
Distribution and Morphology of Coscinodiscus species from the Surface Water of Dhamra Coast, Bay of Bengal (Odisha)
*Corresponding Author(s):
Lipika PatnaikEnvironmental Science Laboratory, Department Of Zoology Centre Of Excellence In Environment And Public Health Ravenshaw University, Cuttack – 753003 Odisha, India
Email:lipikapat@gmail.com
Abstract
A study on the diversity of diatom Coscinodiscus species was carried out in the coastal water of Dhamra during pre-monsoon, monsoon, and post-monsoon period (2021-2022). Surface water was collected from 6 different stations with the help of Niskin water sampler with capacity of 1.5L. Sea Surface Temperature (SST), pH, transparency were measured on the site and other parameters like, abundance, salinity, Dissolved Oxygen (DO), silicate, nitrite, nitrate and Coscinodiscus species identification were done after returning to the laboratory. A total of 6 species C. centralis, C. granii, C. marginatus, C. wailessi, C. radiatus and C. oculo-iridis were observed. Dhamra coastal water was dominated by Coscinodiscus species and the species diversity was high during post-monsoon period as compared to the monsoon and pre-monsoon. Positive correlations between different water parameters were observed.
Keywords
Coscinodiscus diversity; Coastal waters; Dhamra
Introduction
Phytoplankton play a major role in the marine ecosystems as they initiate the food chain, maintain global carbon cycle, energy flow, nutrient cycling and also regulate the abundance and diversity of other marine communities [1-3]. Coastal and estuarine ecosystems are economically important and are the most productive ecosystems [4,5]. Distribution and diversity of phytoplankton species are affected by hydrology and atmospheric factors, hence continuous observation can provide valuable information about the water quality, thus making them good bio-indicators of water pollution [6,7]. Phytoplanktons maintain the global carbon cycle or the Red field ratio (C:N:P= 106:6:1) and are excellent source of fossil fuel. Diatoms are commercially important as diatomaceous earth which is a congregation of dead silica rich frustules. They are used in the boilers, electric oven as insulators, as polishing agent for metals, manufacture of concrete, in the separation of paraffin wax from petroleum, filtration of fruit juice and syrup [8]. Diatoms play an important role in marine productivity and carbon fixation [9]. The frustules of diatoms are impregnated with huge amount of silica and various photosynthetic pigments, which appear as yellow-brown pigments. They play major role in biogeochemical cycling like Si, N, P, C and mostly depend on silica for their growth and metabolism. Diatoms can act as bio-indicator species due to their sensitivity towards any change in the surrounding water [10]. Diatoms have a hydrocarbon compound known as diatomin, which isused as an indicator of rich petroleum grounds and the small free-floating producers act as primary precursor of petroleum rich zones [8].
Odisha has a coastline of over 480 kms, stretching from Subarnarekha River in the north to Rushikulya in the south, and has been one of the major hubs of intense fishing and related activities. Dhamra has Adani port located between the main land and Kanika Island of Bay of Bengal which give anchor to vessels coming from different places. Hence, Dhamra coastal water is considered ecologically sensitive area due to industrialization and commercial activities. Very few plankton studies have been carried out in the coastal waters of Dhamra [11-13]. Physicochemical variables like pH, water temperature, Dissolved oxygen, salinity, nutrients are important factors controlling phytoplankton growth and diversity in marine environments [14,15]. Coscinodiscus is a centric diatom which plays a crucial role in marine primary productivity.As oceans get subjected to continuous change due to fluctuations in weather conditions, it also influences phytoplankton dynamics. Hence the present study was undertaken to look into the diversity of Coscinodiscus species and also understand the role of nutrients and its correlation with phytoplankton diversity.
