
Dose-Dependent Anticonvulsant and Protective Effects of Metformin in Kainate Induced Temporal Lobe Epilepsy
*Corresponding Author(s):
Farnaz NikbakhtAssociate Professor Of Physiology, Cellular And Molecular Research Center And Department Of Physiology, Iran University Of Medical Sciences, Tehran, Iran, Islamic Republic Of
Tel:8618980602201,
Email:farnazinikbakht@yahoo.com
Abstract
Introduction: Metformin, the most commonly prescribed antidiabetic drug, has been shown to be effective in controlling seizures in some studies. However, there are some reports of proconvulsant activity of metformin in diabetic patients. The aim of the present study was to investigate the effects of metformin dosage on anti-seizure and neuronal protective activity.
Method: Male Wistar rats were randomly divided into 5 groups as follows: 1-control/vehicle group, 2- Kainic acid (KA), 3-metformin+KA (50 mg/kg), 4-metformin+KA (100 mg/kg), 5- metformin+KA (200 mg/kg). Temporal lobe epilepsy (TLE) was induced by injection of 0.5 μg kainite into the left lateral ventricle.Metformin was administered orally for two weeks before the induction of TLE.
Results: We found that metformin at higher doses (100 and 200 mg/kg) significantly suppressed the progression of seizure in TLE and ameliorated the neuronal loss in the hippocampus induced by KA. However, the low dose of metformin had no effect.
Conclusion: We concluded that metformin may be a potential agent for the treatment of epilepsy and seizure and this effect is dose-dependent.
Keywords
Metformin; Epilepsy; Dose dependent; Neuroprotection; Anticonvulsant
INTRODUCTION
Epilepsy is a severe neurological disease which characterized by spontaneous recurrent seizures [1]. Temporal lobe epilepsy (TLE) is one of the most common and drug resistant forms of epilepsy in human with the consequence of hippocampal sclerosis. Hippocampal sclerosis is associated with vast neuronal loss in CA1, CA3 and the hilar areas of the hippocampus and pathological synapses connections within the hippocampus [2]. Status epilepticus and long-lasting seizures also cause neuronal loss in the hippocampus in TLE [3]. Any medication which can control seizures or prevents neuronal loss may be helpful in TLE. It is worth mentioning that about one-third of epileptic patients are resistant to available medications [4]. As many epileptic patients benefit the use of ketogenic diet [5] we hypothesize that medications which can control the body’s energy levels might also control epilepsy. Metformin is a common diabetes drug which can modulate AMPK, a key energy sensor in the brain [6]. It has been reported that chronic metformin treatment in PTZ kindling model, could terminate seizures [7].
The systemic protective effects of metformin are probably due to the improvement in endothelial function [8], anti-oxidative and anti-inflammatory functions [9]. However, in scopolamine-induced cognitive deficit, metformin show adose–dependent protective effects [10].
Another study also reveals a dose-dependent relationship between metformin and colorectal cancers in patients with type 2 diabetes [11].
Regarding previous data, in this study, we try to investigate the dose–dependent anticonvulsant effects of metformin in a model of temporal lobe epilepsy.
MATERIALS AND METHODS
Animals
Male Wistar rats (200-250 g) were obtained (n=40) from Iran University of Medical Sciences experimental studies, Tehran, Iran. The animals were kept in standard conditions as follows: 12-h light/dark cycle (lights on from 7:00 AM to 7:00 PM), environmental temperature 22±2 °C, and humidity 40-50%. The food and water were available throughout the study. All the experimental protocols and procedures were compiled according to guidelines for the care and use of laboratory animals stipulated by National Institutes of Health (NIH).
