
Early Success Using Larval Stages of White Shrimp Litopenaeus vannamei as Seahorse Feed: Indicative for a Simultaneous Mariculture?
*Corresponding Author(s):
Rosana Beatriz SilveiraHippocampus Institute, Marine Aquaculture Laboratory, Porto De Galinhas, Brazil
Tel:+55 81987912677,
Email:rosanasilveira@projetohippocampus.org/labaquac@yahoo.com
Abstract
The seahorse Hippocampus reidi (Syngnathidae) is classified as “data deficient” on the IUCN Red List (International Union for Conservation of Nature) and as “vulnerable” on the official Brazilian list. In order to seek alternative live feed for captive seahorse cultivation, H.reidi newborns were fed for the first time using larval stages of sea shrimp Litopenaeus vannamei and the conventional Artemia salina and rotifer Brachionus plicatilis diets in Short-Duration experiments - SD (up to 11 days) and Medium-Duration MD (up to 60 days). Treatments used each food item separately or combined with another. The experiments were carried out in closed systems with recirculation and partial seawater exchange (salinity 30), 12 h photoperiod and controlled temperature (25°C±1). The crustacean nauplii and rotifers were fed with the microalgae Chaetoceros gracilis. For SD experiments, the use of B.plicatilis up to 10°C and the addition of Artemia salina nauplii from the 5th day of life obtained a good result with a survival rate of 72%. For the MD experiments, the combination of L.vannamei nauplii and rotifers had a survival rate up to 20 days of life of 89.2% and varied between treatments from 50 to 86% until 60 days of life. The development in weight and height reached the highest values in the treatments that used L.vannamei nauplii.
Keywords
Artemia; Culturing; Fish; Prawn; Rotifer
INTRODUCTION
Seahorses are bony fishes and all species are included in a single genus, Hippocampus, which belongs to the family Syngnathidae. Species of Hippocampus are listed by the IUCN Red List (International Union for Conservation of Nature) as Vulnerable (VU) or Data Deficient (DD) [1]. There are three species native to Brazil: Hippocampus reidi; H.erectus and H.patagonicus [2-4]. In Brazil, the three species have been listed as VU by the Ministry of Environment, through Ordinance 455/2014 [5].
According to Vincent [6], aquarists and people involved with traditional medicine, mainly traditional Chinese medicine, started to become interested in seahorses many decades ago. The earliest record of cultivating these fishes is from 1950 [7]. The first attempts to commercially cultivate seahorses were during the 1970s; in the 1990s, the aquaculture of seahorses began in Asia and, then in 1996, could be found in Australia and the United States [8].
In Brazil there are very few records regarding the true sale of live seahorses and nothing regarding the trading of dried seahorses. According to Baum and Vincent [9], 100 kg of seahorses were sold in 1990 in this country. It is also known that dried seahorses are used throughout Brazil by individuals practicing traditional medicine in, for example, “seahorse tea” which is used to treat asthma and fatigue [10,11].
In addition to the trade of dried seahorses in Brazil, the trade of live seahorses for aquaria has a quota of 250 seahorses/species/year/company [12]. According to Baum and Vincent [9], Brazil is the largest exporter of seahorses in Latin American. These are reasons that make seahorse aquaculture of vital importance for supplying the world market and for maintaining natural stocks.
A comprehensive review of seahorse aquaculture was compiled and summarized by Koldewey and Martin-Smith [8], in which the authors discuss the use of live and frozen diets. These authors found that mysids, brine shrimp, amphipods, copepods, rotifers and wild zooplankton are the most common food sources used. The authors also comment on the importance of economic feasibility studies in seahorse cultivation.
In the absence of a literary record on the use of sea shrimp nauplii as food for early stages of seahorses, the purpose of this manuscript was to draw attention to alternative living diets using Litopenaues vannamei sea shrimp for this purpose.
