
Concentration Effect on Foaming and Stability of Isolates from Two Varieties (DAS & BS) of Nigerian Cultivated Solojo Cowpea (Vigna Unguiculata L. Walp)
*Corresponding Author(s):
Henry O ChibudikeDepartment Of Chemical, Fiber And Environmental Technology, Federal Institute Of Industrial Research, Oshodi, Lagos, Nigeria
Tel:+234 8023225788,
Email:henry.chibudike@fiiro.gov.ng / henrychibudike@gmail.com
Abstract
Concentration Effect on foaming capacity and stability of the various samples were investigated. Foaming capacity of raw (native/ control) and germinated Dark-ash Solojo Cowpea (FFDAS, DFDAS, FFBS and DFBS flours; DAS and BS protein isolate) were all concentration dependent. The four flour samples, FFDAS, DFDAS, FFBS and DFBS as well as the two protein isolates exhibited rise in foam capability with rise in concentration but to different degrees. The difference was manifested not only between the full fat and defatted but also varietal wise. The foaming capacity of the Raw FFDAS, 6h FFBS and Raw DFBS increased only up to 4% concentration before experiencing a decrease in foam capacity. FFDAS 24h; DFDAS Raw and 48 h; FFBS Raw, 24h, 36h and 48h; DFBS 24h; DAS isolate Raw and 36 h; BS isolate 24h, 36h and 48h all had their foaming capacity going up to 6% concentration. FFDAS 6h, 36h, 48h and 72h; DFDAS 6h and 72h; FFBS 72h, DFBS 36h and 72h; DAS 24h, 48h and 72h; BS Raw, 6h and 72h all had their foaming capacity increasing up to 8% concentration, while only DFDAS 24h, 36h and DFBS 6h and 48h had their foaming capacity increase to 10% concentration. The FC ranged between 17.30±1.52 – 88.68±1.65%; 61.78±0.21 – 106.83± 1.06%; 37.62±0.98 – 92.08±1.01%; 64.15±0.34 – 107.27±0.07%; 94.62±2.23 – 188.30± 1.57%; 109.66±3.29 – 151.67±2.52% for FFDAS, DFDAS, FFBS, DFBS, DAS and BS respectively. The Foaming Capacity (FC) for FFDAS increased with germination except at lower concentration of 2-4%. That of DFDAS showed a better response. The FC was higher than that of the FFDAS, this could be as a result of the exposure of more hydrophilic sights as a result of defatting. The DFBS likewise exhibited a higher FC compared to that of FFBS. The FC of the flours of brown solojo was found to be higher than that of the dark-ash solojo cowpea. The isolates had higher FC, with DAS having higher FC than the BS. Increase in concentration enhances greater protein-protein interaction, which increases viscosity and facilitates formation of multilayer protein film at the interface. The formation of cohesive multilayer film offers resistance to disproportional and coalescence of bubbles. In addition, increase in concentration could lead to formation of thicker films, which limits the effect of drainage of protein from films.
Keywords
BS; DAS; Essential amino acids; Food industry; Nutraceuticals; Solojo Cowpea; Under-utilized legumes
Introduction
Cowpea (Vigna unguiculata) is among the pulse’s species of greatest economic and social importance. This legume is strategic for the food security and health of millions of people in the world. Cowpea is rich in nutraceutical compounds such as dietary fibre, antioxidants and polyunsaturated fatty acids and polyphenols, whose health benefits and use in the food industry have been extensively studied. However, research on the identification of functional proteins from cowpea, their metabolic functions and applications in the food, health and other industries are still scarce.
The whipping character and potential of flours and protein isolates of legumes is depicted by foaming property of the legume. This is because, proteins foam on whipping due to their surface-active properties [1]. The inter-facial scope that can be formed by the protein determines the foaming capacity (FC) of the protein. While capability of protein to maintain foam contrary to environmental pull and internal forces is foaming stability [2]. Foaming capacity is hinged on the capability of proteins to imbibe swiftly at the air- water interface during vigorous mixing, while stability of foam is dictated by the attribute of the various layers of consistent films around the air suds that gives protection contrary to fluid leakage and droplet fusion. Foaming capability and stabilization power are restricting factors in the classification of the functionality of proteins [3].
The interfacial (surface) layer produced by proteins that sustains the froths in suspension and reduce the extent of coming together of the bubbles determines foaming capacity. The characteristic of any food foam is determined not only by the proteins but by other composition of the matrics, carbohydrates and fat existing in the flours as an example [4-6].