Materials And Methods
Sampling and measurements were carried out at six stations using GPS based geographical coordinates between 20º46´8.70´´N, 86º51´2.19´E and 20º51´5.12´´N, 87º02´4.54´E in coastal waters of Dhamra during March 2021 and February 2022 (Figure 1). Water samples were collected with the help of vertical Niskin water sampler. Water from the surface was collected for the analysis of various hydrological parameters. Sea surface temperature was recorded using a 0.01° C precision thermometer. pH was measured using micro-processor pH meter. Transparency was measured with the help of a Secchi disc. Water samples were fixed following the modified Winkler’s method for the analysis of dissolved oxygen. Remaining water samples were transferred to tarson nutrient containers to be analyzed in the laboratory. Dissolved oxygen, salinity and nutrients like silicate, nitrite, and nitrate were analyzed following the procedures as described in Strickland and Parsons (1972) [16]. Dissolved oxygen was analyzed by modified Winkler’s method; salinity by argentometric method; silica by silico-molybdic method; nitrate and nitrite by Azo-dye method. 50L of water sample was filtered on board using plankton net and then fixed in 2% Lugol’s iodine solution for identification and enumeration. 4% buffered formaldehyde solution was added to the water samples for long term preservation. Phytoplankton counting was done with the help of Sedgwick - Rafter counting chamber. Photographs were taken with the help of light microscope Magnus MLX PLUS at 40X magnification for taxonomic identification of various species. Plankton samples were dried and spread over a double-sided adhesive carbon tape on an aluminum stub and the samples were examined under SEM (Gemini SEM 300, Zeiss) at Ravenshaw University, Odisha, India. Identification was done using monographs, NIO identification manual and from marine species.com.
Box plots were plotted using Graph Pad Prism 6 for graphical representation of various physico-chemical water parameters. One-way ANOVA was performed to establish the difference in Coscinodiscus number based on season. Pearson correlation matrix was calculated to determine the impact of various water quality parameters on Coscinodiscus abundance. The relationship between Coscinodiscus abundance and environmental factor silicate was analyzed through regression plot in excel. Cluster analysis was carried out to verify the similarity and dissimilarity among various water quality parameters. Biodiversity professional software was used for cluster analysis. Shannon Wiener diversity index (H’) was calculated using the following formula
Shannon Wiener diversity index (H’) = -∑piln pi
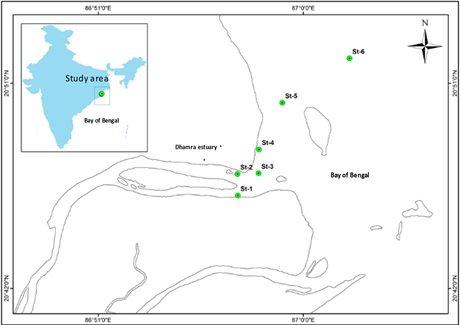 Figure 1: Map showing study area and station locations.
Figure 1: Map showing study area and station locations.
Result
Hydrological parameters
Variations were observed in SST in coastal waters of Dhamra with highest values during monsoon (32 ± 0.63°C) followed by pre-monsoon (27.33 ± 0.82°C) and post-monsoon (26.33 ±1.03°C). Water was observed to be alkaline in nature with highest during the post-monsoon (pH 8.22 ± 0.08) period followed by pre-monsoon (pH 8.21 ± 0.04) and monsoon period (pH 7.98 ± 0.20). Water transparency was most in post-monsoon (0.82 ± 0.13 m) than compared to pre-monsoon (0.53 ± 0.47 m) and monsoon (0.53 ± 0.31 m). The sea surface salinity varied from 17.12 to 29.46 psu in pre-monsoon period, 0.79 to 21.76 psu in monsoon and 14.86 to 26.54 psu in post-monsoon period. Highest salinity was observed during pre-monsoon period (22.85 ± 4.40 psu) followed by post-monsoon (20.17 ± 4.71 psu) and monsoon (10.40 ± 9.18) periods. Dissolved oxygen concentration was highest during monsoon period (7.23 ± 0.34 mgL-1) in comparison to pre-monsoon (6.73 ± 0.33) and post-monsoon (5.40 ± 0.28) periods. Variations in dissolved oxygen ranged from 6.4 – 7.2 mgL-1 in pre-monsoon; 6.8 – 7.6 mgL-1 in monsoon and 5.1 – 5.8 mgL-1 in post-monsoon periods. The average silicate concentration varied from 0.04 to 0.48 mgL-1 (Figure 2). The silicate concentrations ranged from 0.026 to 0.055 mgL-1 in pre-monsoon period, 0.101 to 0.816 mgL-1 in monsoon period and 0.032 to 0.074 mgL-1 in post-monsoon period.
