
Determination of Solvents in Ballpoint Pen Ink by Gas Chromatograhy Mass Spectrometry (GC-MS)
*Corresponding Author(s):
Eda KirisIstanbul University-Cerrahpasa Institute Of Forensic Sciences And Legal Medicine, Department Of Science, Istanbul, Turkey
Email:eda.kiris@ogr.iuc.edu.tr
Abstract
Forensic Document examination is a specialized area that focuses on examining the alterations that have been made to legal documents, as well as identifying their contents. The primary inquiry that forensic document experts are called upon to answer by the legal system is the date when a document was created. To determine this, specialists in forensic document examination have conducted research on how dyes, resins, and solvents present in ink change over time. They have attempted to create several techniques and protocols for identifying these changes. One of these approaches is solvent analysis, which examines how ink ages over time. However, the aging process of ink is affected by various factors such as temperature, light, and humidity, which makes it quite challenging to determine the exact date of a document. Recent research indicates that phenoxyethanol is useful in determining the age of ink since it undergoes time-dependent changes. The purpose of study is to develop a single method that analyze phenoxyethanol (PE), phenoxyetoxyethanol (PEE), Proplylene Glycol (PG) and etoxyethanol (EE) with Gas Chromatography Mass Spectrometry (GC-MS).In this study, a method that allows analysis of solvents in blue ballpoint ink that are phenoxyethanol (PE), phenoxyetoxyethanol (PEE), Proplylene Glycol (PG) and etoxyethanol (EE) with Gas Chromatography Mass Spectrometry (GC-MS) for use of forensic science laboratories has been develop and validated. Also age curve of phenoxyethanol (PE) was drawn up to 280 days and age curve of phenoxyetoxyethanol (PEE) was drawn up to 230 days by using developed method. Further studies in ink aging analysis can be extended by adding different color of ballpoint pens, different types of pens such as gel pens or analysing different solvents in ink composition. Thus, justice will be served by clarifying more document forgery cases.
Keywords
Forensic science; GC-MS; Ink aging; Questioned document; Solvents
Introduction
One of the most frequently committed offences is document forgery. This type of crime is the subject of forensic document examination and is detected by forensic document experts. The use of methods to determine the time of writing of suspicious writings that are subject of the case in service of judicial system system not only ensures that justice is served, but also reduces the workload of the judiciary. In order to clarify this type of crime, the judicial system asks two main questions to the experts. The first one is who created the document and the second one is when it was created. While there are many optic techniques for answer to first question, the number of both analytical methods and experts for answer to second question is very low. The studies on age determination have been carried out since 1903 [1,2]. The basis of age determination studies is to analyse and interpret the time-dependent changes of the substances in the ink structure by analytical techniques. These changes are degradation of dyes or pigments, polymerisation of resins and evaporation of solvents.
Inks in ballpoint pens generally consist of a mixture of dye-based colourants, resins and solvents that are insoluble in water but soluble in organic solvents or oils. According to Weyerman and Bügler, solvents are 50%, colourants and pigments 25% and resins 25% of the ink. Other components are in very small proportions, e.g. surfactants, corrosion inhibitors, thinning agents, etc. [3]. Solvents are used to lighten the colour of ink and to ease the transition from ink to paper. Oil-based solvent, originally used as a solvent for ballpoint pens, has a higher density than water and therefore has a low boiling point and vapour pressure, so it remains in the pen chamber for a long time and is used. Glycol-based solvents keep the ink in the cartridge fluid, but allow the ink to dry quickly after application to the paper. Initially olefins, castor oil or mineral oils were used as solvents. Today, chemicals such as phenoxyethanol, phenoxyethoxyethanol, propylene glycol, benzyl alcohol, butylene glycol, etc. are preferred as ink solvents. Chemical structure of these solvents are shown (Figure 1).
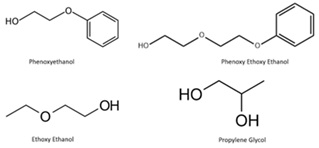 Figure 1: Chemical structure of solvents in ballpoint pen ink.