According to Adebowale and Lawal [7], foam formation generally increases till it reaches a peak value as the quantity of protein rose and thereafter fell. This was possibly as a result of the high lipid content at the higher concentration. Fats, when existing at quantities in excess 0.5% considerably debilitate the foaming capacity of protein for the reason that oils are more facially energetic than are proteins because of their hydrophobicity and so interact better with the air and so easily absorb at the junction between air water (interface) and prevents absorption of proteins at the time of foam formation [8].
Lawal et al. [9], similarly, observed decrease in foam stability for chemically modified bambara groundnut protein, likewise, Lawal and Dawodu [10], observed same for both native and maleylated protein derivatives of African locust bean protein isolates. This was attributed to increase in charge density of succinylated proteins, since protein and protein interaction was inhibited by it. Lawal et al. [11], also noticed a similar reduction in foam stability with rise in concentration for full fat African locust bean flour. Oil films lack the close-knit and visco-elastic properties of the foam bubbles, they swiftly expand and then breakdown; this could be the reason for decrease in FS with rise in concentration. Reduction in FS might also be due to protein unfolding.
Various foaming capacity ranges have been reported for different legumes, 30.4% - 44.3% for Chickpea cultivars [1]; while Adebowale and Lawal [7], reported a value of 58% for Mucuna bean protein concentrate. Germination was also noticed as improving the FC and FS for the BS samples than the DAS samples. Bamdad et al. [12], likewise observed an improvement in FC of germinated lentil when compared with the ungerminated. The germinated isolate samples exhibited similar trend to that shown by chemically modified isolates as well as the enzymatically modified protein isolates.
Materials And Methods
Two varieties of the underutilized cowpea (V. unguculata) found in South west region of Nigeria where it is called ‘Solojo’ were used. Seeds obtained from Bodija market in Ibadan, Western Nigeria, were screened to get rid of every irrelevant materials and unwholesome seeds. The beans were then portioned into six (6). The Solojo seeds for germination were sterilised by soaking in 0.07% Sodium hypochlorite for 30min, then, it was rinsed thoroughly. The Solojo seeds were then immersed for 6h in distilled water at ambient temperature (1:10 w/v) (~25°C), then placed in a colander and germinated under subdued light in an open laboratory for, 24, 36, 48 and 72h.
Preparation of flours
Raw flour: The grains were segregated to remove the spoilt ones; then dry dehulled with a mechanical dry dehuller (Fabricated in FIIRO), dried at 40°C and later milled dry to powder then sifted using 80µm mesh. The flour was stored in flexible bags and preserved at 4°C preceding utilization in a refrigerator freezer.
6h soaked flour: The seeds were segregated to remove the unwholesome ones, then immersed for 6h in the ratio (1:10 w/v) (seed/water). The grains were then frozen to prevent germination from setting in, then the hull was removed manually, dried for 48h at 40°C later milled dry to smooth powder prior to sieving using 80µm mesh screen. The resulting flour was packaged in plastic pack and preserved in a fridge- freezer at 4°C pending utilization.
Germination of seed: This was implemented by the method of Mubarak AE [13], with minor adjustment. The seeds for germination were disinfected by soaking in 0.07% Sodium hypochlorite Rumiyati, AP and James VJ [14], for 30mins, then, it was rinsed painstakingly. The Solojo seeds were then immersed for 6 hours at ambient temperature in water in the ratio (1:10 w/v) (seed/water) (~25oC), then placed in a colander and germinated under subdued light in an open laboratory Rusydi MR, Noraliza CW, Azrina A and Zulkhairi A [15], for various hours such as 24, 36, 48 and 72h. The process of germination was terminated by freezing, the seeds were manually dehulled, dried in a draught oven (Schutzart DIN EN 60529-IP 20. Memmert, Germany) at 40°C for 48h, cooled, milled and packaged in an air tight plastic bag in the refrigerator pending analysis (Figures 1-3).
 Figure 1: Brown Solojo Cowpea.
Figure 1: Brown Solojo Cowpea.  Figure 2: Dark-Ash Solojo Cowpea.
