oshitari T, Tatsumi T, Takatsuna Y, Arai M, et al. (2017) Efficacy of One-Year Treatment with Aflibercept for Diabetic Macular Edema with Practical Protocol. Biomed Res Int 2017: 7879691.
- Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, et al. (2015) Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology 122: 2044-2252.
- Ito Y, Saishin Y, Sawada O, Kakinoki M, Miyake T, et al. (2015) Comparison of single injection and three monthly injections of intravitreal bevacizumab for macular edema associated with branch retinal vein occlusion. Clin Ophthalmol 9: 175-180.
- Ahn SJ, Ahn J, Woo SJ, Park KH (2013) Initial dose of three monthly intravitreal injections versus PRN intravitreal injections of bevacizumab for macular edema secondary to branch retinal vein occlusion. Biomed Res Int 2013: 209735.
- Miwa Y, Muraoka Y, Osaka R, Ooto S, Murakami T, et al. (2017) Ranibizumab for macular edema after branch retinal vein occlusion: one initial injection versus three monthly injections. Retina 37: 702-709.
- Bayat AH, Çak?r A, Özturan ?G, Bölükba?? S, Erden B, et al. (2018) Comparison of one and three initial monthly intravitreal ranibizumab injection in patients with macular edema secondary to branch retinal vein occlusion. Int J Ophthalmol 11: 1534-1538.
Citation: Nagino Y, Tatsumi T, Oshitari T, Takatsuna Y, Arai M, et al (2020) Comparison of One Injection to Three Monthly Injections of Anti-Vascular Endothelial Growth Factor Agents for Macular Oedema Associated with Branch Retinal Vein Occlusion. J Ophthalmic Clin Res 7: 063.
Copyright: © 2020 Yu Nagino, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