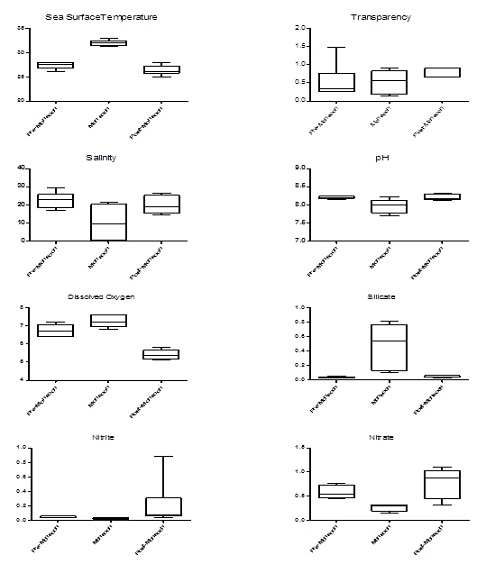 Figure 2: Hydrography results of Dhamra coastal waters during different seasons.
Figure 2: Hydrography results of Dhamra coastal waters during different seasons.
Coscinodiscus diversity and correlated biological parameters
A significant seasonal variation was observed in the distribution and diversity of Coscinodiscus sp. in coastal waters of Dhamra during the study period. A total of 6 species belonging to Coscinodiscus genera were reported from different sampling stations. Highest number of Coscinodiscus was observed at station 2 during the post-monsoon period whereas lowest number of Coscinodiscus was recorded at station 4 during the monsoon period (Figure 3). The relationship between silicate concentration and Coscinodiscus abundance has been depicted though linear regression graphs in (Figure 4). The graphs clearly show the positive correlation between silicate and Coscinodiscus in monsoon season and subsequent uptake of silicate by diatoms for their growth which leads to a negative correlation during the post-monsoon period. The observations show that Coscinodiscus abundance were more in winter (730/ml) as compared to monsoon season (497/ml). Our study has reported a strong positive correlation (p ≤ 0.05) between Coscinodiscus abundance with sea surface temperature (r = 0.955), silicate (r = 0.861) and nitrite (r = 0.937) in the pre-monsoon period. Our study shows strong negative correlation between Coscinodiscus abundance and pH (r = -0.813) but no significant correlation was found to exist between Coscinodiscus density and transparency, salinity, dissolved oxygen and nitrate in the pre-monsoon period (Table 1). A strong positive correlation (p ≤ 0.05) between Coscinodiscus abundance with transparency (r = 0.854), salinity (r = 0.720) and silicate (r = 0.884) in the monsoon period. Our study shows strong negative correlation between Coscinodiscus abundance and nitrite (r = -0.813) but no significant correlation was found to exist between Coscinodiscus density and temperature, pH, dissolved oxygen and nitrate in the monsoon period (Table 2). Our study shows strong negative correlation between Coscinodiscus abundance with transparency (r = -0.647) but no significant correlation was found to exist between Coscinodiscus density and temperature, salinity, pH, dissolved oxygen, nitrite and nitrate in the post - monsoon period (Table 3). Strong negative correlation was found between Coscinodiscus abundance and silicate in the post-monsoon season (r = -0.659). (Figure 5) also shows the similarity between various physico-chemical parameters of the coastal waters of Dhamra spread over seasons. A strong correlation was observed in the cluster matrix graph between Sea Surface Temperature (SST) and salinity. Similarly, strong correlation was observed between pH and dissolved oxygen. (Figure 6) represents few species of Coscinodiscus observed at the sampling area during the study period. With the help of Scanning Electron microscope, the pore pattern of various samples were observed and about 6 species of Coscinodiscus were identified. The six prominent species of Coscinodiscus spotted in coastal waters were C. marginatus, C. centralis, C. oculusiridis, C. wailesii, C. Radiatus and C. granii (Figure 6). The entire structure of the frustule was also visualized using Scanning Electron Microscope (SEM), wherein a more detailed and intricate pattern of both valve and the girdle view was obtained (Figure 7a&7b). Pattern change in the outer and inner surface of the complex structure of the frustule pattern was observed in different species. The Shannon’s species diversity index (H’) was calculated to be 0.756 in pre-monsoon, 0.683 in monsoon and 0.646 in post-monsoon.
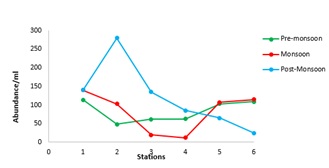 Figure 3: Station wise abundance of Coscinodiscus during different seasons.