Figure 1: Chemical structure of solvents in ballpoint pen ink.
Avci et al. found the equation in (Figure 2) which explains the change of evaporation of volatile solvents from non-volatile solvents with respect to time developed at the end of 1990s [4].
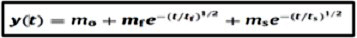 Figure 2: The equation that was found by Avci et al.
Figure 2: The equation that was found by Avci et al.
In his 2012 paper, Cantu showed that, based on this equation, if the non-volatile solvent in Avci's model is replaced by the cellulose of the paper and the volatile solvent is replaced by phenoxyethanol, the solvent of the ink, the ink aging curve obtained in practice is in accordance with the mathematical equation in the model [5]. (Figure 3) shows the ink aging curve of Cantu.
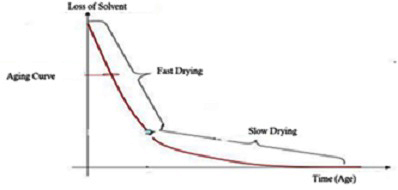 Figure 3: The ink aging curve of Cantu.
Figure 3: The ink aging curve of Cantu.
In order to determine the creation time of the document, the dyestuffs [6-11] and resins [12-15] in the ink were also analysed. Since the time difference required for dyestuff and resin analyses is greater than the time difference required for solvent analysis, it is used in the analysis of documents 4 years or older [2]. Solvent analysis is used in the chronological order of inks used on the same document at different times within the same year. Also, result of literature review, it was determined that there are more studies on the time-dependent change of phenoxyethanol [16-25]. However recent studies focuses ink aging analysis by using different analytical instruments and different componenets in ink structure such as dyes, solvents and resins [26-28].
This study aims to develops a method for analyse the detection of phenoxyethanol, phenoxyethoxyethanol, ethoxyethanol and propylene glycol solvents using GC-MS. In addition to drawing the age curve of phenoxyethanol solvent, it was aimed to draw the age curves of phenoxyethoxyethanol, ethoxyethanol and propylene glycol solvents in the structure of ballpoint pen ink to increase the discrimination power of suspicious writings in suspicious documents.
Materials And Methods
Materials
Chemicals used as referece were pure phenoxyethanol (PE), ethoxyethanol (EE), Propylene Glycol (PG) purchased from Sigma-Aldrich,Germany and phenoxyethoxyethanol (PEE) purchased from TCI, Japan. 10 blue ballpoint pens of different brands and models (Table 1) were collected from the local markets. Blue ballpoint pens were applied on standart white A4 office paper from CopierBond (80 g/m2, Türkiye). Extraction of solvents from ballpoint pens that applied on A4 paper were made with dichloromethane (DCM, Merck, Germany) containing internal standart 1,3-benzodioxol-5-methanol (IS, Sigma-Aldrich,Germany). Extraction was made in 1.5 ml glass vials (Agilent, USA).
|
Brand and Model of Blue Ballpoint Pens |
|
Bic Round Stic |
|
Faber Castell 1425 |
|
Gestetner |
|
Mikro M-25 |
|
Office Time |
|
Pensan Büro |
|
Pensan My Tech |
|
Pensan Ofis |
|
Scrikss F108 |
|
Uni-Ball Laknock |
Table 1: Brand and Model of Blue Ballpoint Pens.