Figure 2: Dark-Ash Solojo Cowpea. 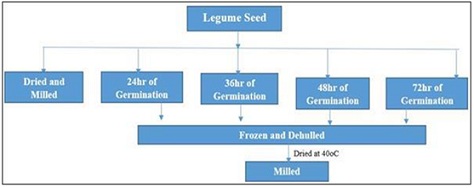
Figure 3: Preparation of Beans Flour/Schematic representation.
Discussion Of Experimental Results
The impact of concentration on foaming characteristics is presented in tables 1-12. A similar observation of increase in foaming capacity up to a certain concentration before falling was observed by Lawal et al. [11], for Parkiabiglobosa flour as reported by [16].
|
FFDAS |
2% |
4% |
6% |
8% |
10% |
|
Raw |
67.21±1.24a |
69.42± 1.95a |
59.56± 1.36c |
71.09± 1.55e |
62.02±1.77cd |
|
6h |
66.04±2.33ab |
70.96±2.41b |
80.14±3.73b |
84.25±0.52a |
81.55±1.68a |
|
24h |
63.16±3.29b |
67.31±0.31b |
88.68±1.65a |
70.97±4.93c |
63.69±4.80cd |
|
36h |
38.48±1.54c |
58.89±2.42c |
67.95±2.88c |
78.72±1.56b |
65.76±0.30c |
|
48h |
17.30±1.52d |
30.55±3.60d |
53.71±0.50d |
63.64±0.58d |
60.72±0.54d |
|
72h |
39.04±0.58c |
77.78±0.93a |
79.49±5.00b |
82.32±0.87ab |
74.24±1.61b |
Table 1: Effect of concentration on foaming capacity (%) of (FFDAS).
Note: FFDAS- Full fat dark ash Solojo Cowpea; Means in colomn not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
The foaming capacity increased to 6% before falling. The foaming capacity ranged between 47.8±0.9 and 79.1±1.9% for the undefatted African locust bean and between 66.4±0.8 to 93.6±2.1% for the defatted African locust bean flour. Yellavila et al. [6], reported the foaming capacities of the different legume flours of five lima beans sample to range from 19.21% to 22.13%. These observed values were lower than that obtained for the germinated samples both for the full fat and defatted. While that of African locust bean compared favorably with those of the flours. However, Akubor and Badifu [17], recorded a FC value of 40% for Wheat flour.
|
DFDAS |
2% |
4% |
6% |
8% |
10% |
|
Raw |
72.41±1.26c |
78.44±0.94f |
86.55±0.95d |
78.95±0.18f |
84.76±0.83d |
|
6h |
71.97±1.19c |
88.13±1.05b |
93.17±1.06b |
97.60±1.01b |
94.59±1.75b |
|
24h |
69.56±1.17d |
80.31±1.02d |
91.39±1.05c |
94.51±3.12c |
94.57±1.72b |
|
36h |
61.78±0.21e |
86.46±1.11bc |
86.58±1.09d |
91.47±1.04d |
91.49±1.07c |
|
48h |
72.15±1.03c |
84.74±1.10c |
93.38±1.10b |
87.95±0.99e |
100.61±1.05a |
|
72h |
85.89±1.24b |
97.48±1.12a |
97.81±0.53a |
106.83±1.06a |
101.22±1.05a |
Table 2: Effect of concentration on foaming capacity (%) of (DFDAS).
Note: DFDAS- Defatted dark ash Solojo Cowpea; Means in colomn not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
The observed increase in foaming capacity of the germinated DAS and BS protein isolate is also similar to that reported by various other researchers such as; [9,10,18], for Mung bean protein isolate; succinylated and acetylated protein isolates; native and maleylated protein derivatives of African locust bean protein isolates) they all reported significant increase of foaming capacity and stability with rise in sprouting period and increase in chemical modification respectively. A similar increase was also reported for microbial transglutaminase treated Cajanuscajan and Lablab purpureus protein isolates by Ali et al. [19]. These could be due to increase solubility of protein.
|
FFBS |
2% |
4% |
6% |
8% |
10% |
|
Raw |
71.88±1.11a |
73.09±0.86c |
75.42±2.03c |
72.29±2.47c |
67.25±0.78d |
|
6h |
64.85±1.22b |
84.76±0.15a |
84.54±0.51a |
83.90±1.12b |
84.34±0.98b |
|
24h |
42.86±0.48d |
51.77±0.27f |
64.88±0.79d |
62.85±0.76e |
62.42±1.05e |
|
36h |
37.62±0.98e |
64.71±0.19e |
65.64±1.16d |
65.25±1.53d |
68.29±0.93d |
|
48h |
43.41±0.99d |
66.77±0.48d |
80.13±1.03b |
73.86±0.90c |
70.21±0.98c |
|
72h |
54.55±0.61c |
78.23±1.05b |
85.49±1.13a |
92.08±1.01a |
90.28±1.03a |
Table 3: Effect of concentration on foaming capacity (%) of full fat brown solojo cowpea (FFBS).