Figure 3: Station wise abundance of Coscinodiscus during different seasons.
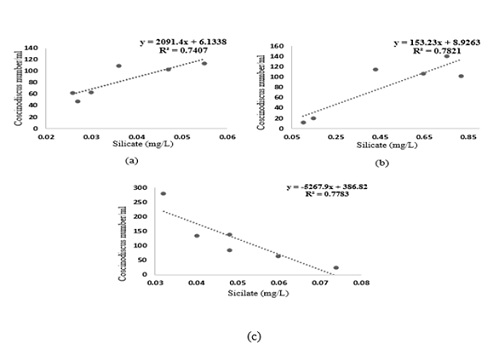 Figure 4: Linear correlation between silicate and Coscinodiscus abundance during (a) pre-monsoon (b) monsoon and (c) post-monsoon in coastal waters of Dhamra in 2021–2022.
Figure 4: Linear correlation between silicate and Coscinodiscus abundance during (a) pre-monsoon (b) monsoon and (c) post-monsoon in coastal waters of Dhamra in 2021–2022.
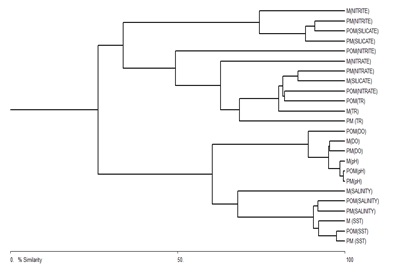 Figure 5: Cluster matrix showing the interrelationship between physicochemical parameters of coastal waters during different seasons [PM: Pre-monsoon, M: Monsoon, POM: Post-monsoon].
Figure 5: Cluster matrix showing the interrelationship between physicochemical parameters of coastal waters during different seasons [PM: Pre-monsoon, M: Monsoon, POM: Post-monsoon].
|
Variables |
SST |
Transparency |
Salinity |
pH |
DO |
Silicate |
Nitrite |
Nitrate |
Coscinodiscus |
|
SST |
1 |
|
|
|
|
|
|
|
|
|
Transparency |
0.376 |
1 |
|
|
|
|
|
|
|
|
Salinity |
-0.497 |
0.249 |
1 |
|
|
|
|
|
|
|
pH |
|
-0.131 |
0.385 |
1 |
|
|
|
|
|
|
DO |
-0.500* |
-0.263 |
0.711* |
0.581* |
1 |
|
|
|
|
|
Silicate |
0.776* |
-0.082 |
-0.426 |
-0.903* |
-0.326 |
1 |
|
|
|
|
Nitrite |
0.832* |
0.506* |
-0.22 |
-0.677* |
-0.246 |
0.727* |
1 |
|
|
|
Nitrate |
-0.07 |
-0.47 |
0.042 |
-0.432 |
0.26 |
0.530* |
0.248 |
1 |
|
|
Coscinodiscus |
0.955* |
0.388 |
-0.381 |
-0.813* |
-0.418 |
0.861* |
0.937* |
0.177 |
1 |
Table 1: Pearson Correlation matrix between hydrology and biological parameters of the coastal waters of Dhamra during pre-monsoon period. *Significant at p ≤ 0.05.
|
Variables |
SST |
Transparency |
Salinity |
pH |
DO |
Silicate |
Nitrite |
Nitrate |
Coscinodiscus |
|
SST |
1 |
|
|
|
|
|
|
|
|
|
Transparency |
-0.01 |
1 |
|
|
|
|
|
|
|
|
Salinity |
-0.233 |
0.930* |
1 |
|
|
|
|
|
|
|
pH |
-0.644* |
0.311 |
0.394 |
1 |
|
|
|
|
|
|
DO |
-0.351 |
0.252 |
0.263 |
0.660* |
1 |
|
|
|
|
|
Silicate |
0.601* |
0.595* |
0.541* |
-0.37 |
-0.409 |
1 |
|
|
|
|
Nitrite |
-0.518* |
-0.523* |
-0.351 |
0.619* |
0.454 |
-0.742* |
1 |
|
|
|
Nitrate |
0.623* |
-0.755* |
-0.802* |
-0.765* |
-0.462 |
-0.011 |
-0.026 |
1 |
|
|
Coscinodiscus |
0.41 |
0.854* |
0.720* |
-0.139 |
-0.208 |
0.884* |
-0.813* |
-0.362 |
1 |
Table 2: Pearson corelation matrix between hydrology and biological parameters of the coastal waters of Dhamra during monsoon period. *Significant at p ≤ 0.05.