MATERIALS AND METHODS
Short-duration experiment - 1SD- up to 11 days old
A short-term experiment was conducted with two treatments (A and B) in a climate-controlled environment (25±1°C), with newborns of the H.reidi from the same brood. The breeders were also born and raised in our laboratory. For the present cultivation experiment, a density of 10 newborn seahorses per liter was maintained.
In the treatment A, they were fed the rotifer Brachionus plicatilis for 10 days. In treatment B they were fed B.plicatilis for 11 days and newly hatched Artemia salina nauplii from day 5 to day 11. Artemia salina cysts (INVE) were obtained from local shops and enriched on a diet of the microalga Chaetoceros gracilis in 300-L tanks. The experiments were conducted in 1-L aquaria using 12 h photoperiod, natural seawater (salinity 30) that was sterilized with chlorine, and received moderate aeration. The water was replaced each morning, which was important for removing feces and old food from the aquaria and keeping the levels of ammonia and nitrite at zero and the pH at 8.2. In all experiments Ammonia and nitrite were monitored with colorimetric tests (Alcon®, Brazil), the pH with digital phmeter (Analyzer®, Brazil) and salinity with automatic temperature-compensated analogical refractometer (Instruterm®, Brazil) and temperature with digital thermometer.
The rotifers were fed the microalga C.gracilis. Each treatment was replicated nine times. The zooplankton used had a concentration of 80 ind/mL B.plicatilis and 50 ind/mL A.salina, and they were offered to the seahorses in volumes of 100 and 70 mL, respectively, three times per day (9:00 a.m.; 1:00 p.m. and 5:00 p.m.).
Medium-duration experiments-MD- up to 60 days old
Two medium-term experiments were conducted using five 40-L aquaria each, equipped with an external filter and screens so the fish would not be sucked into the filter. The alevins of H.reidi used for each experiment were obtained from the same parents. The water used was seawater (30 ppm) sterilized with chlorine; 10% of the volume of the tank was changed each day. A 12-h photoperiod was used and the tanks were kept at a pH of 8.2, 0 mg.L-1 ammonia and nitrite levels between 0 and 1.8 mg.L-1. When the nitrite concentration increased to more than 0.5 mg.L-1, 50% of the water was replaced. The experiment took place in a warm room, 25°C±1.
Experiment 1MD- Newborn H.reidi seahorses were stocked at a density of 1.25 fish per liter (50 seahorses in a 40-L aquarium). Each day, the seahorses were fed an exclusive diet of white shrimp (Litopenaeus vannamei) nauplii that were 1 to 2 days old. The nauplii were kept at an average concentration of 60 ind/mL, and given to the seahorses in 500-mL batches, twice a day (9:00 am and 2:00 pm.), for the first 22 days. Afterwards, the young seahorses were fed A.salina twice a day, which were up to 2 mm long and at a concentration of 10 ind/mL. The microcrustaceans were fed the microalga C.gracilis and the shrimp commercial liquid feed (rich in polyunsaturated fatty acids), Epifeed® LHF1 3 times per day.
Experiment 2MD- The newborn seahorses (n=100) were fed the rotifer B.plicatilis and nauplii of the white shrimp L.vannamei that were 1 to 2 days old. The rotifers were provided under the same conditions as in the short-term experiment until the seahorses reached 6 days of life, at which time the seahorses were fed white shrimp nauplii until day 20. After day 20 the seahorses were divided into three treatments (30 seahorses per 40-L tank):
- Shrimp treatment- 30 seahorses fed only vannamei nauplii (CAM).
- Shrimp+Artemia treatment- 30 seahorses fed vanamei nauplii (50%) and fed A.salina nauplii and juveniles (50%) (CAM+ART).
- Artemia treatment- 30 seahorses fed only salina nauplii and juveniles (ART).
As the medium-term experiment was dependent on external supply to our laboratory (supply of sea shrimp nauplii by the partner mariculture, far from our laboratory), they had no replication due to the difficulty of obtaining sufficient quantities of sea shrimp nauplii in the periods when necessary until the conclusion of the experiment.