Note: FFBS- full fat brown Solojo Cowpea flour.
A very important factor in foaming capacity is the rapidity in which protein lowers the surface tension, smaller, elastic and transformed proteins diminish the surface pressure more readily and easily, compared with other firmer and bigger proteins. The prominent protein fraction in the isolate also affects the foaming capacity, for example if the globulin fraction is higher than the albumin, the foaming capacity is found to be less than the isolate with higher albumin. This is because, albumins are more water soluble than globulins. The observed reduction in foaming capacity at 6% and 8% may be due to insufficient electrostatic repelling, cummulating in insufficient solubility and hence too much protein-protein interplay. The rate of foaming of flour has also been associated with the quantity of native protein in the sample.
|
(DFBS) |
2% |
4% |
6% |
8% |
10% |
|
Raw |
78.24±1.54bc |
93.12±2.89b |
91.95±1.05b |
87.95±0.99e |
88.50±1.94e |
|
6h |
80.95±0.18a |
96.89±1.06a |
100.02±3.25a |
101.84±0.02c |
103.04±1.09b |
|
24h |
76.92±0.22c |
92.45±0.07b |
101.85±0.02a |
94.59±0.05d |
98.19±0.02c |
|
36h |
73.71±1.33d |
85.18±0.14d |
88.99±0.10b |
101.82±0.02c |
94.55±0.05d |
|
48h |
79.24±0.20b |
89.09±0.10c |
100.02±3.16a |
107.27±0.07a |
107.27±0.07a |
|
72h |
64.15±0.34e |
85.58±0.01d |
89.09±0.09b |
105.36±0.05a |
94.59±0.05d |
Table 4: Effect of concentration on foaming capacity (%) of defatted brown solojo cowpea.
Note: DFBS-Defatted brown Solojo Cowpea flour; Means in colomn not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
|
DAS |
2% |
4% |
6% |
8% |
10% |
|
Raw |
94.62±2.23d |
144.09±3.24b |
152.43±3.16b |
142.51±2.04d |
140.19±2.42c |
|
6h |
131.33±1.15a |
152.67±3.06a |
155.33±5.03b |
162.00±2.00c |
175.33±1.15a |
|
24h |
117.33±2.31c |
125.00±1.73cd |
128.67±1.15c |
132.83±1.04d |
128.92±1.01d |
|
36h |
116.67±1.15c |
121.33±2.31d |
132.67±1.15c |
124.33±0.58e |
124.17±3.62e |
|
48h |
118.00±2.00bc |
128.33±1.53c |
161.67±1.53a |
188.30±1.57a |
143.33±3.06bc |
|
72h |
121.00±1.00b |
122.83±2.57d |
152.67±1.15b |
165.00±1.73b |
145.22±1.35b |
Table 5: Effect of concentration on foaming capacity of (%) DAS protein isolate.
Note: Dark-ash Solojo Cowpea protein isolate; DAS Means in colomn not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
Foaming ability is not only influenced by the nature of protein and fat, it is also affected by pH, method of processing, temperature, whipping method, presence or absence of sugar and salt such as calcium ion, duration of heating as well as solubility. Foam capacity was also observed to reduce with germination time up to 36hrs, but increased again from 48h. This was also observed by Akaerue and Onwuka [20], for germinated Mung bean. Igbabul et al. [21], likewise expressed decrease in FC with rise in fermentation time for Mucunasloanei and Detariummicrocarpum.
|
BS |
2% |
4% |
6% |
8% |
10% |
|
Raw |
109.66±3.29c |
123.35±2.25cd |
127.28±2.36c |
137.55±3.17b |
120.83±2.03d |
|
6h |
114.67±3.06bc |
126.67±3.06bc |
140.00±2.00ab |
151.67±2.52a |
136.67±1.15b |
|
24h |
131.33±4.16a |
140.67±3.06a |
143.33±4.16a |
131.33±4.16c |
143.33±3.06a |
|
36h |
116.00±2.00b |
122.00±2.00d |
127.33±3.06c |
123.33±3.06d |
118.00±2.00d |
|
48h |
113.33±1.15bc |
128.00±2.00b |
144.67±3.06a |
130.67±1.15c |
136.67±1.15b |
|
72h |
127.00±3.00a |
129.67±2.08b |
137.33±1.15b |
149.67±1.53b |
130.00±2.00c |
Table 6: Effect of concentration on foaming capacity (%) of BS protein isolate.