|
Variables |
SST |
Transparency |
Salinity |
pH |
DO |
Silicate |
Nitrite |
Nitrate |
Coscinodiscus |
|
SST |
1 |
|
|
|
|
|
|
|
|
|
Transparency |
-0.500* |
1 |
|
|
|
|
|
|
|
|
Salinity |
0.317 |
0.116 |
1 |
|
|
|
|
|
|
|
pH |
-0.104 |
0.432 |
0.901* |
1 |
|
|
|
|
|
|
DO |
-0.351 |
0.695* |
-0.382 |
-0.126 |
1 |
|
|
|
|
|
Silicate |
0.2 |
0.541* |
0.775* |
0.774* |
-0.019 |
1 |
|
|
|
|
Nitrite |
0.780* |
-0.655* |
-0.116 |
-0.513* |
-0.530* |
-0.148 |
1 |
|
|
|
Nitrate |
-0.26 |
-0.226 |
-0.987* |
-0.916* |
0.35 |
-0.846* |
0.128 |
1 |
|
|
Coscinodiscus |
-0.02 |
-0.647* |
-0.33 |
-0.364 |
-0.107 |
-0.659* |
-0.042 |
0.464 |
1 |
Table 3: Pearson Coleration matrix between hydrology and biological parameters of the coastal waters of Dhamra during post-monsoon period. *Significant at p ≤ 0.05.
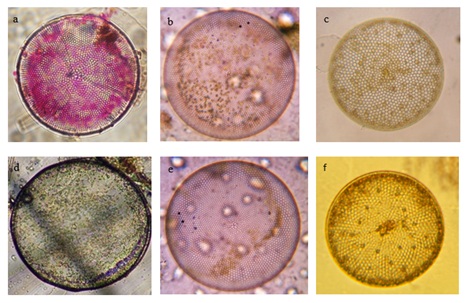 Figure 6: Coscinodiscus species in Dhamra coastal waters. A C.marginatus b C.centralis c C. oculusiridis d C.wailesiie e. C.radiatus f C.granii (photographs taken at 40× magnification).
Figure 6: Coscinodiscus species in Dhamra coastal waters. A C.marginatus b C.centralis c C. oculusiridis d C.wailesiie e. C.radiatus f C.granii (photographs taken at 40× magnification).
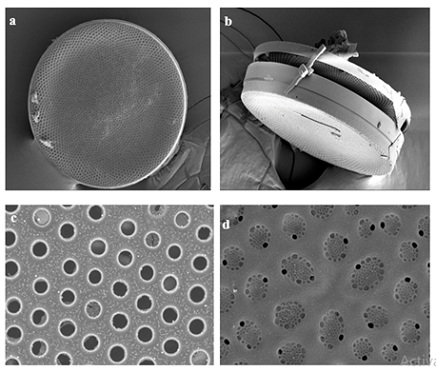
Figure 7: Scanning Electron Microscopy (SEM) images of Coscinodiscus sp (a) SEM image of the of the whole Coscinodiscus sp. (b) whole frustule (c) porous structure of the Coscinodiscus sp from the inner side and (d) outer side of the valve.
Discussion
The physico-chemical parameters like SST, pH, salinity, transparency, DO, silicate, nitrate, and nitrite exhibited different seasonal variations. Lowest water transparency was observed during pre-monsoon period as fresh water runoff from river was comparatively lower. Highest salinity was observed during the pre-monsoon period which can be attributed to increase in solar radiation and evaporation from the water surface. Lower salinity was observed during monsoon period because of ingress of both rain water and fresh water into the sea water. Similar results were observed in the studies carried out by Dash et al. 2017 [17] and Jyothibabu et al. 2008 [18]. Dissolved oxygen was maximum during the monsoon period, probably due to heavy rainfall and high wind velocity. Lower concentration of dissolved oxygen during the pre-monsoon period was due to more abundance of diatom species at the sampling stations. Stations close to the coastal region showed fluctuation in DO values due to influx of fresh water. Our results showed negative correlation of SST with dissolved oxygen across all seasons indicating increase in temperature contributed towards decreased solubility of oxygen in coastal waters of Dhamra. Concentration of silicate was maximum during the monsoon period, indicating increased abundance of diatom species. Lower silicate values during post-monsoon period might be due to uptake of silicate by diatoms for their biological activity. Higher concentrations of nitrite and nitrate were observed during post-monsoon periods which might be due to release from bottom sediments and similar observations were observed by studies carried out by Shirodkar et al. 2009 [19].The species composition, growth, production and diversity of diatoms in marine water is greatly influenced by various physico-chemical parameters of its particular environment. The diatoms were more abundant in post-monsoon season which could be correlated with increased amounts of silicate during monsoon period which were evident in our results. The enhanced amounts of silicates during the monsoon period might have led to increase in abundance of Coscinodiscus in the post-monsoon period.