Instrumentation
For better dissolution samples were vortexed VTX-3000 L from Mixer Uzusio (LMS, Japan) and kept in water bath BM-302 from Nüve (Nüve, Türkiye). Analysis of the solvents was made on a Gas Chromatography-Mass Spektrometer 7820A/5977E MSD from Agilent (Agilent Technologies, USA). Separation was carried out on a HP 5MS capillary column from Agilent (Agilent Technologies, USA). The column was 30 m long and had an internal diameter of 0.25 mm and film thickness of 0.25 µm. The chromatographic elution was temperature programmed as follows: at 400C for 2 min, then from 400C to 2000C at a rate of 10 0C/min, and finally at 4000C for 2 min. Totaly analysis takes 11 minutes. The carrier gas was helium with a constant flow of 1 ml/min. For the chromatographic separation, a solvent delay of 5 min. was chosen. The sample was injected in the splitless mode and the injector temperature was maintained at 2500C. The MS part of the GC/MS was a highly sensitive quadrupole instrument with a mass range up to 1000 u. For qualitative analysis, the instrument was used in the selected ion monitoring (SIM) mode. 12 ions were selected and monitored, corresponding to the masses: 43, 45, 59, 65, 72, 77, 93, 94, 135, 138, 152 and 182 u.
Sample Preparation
Lines were drawn on paper with blue ballpoint pens and the help of a ruler by the same person every month to eliminate the press difference. Lines were about 0.5 mm wide, 20 cm long and 5 cm intervals (the reason for this distance is that there is no interference due to the horizontal and vertical diffusion of the solvents in the ink on the paper surface). In order to prevent solvent contamination in created documents, they were placed one by one in transparent files and filed. They were stored at a temperature of 23-25oC and a humidity range of 45-55%. These values were measured with a temperature-humidity meter. To determine calibration curves, pure solvents were dissolved in dichloromethane at concentrations of 0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 mg/ml with 0.05 mg/ml internal standard concentration. Then validation of the method was performed. After method validation samples of 1 mm x 5 mm, 20 rectangles were taken from aged documents at the time of first drawing and then every month. The samples were placed in 1.5 ml vials with DCM and internal standard. To increase dissolution samples were vortexed for 5 min. and kept in a water bath at 80oC for an hour. Then samples analysed by GC-MS.
Results
For specificity and selectivity parameters, the internal standard was added on the blank paper sample and analyzed by GC-MS to detect whether there was interference from the paper at the retention times of the solvents. Then 0.1 mg/ml mixture solution was prepared and analyzed. The chromatogram of the blank paper is shown in (Figure 4) and the chromatogram of the mixture is shown in (Figure 5). As a result, it was determined that retention time and specific ions of solvents. It was shown in (Table 2).
 Figure 4: Chromatogram of blank paper sample containing internal standard.
Figure 4: Chromatogram of blank paper sample containing internal standard.
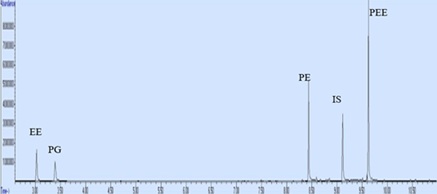 Figure 5: Chromatogram of 0.1 mg/ml mixture of solvents containing internal standard.
Figure 5: Chromatogram of 0.1 mg/ml mixture of solvents containing internal standard.
|
Analytes |
EE |
PG |
PE |
IS |
PEE |
|
Retention Time (min) |
3.026 |
3.391 |
8.447 |
9.115 |
9.626 |
|
Specific Ions (m/z) |
59, 72, 45, 43 |
45 |
94, 138, 77 |
152, 135, 93, 65 |
94, 45, 182, 77 |
Table 2: Retention times and specific ions of analytes.
For Limit Of Detection (LOD) and Limit Of Quantitation (LOQ) was obtained by preaparing phenoxyethanol, phenoxyethoxyethanol, propylene glycol and ethoxyethanol mixture solutions at the concentrations of 0.1 mg/ml, 0.05 mg/ml and 0.025 mg/ml and analyzed in GC-MS. Result of this, LOD and LOQ values were determined as 0.025 mg/ml. Result was shown in (Figure 6). Mixture solutions of 0.1, 0.3 and 0.5 mg/ml were prepared by the same analyst on the same day and given to the system in 3 consecutive repetitions. The mean peak area, Standard Deviation (SD) and Relative Standard Deviation (%RSD) values were calculated and shown in (Table 3).
 Figure 6: Superimposed chromatograms of 0.1 mg/ml, 0.05 mg/ml and 0.025 mg/ml mixture solutions.