Experimental procedure
The rats were randomly divided into 5 groups as follows: 1-control/vehicle group, 2- Kainic acid (KA), 3-metformin+KA (50 mg/kg), 4- metformin+KA (100 mg/kg), 5- metformin+KA (200 mg/kg). For induction of TLE, rats were first anesthetized by the intraperitoneal (IP) injection of ketamine (100 mg/kg) and xylazine (20mg/kg) and fixed on a stereotaxic apparatus (Stoelting Co, USA). After a midline sagittal incision in the scalp, the left lateral ventricle was targeted at the following coordination from bregma; anterior-posterior: -1 mm; lateral, -1.5 mm and height: 3.5 mm below the Dura. 1.8 μl of sterile normal saline solution containing 0.5 μg of kainic acid was injected unilaterally into the left lateral ventricle 4 using a 5 μl Hamilton syringe. The needle remains in the place for five minutes then removed slowly to minimize the withdrawal of the drug. Metformin was dissolved in distilled water and was administered orally at doses (50, 100, 200 mg/kg) for two weeks. The last dosage of metformin was administered thirty min before microinjection of KA. Fourteen days after oral administration of metformin and before TLE induction, glucose was determined by glucometer. Blood samples were collected tail veins.
Assessment of epileptic behaviors
Following the microinjection of KA the rats were video monitored for the seizure behaviors for three hours and the outcome was assessed by a person blinded to the group experiments. Seizure severity was rating according to Racine’s modified classification [12].The scores were as following:
0: no response; 1: Stereotypic mounting, eye blinking, and /or mild facial clonus; 2: head nodding and/or multiple facial clonus; 3: myoclonic jerks in the forelimbs; 4: clonic convulsions in the forelimbs with rearing and 5: generalized clonic convulsions associated with loss of balance. Additionally, other parameters were monitored including latency to the first convulsion and duration of tonic-clonic seizures(Figures 1 and 2).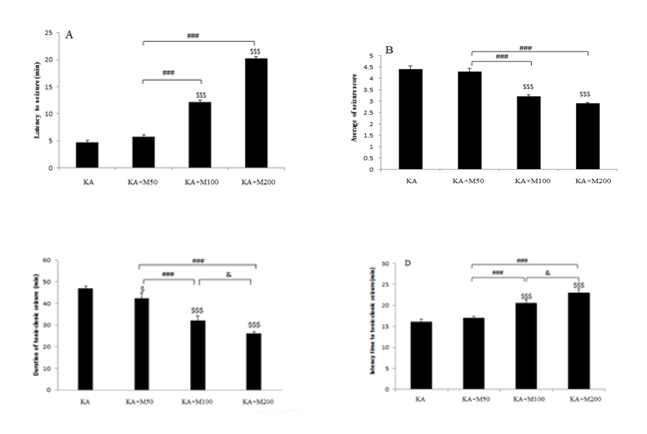 Figure 1: The anticonvulsant effects of Metformin at doses 50,100 and 200 mg/kg. (A) Latency to first convulsion, (B) Average of seizure score, (C) duration of tonic-clonic seizure and (D) latency time to tonic-clonic seizure. All data are presented as mean ± SEM$$$ (P<0.001) vs. KA group, ### (P<0.001) vs. M50 group, & (p?0.05) vs. M200 group.
Figure 1: The anticonvulsant effects of Metformin at doses 50,100 and 200 mg/kg. (A) Latency to first convulsion, (B) Average of seizure score, (C) duration of tonic-clonic seizure and (D) latency time to tonic-clonic seizure. All data are presented as mean ± SEM$$$ (P<0.001) vs. KA group, ### (P<0.001) vs. M50 group, & (p?0.05) vs. M200 group.
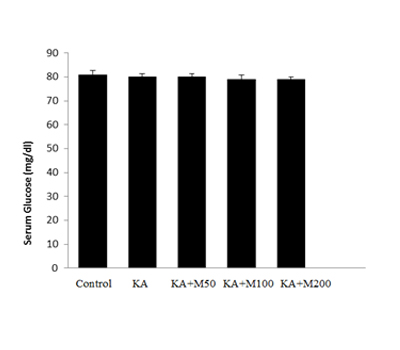 Figure 2: The glucose levels of rats which received different doses of metformin. No significant difference was observed between groups. Data expressed as mean± SEM.
Figure 2: The glucose levels of rats which received different doses of metformin. No significant difference was observed between groups. Data expressed as mean± SEM.