Silicate was found to be negatively correlated with diversity during post monsoon indicating high uptake by few species. Similar results have been observed by Sas et al. 2022 [20], wherein silica was found to be negatively correlated with epipelic diversity of diatom due to the intensive uptake by some group or species. Shannon diversity index was lower during post-monsoon period and higher during pre-monsoon and monsoon period. The higher the value of H, higher will be the diversity of a species. The lower the value of H, the lower will be the diversity and a value of H=0 indicates presence of only one species. Delayed monsoon effects like upwelling and land runoff favours the growth and proliferation of diatom species. Similar results regarding influence of chemical parameters on phytoplankton diversity were also noticed in coastal waters of Parangipettai, Bay of Bengal [15]. Diatoms including several species of Coscinodiscus are reported to be Harmful Algal Bloom species (HABs). Coscinodiscus wailesii mostly occurs in warm waters which produces profuse amount of mucilage which aggregate, sink and cover the seabed and in turn affecting the benthic habitat. It also harms the microalgae cultivation, fish and shellfish farming etc [21]. These are also highly tolerant to heavy metals. Its high density leads to depletion of oxygen and nutrients [22,23]. Coscinodiscus centralis have been reported causing blooms have been reported causing blooms near the coastal region of south Andaman Sea [24]. In the shallow estuarine regions of Amba river of West Coast of India, Coscinodiscus oculusiridis are found extensively forming algal blooms [25]. Similar species of Coscinodiscus were also found in the coastal waters of Dhamra which can cause algal blooms, thus affecting food web patterns. Diatom frustules are highly ornamented, forming an amazing range of forms and the shape of the diatom frustule is species specific [26]. Centric diatoms thrive well in coastal waters due to buoyancy and can also survive in turbulent waters [27]. Study carried out by Kraberg et al. 2010 [28] and Kuhn & Kohler-Rink 2008 [29] reported toleration of wide range of temperature variation and resistance against parasitic infection by Coscinodiscus granii. Occurrence of Coscinodiscus species is an indication of rise in temperature and it was observed as bloom forming species during the sample collection period. The occurrence index of plankton at the sampling stations showed that Coscinodiscaceae (Coscinodiscus sp.) dominated the plankton groups [30] and the dominance of family Coscinodiscaceae across different sampling stations was similar to the findings of Varadharajan and Soundarapandian, 2015 [31]. Diatoms dominance could be the result of their ability to tolerate wide geographical and climatic conditions [32]. Increase in Coscinodiscus sp. might have been triggered by the presence of nutrients coming as runoffs with rain water. Similar bloom of Coscinodiscus centralis was observed by Kartik et al. (2014) [33] in coastal waters of South Andaman Sea under the influence of nutrients coming as runoffs. Significantly higher diatom might lead to increase in fish production as diatoms form the base of the food web. Diatoms are important components which contribute towards the stability of the intertidal mudflats and they release polysaccharides, which help to trap sediment grains and in turn stabilize the sediment [8]. Data about phytoplankton can help in better management of fishery resources and understanding of the ocean’s biogeochemical cycles [34-36].
Conclusion
The present work has reported about six species of Coscinodiscus from the coastal waters of Dhamra. The centric diatoms are ecologically significant as they contribute more than 20% of the total primary productivity and are a major dominating group under micro phytoplankton. Reported centric diatoms Coscinodiscus species are generally found in nutrient rich water. It can be concluded that Dhamra coastal water is favorable for diatoms as evident from the various physicochemical parameters and their correlation with the abundance of centric diatoms.
Declaration of Competing Interest
Authors report there are no competing interests to declare.