Figure 6: Superimposed chromatograms of 0.1 mg/ml, 0.05 mg/ml and 0.025 mg/ml mixture solutions.
|
Cons. (mg/ml) |
Solvents |
1st Analyze |
2nd Analyze |
3rd Analyze |
Mean Area |
SD |
%RSD |
|
0.1
|
EE |
3281608 |
3154131 |
3265159 |
3233633 |
69340 |
2,144 |
|
PG |
2228935 |
2315968 |
2478337 |
2341080 |
126583 |
5,407 |
|
|
PE |
5166014 |
4903437 |
5076309 |
5048587 |
133466 |
2,644 |
|
|
PEE |
1082025 |
1043091 |
1121718 |
1082278 |
39314 |
3,633 |
|
|
0.3
|
EE |
7037628 |
8069216 |
8251549 |
7786131 |
654602 |
8,407 |
|
PG |
10606901 |
12332362 |
12223089 |
11720784 |
966197 |
8,243 |
|
|
PE |
13113263 |
14843649 |
14028608 |
13995173 |
865677 |
6,186 |
|
|
PEE |
27676662 |
32728571 |
27041014 |
29148749 |
3116465 |
10,692 |
|
|
0.5
|
EE |
13375306 |
12622643 |
10949090 |
12315680 |
1241894 |
10,084 |
|
PG |
22022801 |
20025194 |
18134474 |
20060823 |
1944408 |
9,693 |
|
|
PE |
20695200 |
18147709 |
19914240 |
19585716 |
1305133 |
6,664 |
|
|
PEE |
41865048 |
43744745 |
39622002 |
41743932 |
2064038 |
4,945 |
Table 3: The mean peak area, Standard Deviation (SD) and Relative Standard Deviation (%RSD) values of Analytes.
Phenoxyethanol, phenoxyethoxyethanol, propylene glycol and ethoxyethanol solutions at 0.5 mg/ml, 0.3 mg/ml and 0.1 mg/ml concentrations were prepared by different analysts and analyzed 3 times using the same method and system. The method was found to be reproducible within the laboratory. The chromatogram of 0.1 mg/ml concentration prepared by two different analysts is shown in (Figure 7). F-test was applied for the analyzes made by both analysts. Since the values we obtained were less than 5.79, they were within the 95% confidence interval (α=0.05).
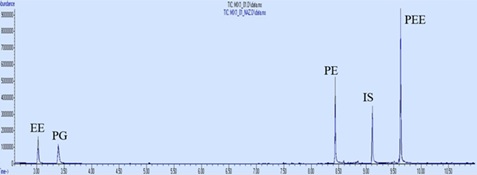 Figure 7: Superimposed chromatograms of 0.1 mg/ml mixture solutions prepared by two different analysts.
Figure 7: Superimposed chromatograms of 0.1 mg/ml mixture solutions prepared by two different analysts.
For solvent analysis from documents, 14 documents between 0-12 months stored at room temperature, 45-55% humidity and in transparent files were analysed by GC-MS. Relative Peak Area (RPA) values were calculated and RPA-time graph was drawn. RPA was calculated with the formula given below. The chromatogram of fresh ink is shown in (Figure 8).
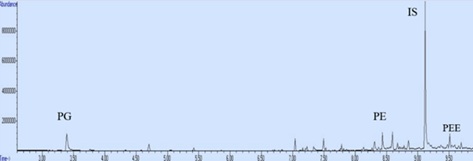 Figure 8: The chromatogram of newly created document.
Figure 8: The chromatogram of newly created document.
 The chromatogram of newly created document (t=0 day) is shown in (Figure 9). The chromatogram of 230 days old and 280 days old document are shown in (Figure 9&10) The time dependent changes of the peak areas of PE obtained from GC-MS analysis and RPA values are given in (Table 4). RPA-Time graph was drawn with the obtained data. RPA-Time graph of PE is shown in (Figure 11).