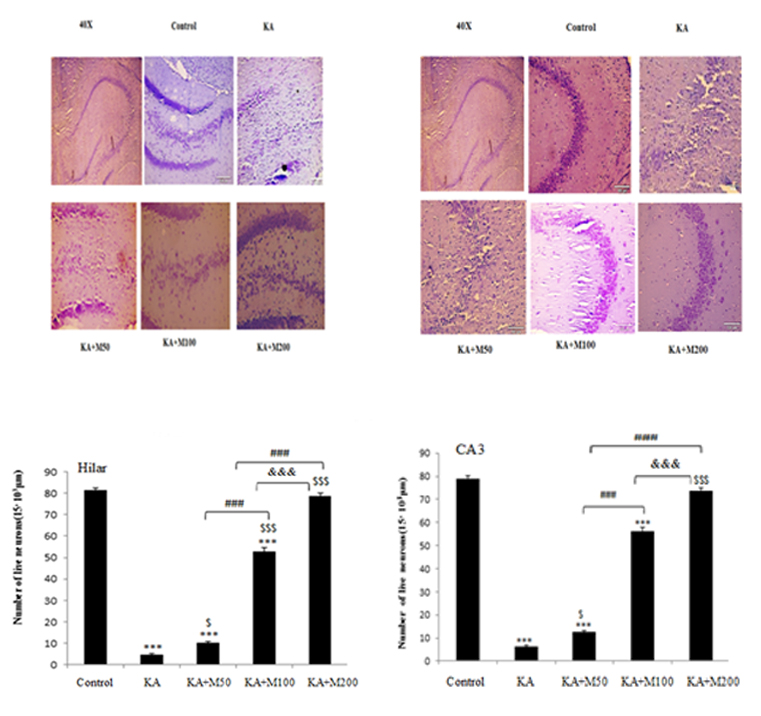 Figure 3:Neuroprotection of metformin on hippocampal neurons. Hippocampal neurons of each group were shown by Nissl staining in CA3 (A) and hilar areas (B). The results showed that metformin could protect neurons in CA3and hilar regions. Scale bars were 100 µm (C and D) Quanti?cation of the survival of neuronal cells in CA3 and hilus of hippocampus.
Figure 3:Neuroprotection of metformin on hippocampal neurons. Hippocampal neurons of each group were shown by Nissl staining in CA3 (A) and hilar areas (B). The results showed that metformin could protect neurons in CA3and hilar regions. Scale bars were 100 µm (C and D) Quanti?cation of the survival of neuronal cells in CA3 and hilus of hippocampus.
*** P<0.001 vs. Control group, $ P<0.05 vs. KA, $$$ P<0.001 vs. KA,&&& P<0.001 vs. KA+m100.
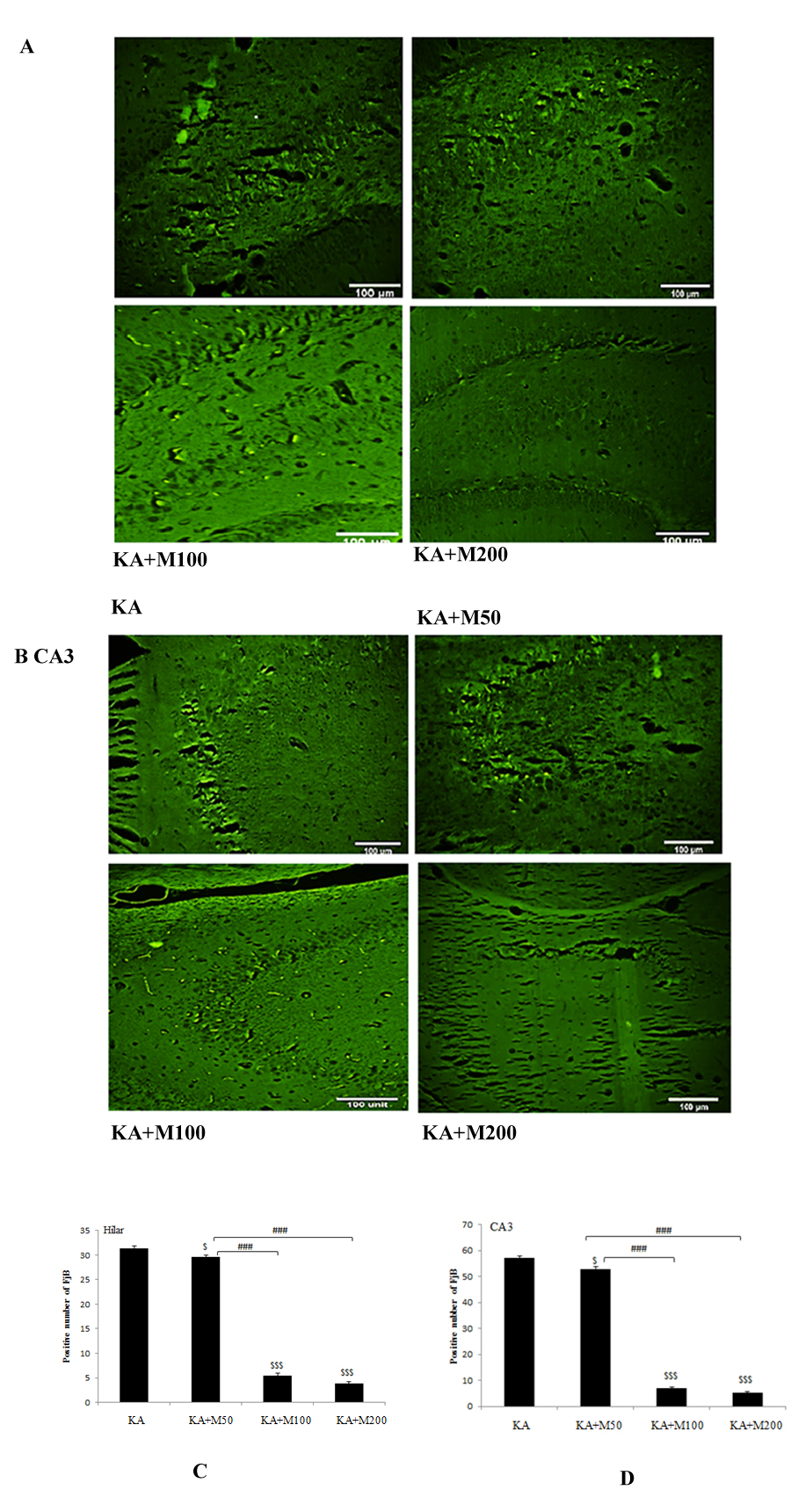 Figure4: Metformin pretreatment dose-dependently decreases neuronal cell death after kainate-induced seizures. A; Representative sections of Fluoro-Jade B staining in dentate of hilus and B; in CA3 areas of the hippocampus of kainite (KA), metformin pretreatment at dose of 50, 100 and 200 mg/kg (M50, M100 and M200 respectively) groups. Abundant Fluoro-Jade B-positive neurons were found in KA in CA3, and hilus but not in metformin-pretreated rats at doses of 100 and 200 mg/kg. Metformin at dose of 50 mg/kg was less protective. C and D; Quantitative analysis demonstrated a significant decrease in Fluoro-Jade B-positive neurons in metformin pretreated rats. P<0.001 M100 and M200 vs. KA, P<0.05 M50 vs. KA.
Figure4: Metformin pretreatment dose-dependently decreases neuronal cell death after kainate-induced seizures. A; Representative sections of Fluoro-Jade B staining in dentate of hilus and B; in CA3 areas of the hippocampus of kainite (KA), metformin pretreatment at dose of 50, 100 and 200 mg/kg (M50, M100 and M200 respectively) groups. Abundant Fluoro-Jade B-positive neurons were found in KA in CA3, and hilus but not in metformin-pretreated rats at doses of 100 and 200 mg/kg. Metformin at dose of 50 mg/kg was less protective. C and D; Quantitative analysis demonstrated a significant decrease in Fluoro-Jade B-positive neurons in metformin pretreated rats. P<0.001 M100 and M200 vs. KA, P<0.05 M50 vs. KA.
Scale bar 100µm for all photomicrographs.