Acknowledgement
We are thankful to Mr Jaywant Sahu for helping us to take photographs using SEM facilities of Department of Chemistry, Ravenshaw University, Cuttack.
References
- Pati A, Behera RK, Dash S, Mohapatra PK, Sarangi RK, et al. (2018) First and New Record of Ceratium vulture v. sumatranum and Pediastrum species from coastal waters of Paradip, Bay of Bengal East Coast of India 41: 1169-1171
- Findlay HS, Gibson G, Kedra M, Morata N, Orchowska M, et al. (2015) Responses in Arctic marine carbon cycle processes: Conceptual scenarios and implications for ecosystem function. Polar Research34: 24252.
- Tian R, An J (2013) Relationship between aerosol transport routes and red tide occurrences in the East China Sea. Environmental earth sciences69: 1499-1508.
- Thillai RK, Rajkumar M, Sun J, Ashok PV, Perumal P (2010) Seasonal variations of phytoplankton diversity in the Coleroon coastal waters, southeast coast of India. ActaOceanologicaSinica 29: 97-108.
- Dash S, Padhan S, Rajhans G, Mohapatra PK, Sarangi RK,et al. (2019) Abundance and diversity of plankton in the coastal waters of Chandipur, Bay of Bengal. Russian Journal of Marine Biology45: 252-261.
- Shen GY, Shi BZ (2002) Marine Ecology.2nd edition. Science Press Beijing 191-192.
- Achary MS, Sahu G, Mohanty AK, Samatara MK, Panigrahy SN, et al. (2010) Phytoplankton abundance and diversity in the coastal waters of Kalpakkam, east coast of India in relation to the environmental variables. The Bioscan2: 553-568.
- Mitra A, Flynn KJ, Tillmann U, Raven JA, Caron D, et al. (2016) Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition: incorporation of diverse mixotrophic strategies. Protist167: 106-120
- Naz T, Burhan Z, Jamal PJA (2013) Seasonal abundance of diatoms in correlation with the physicochemical parameters from coastal waters of Pakistan. Pak J Bot 45: 1477-1486.
- Khokhar FN, Ahmad N, Ali A, Iqbal P, Jan B, et al. (2022) Spatio-Temporal Variations of Diatom Community in the Coastal Waters off Karachi, Northern Arabian Sea. Thalassas: An International Journal of Marine Sciences 1-12.
- Palleyi S, Panda CR (2011) Influence of water quality on the biodiversity of phytoplankton in Dhamrariver Estuary of Odisha Coast, Bay of Bengal. Journal of Applied Sciences and Environmental Management15: 69-74
- Sangita S, Panda S, Satapathy D, Kar RN, Panda CR (2013) Statistical Approach in Assessing Dynamic Variations of Estuarine Water Quality of Dhamra, Bay of Bengal. Asian Journal of Water Environment and Pollution10: 39-50.
- Naik S, Mishra RK, Mahapatro D, Panigrahy RC (2014) Impact of water quality on phytoplankton community and biomass in Dhamara estuary east coast of India. Journal of Environmental Biology35: 229-235
- Jabbari M, Salahi M, Ghorbani R (2008) Spatio-temporal influence of physicochemical parameters on phytoplankton assemblage in coastal brackish lagoon: Gomishan Lagoon, Caspian Sea, Iran. Biodiversitas 19: 2020–2027.
- Vajravelu M, Martin Y, Ayyappan S, Mayakrishnan M (2017) Seasonal influence of physico-chemical parameters on phytoplankton diversity, community structure and abundance at Parangipettai coastal waters, Bay of Bengal, South East Coast of India. Oceanologia 60: 114-127.
- Strickland JDH, Parsons T R (1972) A practical handbook of seawater analysis.
- Dash S, Behera RK, Mohapatra PK, Sarangi RK, Raut D, et al. (2017) Species composition of microzooplanktonTintinnid from the coastal waters of Digha, Bay of Bengal. Environmental Monitoring and Assessment 189: 1-11.
- Jyothibabu R, Madhu NV, Maheswaran PA, Jayalakshmy KV, Nair KKC, et al. (2008) Seasonal variation of microzooplankton (20–200 μm) and its possible implications on the vertical carbon flux in the western Bay of Bengal. Continental Shelf Research28: 737-755.