Note: BS-Brown Solojo Cowpea protein isolate; Means in colomn not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
Foam Stability (FS) for all the samples improved with rise in concentration as shown in Tables 1-6. The FS is essential as the advantage of whipping agents lies on their ability to preserve the whip as long as possible. Foaming Capacity (FC) and Foam Stability (FS) of flours and isolate from different Solojo cowpea accession were also observed to differ significantly, this is also observed for different chickpea cultivars as reported by Kaur and Singh [22]. Sreerama et al. [23], also observed that Vigna unguiculata flour possess higher capacity to create stable and united coating round air bubble thereby creating more opposition to air migration from the bubbles, hence better foam stability.
|
FFDAS |
2% |
4% |
6% |
8% |
10% |
|
Raw |
31.81±3.99a |
37.28±2.23b |
47.01±0.85b |
41.57±0.85b |
41.34±0.57b |
|
6h |
35.62±1.16a |
51.56±1.77a |
53.74±1.90a |
63.99±2.13a |
62.16±1.20a |
|
24h |
31.14±4.25a |
38.46±0.59b |
38.72±3.21c |
40.04±3.77b |
40.63±3.07b |
|
36h |
18.27±1.97b |
27.74±3.05c |
35.54±0.57c |
37.08±3.88b |
34.25±1.98c |
|
48h |
11.88±2.54c |
12.59±1.29e |
24.07±0.70e |
23.94±1.85c |
14.29±1.55d |
|
72h |
17.45±1.83b |
20.00±1.66d |
29.20±2.91d |
37.80±1.93b |
41.73±2.38b |
Table 7: Effect of concentration on foam stability (%) of FFDAS after 4 h at room temperature (30±2oC).
Note: FFDAS- Full fat dark ash Solojo Cowpea; Means in colomn not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
Decrease in foam volume with time was observed with all the samples, this was equally observed by Arawande and Borokini [24], for Canavaliaensiformis, Cajanuscajan and Vignaunguiculata flours with values reducing from 20.67±0.41 to 10.33±0.41; 3.53±0.36 to 3.05±0.10; and 16.33±0.37 to 15.70±0.31% respectively. The foam stability values ranged between 11.88±2.54 - 63.99±2.31%; 15.82±1.39 - 88.57±2.64%; 27.16±0.40 - 60.07±1.01%; 45.74±0.95 - 96.36±0.03%; 57.33±3.06 -125.18±4.60; 63.48±2.93 - 106.67±2.31%. For, FFDAS, DFDAS, FFBS, DFBS, DAS and BS respectively. The values of foam stability were however higher than those obtained by Arawande and Borokini [24], for Canavalia ensiformis and Cajanuscajan but compareable to that of Cowpea, which were 10.33±0.41, 3.05±0.10 and 15.70±0.31% respectively. Also reported values of 1.96±0.36 and 0.16±0.03% respectively for two species of Vigna subterranean VS1 and VS2 respectively.
|
DFDAS |
2% |
4% |
6% |
8% |
10% |
|
Raw |
31.43±2.75b |
37.61±1.54c |
49.84±1.48a |
48.53±1.43a |
48.97±0.69a |
|
6h |
32.48±0.97e |
60.63±1.70d |
77.33±1.31c |
84.96±2.60b |
88.57±2.64a |
|
24h |
30.75±1.08e |
42.15±1.25d |
61.24±1.67c |
79.21±0.89b |
83.06±2.47a |
|
36h |
18.48±2.50e |
31.70±3.04d |
46.34±0.77c |
65.25±1.67b |
72.64±0.14a |
|
48h |
15.82±1.39e |
34.14±2.13d |
48.20±2.38c |
65.09±4.20b |
81.33±0.84a |
|
72h |
44.88±1.77d |
59.13±2.66c |
72.51±2.73b |
81.36±1.93a |
83.54±0.09a |
Table 8: Effect of concentration on foam stability (%) of DFDAS after 4 h at room temperature (30±2oC).