The chromatogram of newly created document (t=0 day) is shown in (Figure 9). The chromatogram of 230 days old and 280 days old document are shown in (Figure 9&10) The time dependent changes of the peak areas of PE obtained from GC-MS analysis and RPA values are given in (Table 4). RPA-Time graph was drawn with the obtained data. RPA-Time graph of PE is shown in (Figure 11).
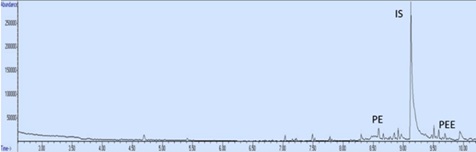 Figure 9: The chromatogram of 230 days old document.
Figure 9: The chromatogram of 230 days old document.
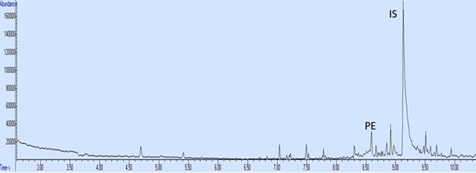 Figure 10: The chromatogram of 280 days old document.
Figure 10: The chromatogram of 280 days old document.
|
Time (Days) |
Area of PE |
Area of IS |
RPA |
|
0 |
248842 |
1075977 |
0,231 |
|
30 |
121088 |
711392 |
0,170 |
|
60 |
102088 |
860394 |
0,119 |
|
80 |
209224 |
2320073 |
0,090 |
|
120 |
50258 |
838066 |
0,060 |
|
140 |
25964 |
528166 |
0,049 |
|
160 |
37568 |
841097 |
0,045 |
|
180 |
38554 |
998926 |
0,039 |
|
210 |
24362 |
705966 |
0,035 |
|
230 |
23077 |
688084 |
0,034 |
|
240 |
23925 |
765768 |
0,031 |
|
260 |
21324 |
758664 |
0,028 |
|
270 |
22723 |
821961 |
0,028 |
|
280 |
148114 |
5432583 |
0,027 |
Table 4: The time dependent changes of the peak areas of PE (Mean PE values of 10 blue ballpoint pens).
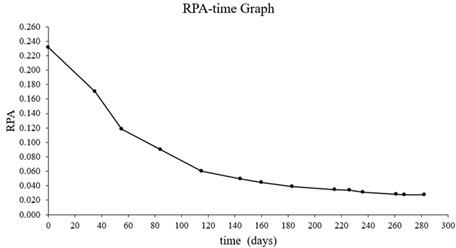 Figure 11: RPA-Time graph of PEE.
Figure 11: RPA-Time graph of PEE.
Also time dependent changes of peak areas of PEE and RPA values are given in (Table-5). RPA-time graph was drawn with the obtained data. RPA-Time graph of PEE is shown in (Figure 12). RPA value was calculated by the same formula given above.
|
Time (Days) |
Area of PEE |
Area of IS |
RPA |
|
0 |
83819 |
1075977 |
0,0779 |
|
30 |
40692 |
711392 |
0,0572 |
|
60 |
35792 |
860394 |
0,0416 |
|
80 |
80043 |
2320073 |
0,0345 |
|
120 |
24472 |
838066 |
0,0292 |
|
140 |
15053 |
528166 |
0,0285 |
|
160 |
23635 |
841097 |
0,0281 |
|
180 |
27870 |
998926 |
0,0279 |
|
210 |
18990 |
705966 |
0,0269 |
|
230 |
18165 |
688084 |
0,0264 |
Table 5: The time dependent changes of the peak areas of PEE (Mean PEE values of 10 blue ballpoint pens).
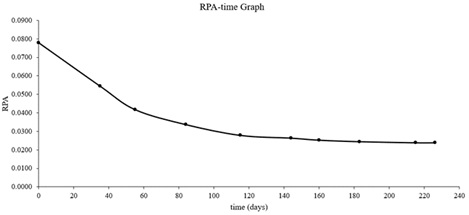 Figure 12: RPA-Time graph of PEE.
Figure 12: RPA-Time graph of PEE.