- Shirodkar PV, Mesquita A, Pradhan UK, Verlekar XN, Babu MT, et al. (2009) "Factors controlling physico-chemical characteristics in the coastal waters off Mangalore-a multivariate approach." Environmental Research 109: 245-257.
- Sas AA, Suriyanti SNP, Das SK, Cob ZC (2022) Effect of Seawater and Surface-Sediment Variables on Epipelic Diatom Diversity and Abundance in the Coastal Area of Negeri Sembilan, Malaysia. Water14: 3187.
- Ferrario ME, Almandoz G, Licea S, Garibotti I (2008) Species of Coscinodiscus (Bacillariophyta) from the Gulf of Mexico. Argentina and Antarctic waters: Morphology 133: 187-216
- Ragueneau O, Conley DJ, Leynaert A, Longphuirt S, Slomp CP (2006) Role of diatoms in silica cycling and coastal marine food webs 163-195.
- Assadi H, Attaran G, Dehghani R (2015) A study on existence of dinoflagellates cysts and detection harmful kind of them in marine sediments of Hormozgan province 10: 1-19
- Raji K, Muthuraj AK, Padmavati G (2014) Silicate as the Probable Causative Agent for the Periodic Blooms in the Coastal Waters of South Andaman Sea. Applied Environmental Research 36: 37-45.
- Karthik R, Robin RS, Anandavelu I, Purvaja R, Singh G, et al. (2020) Diatom bloom in the Amba River, west coast of India: a nutrient-enriched tropical river-fed estuary. Regional Studies in Marine Science 35: 101244.
- Joshi AM, Desai AY, Bhatt AJ, Yusufzai SI, Kardani HK (2019) Checklist of diatoms species available along the Narara and Poshitra Island, Marine National Park, Jamnagar, Gujarat. International Journal of Fauna and Biological Studies 6: 17-20.
- Round FE, Crawford RM, Mann DG (1990) The Diatoms. Biology and Morphology of the Genera.Cambridge University Press Cambridge UK 747.
- Kraberg A, Baumann M, Durselen CD (2010) Coastal Phytoplankton: Photo Guide for Northern European Seas. Verlag Munchen Germany 204.
- Kuhn SF, Köhler-Rink S (2008) pH effect on the susceptibility to parasitoid infection in the marine diatom Coscinodiscus spp. (Bacillariophyceae). Marine Biol 154: 109-116.
- Ajibare AO, Ayeku PO, Akinola JO, Adewale AH (2019) Plankton composition in relation to water quality in the coastal waters of Nigeria. Asian Journal of Fisheries and Aquatic Research 5: 1-9.
- Varadharajan D, Soundarapandian P (2015) Biodiversity and Abundance of Phytoplankton from Muthupettai Mangrove Region, South East Coast of India. Journal of Aquaculture Research and Development 6: 383-389.
- Balogun KJ, Ajani EK (2015) Spatial and temporal variations of Phytoplankton pigments, Nutrients and Primaryproductivity in water column of Badagry Creek, Nigeria. American Journal of Research Communication 3: 157- 172.
- Karthik R, Arun Kumar M, Padmavati G (2014) Silicate as the probable causative agent for the periodic blooms in the coastal waters of south Andaman Sea. Applied Environmental Research36: 37-45.
- Alam MJ, Kamal AM, Ahmed MK, Khondker M, Mondal B, et al. (2021) Abundance, Diversity and Distribution of Phytoplankton in Coastal Water Adjacent to St. Martin’s Island, Bangladesh. The Dhaka University Journal of Earth and Environmental Sciences 10: 21-34.
- Carstensen J, Klais R, Cloern JE (2015) Phytoplankton blooms in estuarine and coastal waters: Seasonal patterns and key species. Coastal and Shelf Science162: 98-109.
- Dey S (2021) THE SEASONAL CYCLE OF THE PHYTOPLANKTON IN THE COASTAL WATERS OF CHATTOGRAM(Doctoral dissertation, Chattogram Veterinary and Animal Sciences University Chattogram-4225 Bangladesh).
Citation: Nayak S, Pradhan SP, Nayak S, Sharma SN, Nayak P, et al. (2024) Distribution and Morphology of Coscinodiscus species from the Surface Water of Dhamra Coast, Bay of Bengal (Odisha). J Aquac Fisheries 8: 082.
Copyright: © 2024 Subhashree Nayak, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