Note: DFDAS- Defatted dark ash Solojo Cowpea; Means in colomn not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
Chinma et al. [25], observed increase in foaming capacity with germination time, with 8.60±0.70% - 12.91±0.61% for the brown variety of tigernut flour, and 7.75±1.50 – 11.40±0.56% for the yellow variety tigernut flour. This value was however found to be lower than that obtained for the samples in this study. According to Arawande and Borokini [24], foaming capacity as low as 16.33±0.37 and 20.67±0.41% can be used as aerifying agents in food arrangement such as “bean balls” and ‘bean pudding’ which needs the production of lasting huge fluffy volumes when whipped. Cucumeropsismannii seed flour protein isolate, with foaming capacity and stability of 30.00±1.00 and 5.00±1.00% have been found to be useful in the production of ice-cream and yogurt which is in agreement with the findings of Ogunbusola et al. [26].
|
FFBS |
2% |
4% |
6% |
8% |
10% |
|
Raw |
36.73±0.92a |
42.38±1.62a |
41.91±2.29c |
45.73±2.58c |
43.87±2.61cd |
|
6h |
27.16±0.40cd |
31.44±2.74b |
29.97±1.57d |
32.50±2.35d |
32.53±0.34e |
|
24h |
27.28±0.30cd |
31.83±0.98b |
50.00±1.26ab |
44.89±1.25c |
41.82±1.82d |
|
36h |
28.94±1.75c |
42.42±1.60a |
50.31±1.62ab |
56.11±1.45b |
51.84±1.61b |
|
48h |
26.69±1.59d |
41.62±0.76a |
48.45±0.97b |
45.29±0.29c |
45.29±1.58c |
|
72h |
31.17±0.35b |
42.59±0.97a |
52.68±1.90a |
60.07±1.01a |
59.88±1.65a |
Table 9: Effect of concentration on foam stability (%) of FFBS after 4h at room temperature 30oC±2.
Note: FFBS-Full fat brown Solojo Cowpea flour; Means in colomn not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
|
DFBS |
2% |
4% |
6% |
8% |
10% |
|
Raw |
45.74±0.95c |
48.75±0.97d |
58.53±1.61e |
59.64±0.62f |
61.24±2.08e |
|
6h |
50.49±2.92b |
70.10±1.75a |
77.27±2.14b |
81.65±0.17c |
80.03±2.81c |
|
24h |
53.21±1.85ab |
60.38±0.37b |
79.63±0.19b |
76.58±0.21d |
87.99±1.05b |
|
36h |
51.92±0.46ab |
54.32±1.15c |
61.47±0.35d |
72.73±0.25e |
70.91±0.26d |
|
48h |
54.72±1.71a |
58.17±2.20b |
83.49±0.15a |
90.91±0.08a |
96.36±0.03a |
|
72h |
47.17±0.50c |
58.56±0.37b |
73.95±1.91c |
89.29±0.10b |
85.58±0.13b |
Table 10: Effect of Concentration on Foam stability (%) of DFBS after 4 h at room temperature 30oC±2.
Note: DFBS-Defatted brown solojo cowpea flour; Means in colomn not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
Reduction in foam quantity with time was observed in all protein isolates. These were also the observation of all previous researchers like [3], for Quinoa protein isolate; for Gourd melon; for Cowpea, Pigeon pea, Pea and Mung bean [11], for native and succinylated Lablab concentrate, Mucuna bean protein concentrate [27,28].
|
DAS |
2% |
4% |
6% |
8% |
10% |
|
Raw |
59.53±1.28e |
114.68±1.33a |
125.18±4.60a |
114.39±4.27b |
106.69±2.42c |
|
6h |
76.33±1.53d |
108.33±1.53b |
120.67±1.15a |
121.33±1.53a |
125.33±2.31a |
|
24h |
57.33±3.06e |
108.33±1.53b |
112.67±1.15b |
116.58±1.23b |
112.67±1.15b |
|
36h |
96.67±1.15a |
101.58±1.42c |
109.67±2.08b |
85.33±2.31d |
76.67±1.15e |
|
48h |
86.53±3.11c |
97.33±2.31d |
98.25±2.05c |
109.67±2.08c |
98.00±2.00d |
|
72h |
92.67±1.15b |
97.00±1.00d |
121.33±2.31a |
124.67±1.15a |
100.67±1.15d |
Table 11: Effect of concentration on foam stability (%) of DAS protein isolate after 4h at room temperature 30oC±2.