Discussion
In this study, in addition to the phenoxyethanol solvent known to be in the structure of the blue ballpoint pen, other solvents such as ethoxyethanol, propylene glycol and phenoxyethoxyethanol, which are also known to be in the blue ballpoint pen, were analysed and ink age determination was made with the data obtained. While phenoxyethanol and phenoxyethoxyethanol were detected in all 10 ballpoint pens studied, propylene glycol was found in only one (Faber Castel 1425). However, no ethoxyethanol was found in any of them. Ethoxyethanol is known to be widely used as a solvent in gel pen inks. Since ethoxyethanol solvent is glycol-based like the solvents used in ballpoint pen inks, it was assumed that it could be used. However, it was not found in the pens we studied.
CP-Sil 8 CB capillary column is generally used in GC-MS ink analysis, but the column we used is HP 5MS column. Due to the unsuitability of the column and the inaccessibility of methods such as thermal desorber or solid-phase micro extraction, no results were obtained from the analysis of a small amount of sample. Accordingly, sample size was increased and 10 cm was taken. Also the reason for not using another solvent other than DCM is that the common solvent in which EE, PG, PE and PEE can be dissolved is chloroform. Chloroform was not preferred because it is toxic and very volatile. In addition, solvents such as acetonitrile, acetone, ethanol and methanol were tried but no significant data were obtained.
The analysis of age curves for phenoxyethanol and phenoxyethoxyethanol revealed that experimental changes persisted for up to 280 and 230 days, respectively, with faster evaporation rates observed on the paper surface during the initial 60-day period that decreased over time due to environmental factors and solvent depletion.
In order to minimise the margin of error in age determination reporting, experts prefer to use time interval instead of direct time. Time interval interpretation for phenoxyethanol if RPA> 0.23, the document is new, if 0.12 < RPA < 0.23, the document is 0-60 days old, if 0.06 < RPA < 0.12, the document is 60-120 days old, if 0.04 < RPA < 0.06, the document is 120-180 days old, if 0.03 < RPA < 0.04, the document is 180-280 days old and if RPA < 0.03, no time interval can be given for the document. Time interval interpretation for phenoxethoxyethanol if RPA>0.078, the document is new, if If 0.042 < RPA < 0.078, the document is 0-60 days old, if 0.029 < RPA < 0.042, the document is 60-120 days old, if 0.028 < RPA < 0.029, the document is 120-180 days old, if 0,026 < RPA < 0,028, the document is 180-280 days old and if RPA < 0.026, no time interval can be given for the document.
Conclusions
In this study, it was aimed to determine the date of creation of the document by analysing the solvents of ethoxyethanol, propylene glycol, phenoxyethanol and phenoxyethoxyethanol in the structure of blue ballpoint pens of different brands and models collected from the market by GC-MS. Since phenoxyethanol and phenoxyethoxyethanol are more abundant in the structure of ballpoint pen ink in the archive samples studied and no solvent was detected in documents older than 280 days. In general, ethoxyethanol could not be detected among the other solvents other than phenoxyethanol in the ink age analysis, while propylene glycol was detected in fresh ink and phenoxyethoxyethanol could be detected up to 230 days.
The reason for investigating other solvents is to confirm the accuracy of the analysis result by having another supporting element in the analysis of the document whose creation time is unknown. In order for the ink age analysis studies to be more comprehensive, the number and types of samples (black, red and green colour) should be increased, appropriate column and method should be selected, thermal desorber, solid phase micro extraction etc. methods should be used. The effect of environmental factors (temperature, light, humidity, etc.) on the aging of the ink on the document, the effect of paper type on ink analysis, the results of the studies to be carried out to determine the ink type by elemental analysis methods can better serve the judicial system. In conclusion, the method developed in this study allows the age determination of 0-12 months in documents created with blue ballpoint pen containing phenoxyethanol and phenoxyethoxyethanol.
Another result of this study is that with the developing technology, chromatographic methods that damage the document in ink aging analysis studies have started to be switched to spectroscopic methods that do not damage the document [29-34].