Nissl staining
5 days after the KA injection, the rats (n=3 for each group) anesthetized with ketamine (100 mg/kg) and xylazine (20mg/kg) and perfused via the left cardiac ventricle with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4). For histopathological process, brains removed and post fixed overnight in 6 ml of the same postfix solution. The brains were then embedded in paraffin for tissue sectioning. The sections (5μm) were cut using a microtome and were mounted on gelatin-coated glass slides for Nissl or Fluoro-Jade B staining. Four periodic Nissl-stained sections at the coronal level of 1 mm posterior to the bregma were used for cell counts and the average was calculated. In the hippocampus, neurons were counted in the pyramidal cell layer of the CA3 and the dentate hilus(Figures 3 and 4). The CA3 subfield was determined as the curve aria of CA3 from the CA3/2 border to the end of the curve. The dentate hilar area was the point where the pyramidal cells enter the hilus till the end of the hilus. The Nissl staining performed according to the 0.1% Cresyl violet protocol. Briefly, the slides were first dehydrated in graded ethanol solutions and xylenes, then stained in 0.1% Cresyl violet for 1 min. Total number of intact neurons in CA3 and hilar areas were counted (15×103µm).
Fluoro-Jade B staining
Four sections of each brain used for Fluoro-Jade B staining. The Fluoro-Jade B staining was performed according to our lab protocol [13]. In brief, the slides were immersed in xylene, 100% ethanol for 3 min and 70% ethanol for 2 min. Further the sections were placed in a solution containing 1% sodium hydroxide in 80% alcohol for 2 min. Subsequently, they were rinsed in dH2O for 2 min before being transferred to a solution containing 0.01 % potassium permanganate (KMnO4) for 15 min. Following rinsing in dH2O, the sections were immersed into a 0.0004 Fluoro-Jade B working solution for about 30 min in darkness. The sections were then air dried in the dark for 20 min. Finally, the dry slides were cleared in xylene and cover slipped with non-aqueous mounting medium, DPX. For observing degenerative neurons a fluorescent microscope with blue (450-490 nm) excitation light was used.
STATISTICAL ANALYSIS
The results were expressed as mean ± S.E.M. Differences were considered statistically significant at p<0.05. Statistical analyses were performed using the SPSS software package version 16. The differences between the groups of animals were tested using one-way ANOVA followed by post hoc Tukey's test.
RESULTS
Effect of different doses of metformin on epileptic behaviors
Epileptic behaviors in each group were assessed by scoring seizure latency, seizure severity, latency to development of tonic-clonic seizures and duration time in tonic- clonic stage (Figures 1A-1D).
Figure1 compares the epileptic behaviors between KA and metformin+ KA groups. As it is shown in (Figure1A), latency to the first seizure was significantly increased in the metformin+KA groups in doses of 100 and 200 mg/kg compared to the KA group (P<0.001). However, metformin at doses of 50 mg/kg had no effect on seizure latency. (Figure 1B)shows the result of average seizure score. The severity of seizure was reduced only in doses of 100 and 200 mg/kg of metformin (P<0.001). Duration of tonic-clonic stage were significantly decreased in all groups of metformin (P<0.05 for the dose of 50 mg/kg and p<0.001for the doses of 100 and 200 mg/kg). However, rats in dose of 200 mg/kg of metformin had the lowest duration time of tonic-clonic stage.Latency time to tonic-clonic stage was demonstrated in (Figure1D). Latency to tonic-clonic stage was significantly increased in the metformin+KA group at doses of 100 and 200 mg/kg compared to the KA group (P<0.001). No significant difference was observed between 50mg/kg metformin with KA group.
Effect of different doses of metformin on blood glucose
As (Figure 2)shown, glucose in control rats was about 81mg/dl. When rats were treated with different doses of metformin, the glucose was slightly lowered to 79 mg/dl which was not significant. There was no significance between the glucose levels in different metformin doses. This indicated that metformin at doses of 50, 100 and 200 mg/kg did not produce hypoglycemia.
Effect of oral administration of metformin on neuronal necrosis in KA-induced rat model of TLE
A large number of neurons were lost in the CA3, and hilar regions in the kainate group. However, no nissl-stained dark neurons were detected in control group Metfromin at dose of 200 mg/kg markedly protected against kainate-induced death of neurons in CA3 as well as hilar regions in the hippocampus ((P<0.001). In contrast, metformin at doses of 50 and 100 mg/kg could not significantly prevent neuronal loss in hilar and CA3 area (P<0.001vs control).