Note: DAS-Dark- ash solojo cowpea protein isolate; Means in colomn not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
|
BS |
2% |
4% |
6% |
8% |
10% |
|
Raw |
63.48±2.93d |
89.40±0.95bc |
91.44±2.58bc |
100.18±2.28a |
106.20±1.09a |
|
6h |
69.33±3.06c |
82.67±2.31d |
95.33±1.15ab |
104.67±4.16a |
106.67±2.31a |
|
24h |
85.33±4.62a |
88.00±3.46c |
96.67±4.16ab |
88.00±2.00b |
92.67±4.16b |
|
36h |
70.00±2.00c |
93.33±3.06ab |
100.67±4.16a |
70.00±2.00c |
66.00±3.46d |
|
48h |
74.67±3.06bc |
97.33±2.31a |
101.33±4.16a |
103.33±3.06a |
96.67±4.16b |
|
72h |
76.33±1.53b |
82.33±2.52d |
88.67±2.08c |
102.33±2.08a |
85.33±1.53c |
Table 12: Effect of concentration on foam stability (%) of BS protein isolate after 4h at room temperature 30oC±2.
Note: BS-Brown solojo cowpea protein isolate; Means in colomn not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
Conclusion and Recommendation
Biochemical modification which involves the activation of the intrinsic enzymes of the Solojo cowpea seed itself by germination was carried out for different hours for the two varieties, i.e. the Dark-Ash and the Brown Solojo beans. This research work shows that biochemical modification (Germination/Malting/ Sprouting) had an enormous impact on the nutritional composition, functional properties, mineral bioavailability, anti-nutrient content and amino assay of Solojo bean, thus, it could be used as protein supplement in infant, young children and geriatric foods.
Efforts should be increased to promote the cultivation, encourage the consumption and industrial application of this underutilized legume by the Government, especially in the south-western region where it can survive the rain fall level. Large scale production of this legume which is gradually going into extinction should be encouraged in order to fight the menace of malnutrition in developing countries where animal protein price is exorbitant; This will ensure food security and also creation of jobs, because people can engage in different aspects of the production process and thereby reducing the rate of unemployment
References
- Kaur M, Singh N (2007) Characterization of protein isolates from different Indian chickpea (Cicer arietium L.). cultivars. Food Chemistry 102: 366-374.
- Ahmed SH, Ahmed IAM, Eltayeb MM, Ahmed SO, Babiker EE (2011) Functional properties of selected legumes flour as influenced by pH. Journal of Agricultural Technology 7: 2091-2102.
- Elsohaimy SA, Refaay TM, Zaytoun MAM (2015) Physicochemical and functional properties of quinoa protein isolate. Annals of Agrictural Science 60: 297-305.
- Ojieh GC, Oluba OM, Ogunlowo YR, Adebisi KE, Eidangbe GO, et al. (2008) Compositional studies of Citrullus lanatus (Egusi melon) seed. The Internet Journal of Nutrition and Wellness 6: 511-521.
- Dossou VM, Agbenorhevi JK, Alemawor F, Oduro I (2014) Physicochemical and functional properties of full fat and defatted ackee (Blighia sapida) aril flours. American Journal of Food Science and Technology 2: 187-191.
- Yellavila SB, Agbenorhevi JK, Asibuo JY, Sampson GO (2015) Proximate composition, minerals content and functional properties of five lima bean accessions. Journal of Food Security 3: 69-74.
- Adebowale KO, Lawal OS (2004) “Comparative study of the functional properties of bambarra groundnut (Voandzeia subterranean), jack bean (Canavalia ensiformis) and mucuna bean (Mucuna pruriens) flours”. Food Research International 37: 355-365.
- Bhat R, Shaharuddin NA, Kuang YT (2014) A promising approach toward exploring nutritional and functional qualities of beko (Oroxy lumIndicum Benth. Ex Kurz) pods for potential food applications. Journal of Food Processing and Preservation 39: 47-55.
- Lawal OS, Adebowale KO, Adebowale YA (2007) Functional properties of native and chemically modified protein concentrate from bambara groundnut. Food Research International 40: 1003-1011.
- Lawal OS, Dawodu MO (2007) Maleic anhydride derivatives of a protein isolate: Preparation and functional evaluation. European Food Research and Technology 226: 186-198.