Ethics approval and consent to participate
Not applicable
Consent for publication
All the authors consent to publish this research paper.
Availability Of Data And Material
Istanbul University-Cerrahpasa Institute of Forensic Sciences and Legal Medicine Toxicology Laboratory, Istanbul, Turkey
Competing interests
The authors declare that they have no competing interests.
Funding
This thesis project was supported by Istanbul University-Cerrahpasa Scientific Research Projects Unit (BAP). Project No:36100
References
- Mitchell CA (1908) English inks: Their composition and differentiation in handwriting. Analyst 33: 80-85.
- Salkim Islek D (2015) Developments and validation of HPLC-uv and TD-GC/MS methods for the identification of ballpoint pen ink components: Study of their decomposition on aging. 27: 215-235.
- Tasdemir K (2013) Belgelerde sahtecilik suçlari. Ütopya Grafik, Ankara.
- Avci A, Can M, Etemoglu AB (2001) A theoretical approach to the drying process of thin film layers. Applied thermal engineering 21: 465-479.
- Cantú AA (2012) A study of the evaporation of a solvent from a solution-application to writing ink aging. Forensic science international 219: 119-128.
- Weyermann C, Kirsch D, Vera CC, Spengler B (2007) A GC/MS study of the drying of ballpoint pen ink on paper. Forensic science international 168: 119-127.
- Costa KF, Brand GD, Groberio TS, Braga JW, Zacca JJ (2019) Document ink dye age estimation by direct injection-mass spectrometry and correlation analysis. Microchemical Journal 147: 1123-1132.
- Saviello D, Trabace M, Alyami A, Mirabile A, Baglioni P, et al. (2019) Raman Spectroscopy and Surface Enhanced Raman Scattering (SERS) for the Analysis of Blue and Black Writing Inks: Identification of Dye Content and Degradation Processes. Frontiers in chemistry 7: 727.
- Cicconi F, Lazic V, Palucci A, Almeida Assis AC, Saverio Romolo F (2020) Forensic analysis of commercial inks by laser-induced breakdown spectroscopy (LIBS). Sensors 20: 3744.
- Alyami A, Barton K, Lewis L, Mirabile A, Iacopino D (2019) Identification of dye content in colored BIC ballpoint pen inks by Raman spectroscopy and surface-enhanced Raman scattering. Journal of Raman Spectroscopy 50: 115-126.
- Burgio L (2021) Pigments, dyes and inks: Their analysis on manuscripts, scrolls and papyri. Archaeological and Anthropological Sciences 13: 194.
- Weyermann C (2009) Documents: Dating a document. Wiley Encyclopedia of Forensic Sciences 3: 684-692.
- Ezcurra M, Góngora JM, Maguregui I, Alonso R (2010) Analytical methods for dating modern writing instrument inks on paper. Forensic Science International 197: 1-20.
- Ortiz-Herrero L, Bartolomé L, Durán I, Velasco I, Alonso ML, et al. (2018) DATUVINK pilot study: A potential non-invasive methodology for dating ballpoint pen inks using multivariate chemometrics based on their UV–vis-NIR reflectance spectra. Microchemical Journal 140: 158-166.
- Koenig A, Magnolon S, Weyermann C (2015) A comparative study of ballpoint ink ageing parameters using GC/MS. Forensic science international 252: 93-106.
- Lociciro S, Dujourdy L, Mazzella W, Margot P, Lock E (2004) Dynamic of the ageing of ballpoint pen inks: quantification of phenoxyethanol by GC-MS. Science & justice: Journal of the Forensic Science Society 44: 165-171.
- Salkim Islek D, Isat E, Cengiz S (2020) Determination of changes in crystal violet and phenoxyethanol (dating ink). Journal of Forensic Sciences 65: 661-663.
- Weyermann C, Almog J, Bügler J, Cantu AA (2011) Minimum requirements for application of ink dating methods based on solvent analysis in casework. Forensic science international 210: 52-62.