Effect of oral administration of metformin on neurodegeneration of hilar and CA3 areas in KA-induced rat model of TLE
Consistent with previous reports of neuronal death in the kainate model, our results demonstrated massive neuronal death by Fluoro-Jade B staining after kainate status epilepticus in neurons from the dentate hilus and CA3 pyramidal regions (Figures 4A-4B). In contrast, in rats pretreated with metformin, 100 and 200 mg/kg had shown only sparse positive degenerating pyramidal neurons. Pre-treatment with metformin 50 mg/kg was not very effective in reducing the number of degenerative neurons.
DISCUSSION
Metformin a medication for treatment of diabetes and control of blood glucose may trigger epileptic behaviors. The anti-seizure activity of metformin has been recently shown [14]. However, there are some reports in clinic showing the pro-convulsing effect of metformin. It seems that the effect of metformin on epilepsy is depending on the dosage. This study was designed to reveal any proconvulsant or anticonvulsant effect of metformin at different doses. We used three different doses of metformin (50,100,200 mg/kg) in a TLE model induced by KA in rat. In our study all three doses did not induce hypoglycemia. This is in accordance with a data showed that the lowest dose of metformin which can induce hypoglycemia in rat is 350 mg/kg [15]. It seems that metformin per se is not proconvulsant but in the condition of hypoglycemia, the low level of glucose trigger seizure. Although epileptic seizures are the rare consequences of hypoglycemia but it has been reported in some cases [16]. In our studies none of the three doses of metformin produced neither hypoglycemia nor seizure behaviors. In contrast, the higher doses (100 and 200 mg/kg) have exerted anticonvulsant and neuroprotective effects. In comparison of the two high doses, the dose of 200 mg/kg was even more effective. The dose dependency of metformin in neuronal protection has been investigated in a study in which two doses of metformin (100 and 300 mg/kg) have been used for two weeks. Their results revealed that metformin at dose of100 mg/kg was very effective in reducing inflammatory factors and amelioration of memory deficit [10]. It seems that metformin, only in the average antidiabetic doses (100 and 200 mg/kg) has anticonvulsant and protective effects against KA-induced TLE. Metformin acts in two different pathways: AMPK dependent pathway and AMPK independent pathway. The AMPK dependent pathway is useful in treating epilepsy. In an in vitro study the dose dependent effect of metformin on adipogenic differentiation has been demonstrated [17].
The authors have concluded that lower concentrations of metformin induce adipogenesis, which could be mediated in an AMPK-independent manner, while higher concentrations of metformin inhibit adipogenesis via AMPK activation.
Our data concerning the anticonvulsant effects of metformin is in accordance with other studies. In mice chronic metformin facilitated seizure termination [7]. In another study, administration of metformin in a post-traumatic brain seizure model induced by FeCl3, inhibited the onset of seizures in rat [18]. Our results also revealed a potent protective role of metformin in CA3 and the hilus area of the hippocampus. Five days after induction of TLE by kainite, the neuronal layer in CA3 have been disrupted. Metformin in two higher doses completely restored this neuronal architecture disruption. However, metformin in lower dose was not at all effective.The neuroprotective effects of metformin can be attributed to different mechanism; inhibition of inflammation pathways through NF?B and MPK signaling [19], lowering the oxidative stress and anti-apoptotic [20, 21] and amelioration of mitochondrial dysfunction [22].
Further investigations need to reveal the exact mechanism of dose dependent effect of metformin in epilepsy.
CONCLUSION
Altogether we conclude that the efficacy of metformin is dose-dependent in TLE. The average doses (100 and 200 mg/kg) which are below hypoglycemic dose works better than low dose.
ACKNOWLEDGEMENT
This research was supported by a grant (No. 29683) sponsored by the Iran University of Medical Sciences.
REFERENCES
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, et al. (2014) ILAE official report: A practical clinical definition of epilepsy. Epilepsia 55: 475-482.
- Thom M (2014) Hippocampal sclerosis in epilepsy: A neuropathology review. NeuropatholApplNeurobiol 40: 520-543.
- Lado FA, Laureta EC, Moshé SL (2002) Seizure-induced hippocampal damage in the mature and immature brain. Epileptic Disord 4: 83-97.
- https://www.uptodate.com/contents/evaluation-and-management-of-drug-resistant-epilepsy
- Williams TJ, Cervenka MC (2017) The role for ketogenic diets in epilepsy and status epilepticus in adults. ClinNeurophysiolPract 2: 154-160.