- Lawal OS (2005) Functionality of reactive and succinylated lab lab bean (Lablab Purpureus) protein concentrate. Food Hydrocolloids 19: 63-72.
- Bamdad F, Dokhani S, Javad K (2009) Functinal Assessment and subunit constituent of Lentil (Leis Culinaris) proteins during germination. International Journal of Agriculture and Biology 11: 690-694.
- Mubarak AE (2005) Nutritional composition and anti nutritional factors of Mung bean seeds (Phaseolus auneus) as affected by some home traditional processes. Food Chemistry 89: 489-495.
- Rumiyati AP, James VJ (2012) Effect of germination on the nutritional and protein profile of Australian sweet lupin (Lupinus angustifolius) Food and Nutrition Science 3: 621-626.
- Rusydi MR, Noraliza CW, Azrina A, Zulkhairi A (2011) Nutritional changes in germinated legumes and rice varieties. International food Research Journal 18: 705-713.
- Chel-Guerrero L, Gallegos-Tintore S, Martinez-Ayala A, Castellanos-Ruelas A, Betancur-Ancona D (2011) Functional properties of proteins from lima bean (Phaseolus lunatus ) seeds. Food science and Technology International 17: 119-126
- Akubor PI, Badifu GIO (2004) Chemical composition, functional properties and baking potential of African breadfruit kernel and wheat flour blends. International Journal of Food Science and Technology 39: 223-229.
- Udensi EA, Okonkwo A (2006) Effect of fermentation and germination on the physicochemical properties of Mucunacochinchinenesis protein isolate: African Journal of Biotechnology 5: 896-900.
- Ali NA, Ahmed SH, Mohamed EA, Ahmed IAM, Babiker EE (2010) Changes in functional properties by transglutaminase cross linking as a function of pH of legumes protein isolate. Innovative Romanian Food Biotechnology 7: 12-20.
- Akaerue BI, Onwuka GO (2010) Evaluation of the yield, protein content and functional properties of Mungbean (Vignaradiata (L) Wilckzek) Protein isolates as affected by processing. Pakistan journal of Nutrition 9:728-735.
- Igbabul B, Hiikyaa O, Amove J (2014) Effect of fermentation on the proximate composition and functional properties of mahogany bean (Afzelia Africana) flour. Current Research in Nutrition and Food Science 2: 1-7.
- Kaur M, Singh N (2006) Relationships between selected properties of seeds, flours, and starches from different chickpea cultivars. International Journal of Food Properties 9: 597-608.
- Sreerama YN, Sashikala VB, Pratape VM (2012) Phenolic compounds in cowpea and horse gram flours in comparison to chickpea flour: Evaluation of their antioxidant and enzyme inhibitory properties associated with hyperglycemia and hypertension. Food Chemistry 133: 156-162.
- Arawande JO, Borokini FB (2010) Comparative study on chemical composition and functional properties of three Nigerian legumes (Jack Beans, Pigeon Pea and Cowpea). Journal of Emerging Trends in Engineering and Applied Sciences (JETEAS) 1: 89-95.
- Chinma CE, Adewumi, O, Abu JO (2009) Effect of germination on the chemical, functional and pastry property of flour from brown and yellow varieties of tiger nut (Cyperus esculentus). Food Research International 42: 1004-1009.
- Ogunbusola EM, Fagbemi TN, Osundahunsi OF (2013) In-vitro protein digestibility, amino acid profile, functional properties and utilization of white melon (Cucumeropsis mannii) protein isolates. Journal of Food Science and Technology 4: 153-159.
- Adebowale KO, Lawal OS (2003) Foaming, gelation and electrophoresic characteristics of mucuna bean (Mucuna pruriens) Protein concentrates. Food Chemistry 93: 237-246.
- Lawal OS (2004) Functionality of African locust bean (Parkia biglobossa) protein isolate: Effect of pH, Ionic strength and various protein concentrations. Food Chemistry 86: 345-355.
Citation: Adeyoju OA, Adebowale KO, Oluwole BO, Ndukwe NA, Chibudike HO, et al. (2022) Concentration Effect on Foaming and Stability of Isolates from Two Varieties (DAS & BS) of Nigerian Cultivated Solojo Cowpea (Vigna Unguiculata L. Walp). J Food Sci Nutr 8: 133.
Copyright: © 2022 Olubamike A Adeyoju, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