- El-sabbah MM, Gomaa AZ, Al-Hawary AS (2019) Dating the ballpoint pen inks using gas chromatography-mass spectrometry technique. Egyptian Journal of Chemistry 62: 385-400.
- Carvallo CMBD, Ortiz RS, Limberger RP (2019) Figures of merit evaluation of gc/ms method for quantification of 2-phenoxyethanol from ballpoint pen ink lines and Determination of The Influence of Support Paper on Solvent Extraction.
- Leal TA, Ferreira C, Quintas A, Bernardo A (2021) 2-Phenoxyethanol derivatization in ink dating determination. Annals of Medicine 53: S74-S75.
- Hoang AD, Tu MB, Ta TT, Hoang MH (2021) Combination of a Green and a Traditional Method for Estimating Relative and Absolute Ink Age: A Case Study of Ballpoint Pen Ink Dating in Vietnam.
- Sharif M, Jalees MI, Ali Shah Tirmazi SA, Athar MM, Durrani AI, et al. (2022) Discrimination of Pakistani fountain pen inks by gas chromatography-mass spectrometry (GC-MS). International Journal of Analytical Chemistry.
- Hu X, Zhang X, Diao Z (2023) Rapid and simultaneous determination of nine volatile solvents in ink entries with improved gas chromatography. Microchemical Journal 190: 108637.
- Ni Y, He N, Lü Y, Zou N, Song H, et al. (2020) Study of ink aging: Targeting triethylene glycol in carbon-based black gel ink strokes on paper. Forensic science international 311: 110296.
- Sabater PQ, Santana OD, Moreno DV (2021) Determining Intersecting Ball-Point Ink Strokes with Different Aging. Journal of Analytical Chemistry 76: 660-670.
- Baygildieva DI, Krylova AS, Baygildiev TM, Shpigun OA, Rodin IA (2019) Studying of Handwritten Strokes Aging Kinetics by High-Performance Liquid Chromatography–Mass Spectrometry. Journal of Analytical Chemistry 74: 1263-1270.
- Lu W, Jiang R, Li X, Qi Y, Ji Z, et al. (2022) Characterization and discrimination of volatile compounds in gel-pen ink via headspace-gas chromatography-ion mobility spectrometry combined with chemometric strategies. Microchemical Journal 182: 107855.
- Khofar PNA, Karim UKA, Elias E, Safian MF, Halim MIA (2022) Trends of Forensic Analysis of Pen Ink Using Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy. Indonesian Journal of Chemistry 22.
- Sharif M, Batool M, Chand S, Farooqi ZH, Tirmazi SAA, et al. (2019) Forensic discrimination potential of Blue, Black, Green, and Red colored fountain pen inks commercially used in Pakistan, by UV/Visible spectroscopy, thin layer chromatography, and Fourier transform infrared spectroscopy. International journal of analytical chemistry.
- Gorshkova KO, Rossinskaya ER, Kirillova NP, Fogel AA, Kochemirovskaia SV, et al. (2020) Investigation of the new possibility of mathematical processing of Raman spectra for dating documents. Science Justice 60: 451-465.
- Ivanova I, Spiridonov I, Lasheva V (2022) Spectroscopic Technique for Studying The Characteristics of Paper, Pigments and Inks in The Aging Process. Journal of Chemical Technology Metallurgy 57.
- Hilario FF, de Mello ML, Pereira-Filho ER (2021) Forensic analysis of hand-written documents using laser-induced breakdown spectroscopy (LIBS) and chemometrics. Analytical Methods 13: 232-241.
- Gautam R, Chauhan R, Kumar R, Sharma V (2021) PLS-DA and infrared spectroscopy based rapid and non-destructive discrimination of black ball and gel pen inks for forensic application. Forensic Science International: Reports 3: 100162.
Citation: Kiris E, Islek DS, Yukseloglu EH (2023) Determination of Solvents in Ballpoint Pen Ink by Gas Chromatograhy Mass Spectrometry (GC-MS). Forensic Leg Investig Sci 9: 082.
Copyright: © 2023 Eda Kiris, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