- Ashabi G, Khodagholi F, Khalaj L, Goudarzvand M, Nasiri M (2014) Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: Interference of AMPK/PGC-1α pathway. Metab Brain Dis 29: 47-58.
- Yang Y, Zhu B, Zheng F, Li Y, Zhang Y, et al. (2017) Chronic metformin treatment facilitates seizure termination. BiochemBiophys Res Commun 484: 450-455.
- Triggle CR, Ding H (2017) Metformin is not just an antihyperglycaemic drug but also has protective effects on the vascular endothelium. ActaPhysiologica 219: 138-151.
- Araújo AA, Pereira ASBF, Medeiros CACX, Brito GAC, Leitão RFC, et al. (2017) Effects of metformin on inflammation, oxidative stress, and bone loss in a rat model of periodontitis. PloS One 12: e0183506.
- Mostafa DK, Ismail CA, Ghareeb DA (2016) Differential metformin dose-dependent effects on cognition in rats: Role of Akt. Psychopharmacology 233: 2513-2524.
- Chang YT, Tsai HL, Kung YT, Yeh YS, Huang CW, et al. (2018) Dose-dependent relationship between metformin and colorectal cancer occurrence among patients with type 2 diabetes-a nationwide cohort study. TranslOncol 11:535-541.
- Lüttjohann A, Fabene PF, van Luijtelaar G (2009) A revised Racine's scale for PTZ-induced seizures in rats. PhysiolBehav 98: 579-586.
- Yeganeh F, Nikbakht F, Bahmanpour S, Rastegar K, Namavar R (2013) Neuroprotective effects of NMDA and group I metabotropic glutamate receptor antagonists against neurodegeneration induced by homocysteine in rat hippocampus: In vivo study. J MolNeurosci 50: 551-557.
- Mehrabi S, Sanadgol N, Barati M, Shahbazi A, Vahabzadeh G, et al. (2018) Evaluation of metformin effects in the chronic phase of spontaneous seizures in pilocarpine model of temporal lobe epilepsy. Metab Brain Dis 33: 107-114.
- Gawler D, Milligan G, Houslay MD (1988) Treatment of streptozotocin-diabetic rats with metformin restores the ability of insulin to inhibit adenylatecyclase activity and demonstrates that insulin does not exert this action through the inhibitory guanine nucleotide regulatory protein Gi. Biochem J 249: 537-542.
- Brennan MR, Whitehouse FW (2012) Case study: Seizures and hypoglycemia. Clinical Diabetes 30: 23-24.
- Chen D, Wang Y, Wu K, Wang X (2018) Dual effects of metformin on adipogenic differentiation of 3T3-L1 preadipocyte in AMPK-dependent and independent manners. Int J MolSci 19: 1547.
- Kumar P, Kale RK, Baquer N (2013) Effect of metformin on seizures in FeCl3 induced epileptic animal models. Journal of the Neurological Sciences 333: e44.
- Tao L, Li D, Liu H, Jiang F, Xu Y, et al. (2018) Neuroprotective effects of metformin on traumatic brain injury in rats associated with NF-κB and MAPK signaling pathway. Brain Res Bull 140: 154-161.
- Hussein AM, Eldosoky M, El-Shafey M, El-Mesery M, Ali AN, et al. (2019) Effects of metformin on apoptosis and α-synuclein in a rat model of pentylenetetrazole-induced epilepsy.Can J PhysiolPharmacol 97: 34-46.
- Patil SP, Jain PD, Ghumatkar PJ, Tambe R, Sathaye S (2014) Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience 277: 747-754.
- Izzo A, Nitti M, Mollo N, Paladino S, Procaccini C, et al. (2017) Metformin restores the mitochondrial network and reverses mitochondrial dysfunction in Down syndrome cells. Hum Mol Genet 26: 1056-1069.
Citation: Vazifekhah S, Nikbakht F, Hashemi P, Khanizadeh AM, Mojarad TB(2019) Dose-Dependent Anticonvulsant and Protective Effects of Metformin in Kainate Induced Temporal Lobe Epilepsy. J Alzheimers Neurodegener Dis 5: 026.
Copyright: © 2019 Somayeh Vazifekhah, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