STATISTICAL ANALYSIS
Short-term survival rates were evaluated at the end of the tenth day and eleventh day of Experiment 1SD. For medium-term Experiments 1MD and 2MD, survival rates were evaluated for the respective treatments at the end of the experiments, and the growth of the seahorses was evaluated in terms of height (cm) and weight (g) on the 30th and 60th day of age in only Experiment 2MD. The seahorses were measured for height according to Lourie et al. [1] and individually weighed using a semi-analytical balance (0.0001 g). To ensure that the seahorses newborns could eat their prey, 1 ml sample of each food item was measured against the size of the mouth of the sea horse, using an ocular micrometer attached to the binocular stereomicroscope, observing the size range of the prey. The evaluation of the survival rate was performed by the Kaplan-Meier method only in the short-term experiment [13]. The Wilcoxon test was used to evaluate the similarity in survival between the series generated [14]. Tukey’s test was used for comparison of average weight and height of different treatments [15]. Proportions were compared using the binomial test, using the software R version 3.6.1. [16].
RESULTS AND DISCUSSION
Short-term experiments
In figure 1, it can be seen that treatments A and B of short-term Experiment 1SD had an average survival rate of 71% (±4.7%) and 75% (±4.6%), respectively, on the sixth day. The Kaplan-Meier assessment of survival rate in this case showed that on the sixth day, treatment A showed survival between 60-79%, and B 64-82%.
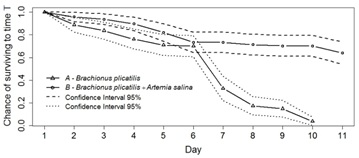 Figure 1: Kaplan-Meier survival rates. A: Newborn seahorses fed Brachionus plicatilis. B: Newborn seahorses fed Brachionus plicatilis+Artemia salina.
Figure 1: Kaplan-Meier survival rates. A: Newborn seahorses fed Brachionus plicatilis. B: Newborn seahorses fed Brachionus plicatilis+Artemia salina.
In treatment A, in which continued the use of only rotifers until the tenth day, there was a significant drop in survival rate relative to treatment B, between the sixth day (p=0.043) and the tenth day (p=0.007), at which point there was only a 1.6% survival rate. In treatment B, in which rotifers were used and then A.salina starting on day 5, the survival average rate was 72% at the end of the experiment (61-79%).
Medium-term experiments
In Experiment 1MD, on the tenth day, 50% of the seahorses died. There were no deaths between day 10 and day 22, but the seahorses began to die again after day 22 when the type of food was changed. Only 20% of seahorses (10 of 50 fishes) lived until the end of the experiment.
In Experiment 2MD, at the end of 20 days, the survival rate was 90%. This showed that the combination of B.plicatilis and L.vannamei was an effective feed. In the Experiment 2 treatments (Shrimp, Shrimp+Artemia and Artemia), the survival rate of the seahorses at 60 days was 50% for the Shrimp treatment and the seahorses that died had symptoms of loss of appetite, slow movement, body covered by a white mucus and necrotic spots on the tail. For the Shrimp+Artemia and Artemia treatments, the survival rates were 86 and 83%, respectively, showing that there was only a significant difference in the Shrimp treatment (Z=4.9439, p<0.0001, figure 2). In addition to the survival rate, the height (cm) and weight (g) of the seahorses in the three treatments were also analyzed. The results are illustrated in figures 3 & 4.
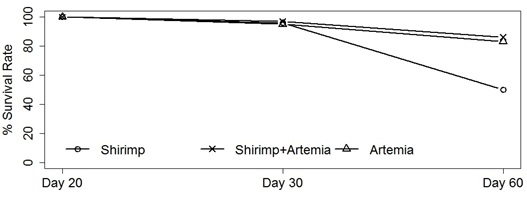
Figure 2: Experiment 2MD: Survival rates of the H.reidi studied, between day 20 and day 60, using different diets. Shrimp, Litopnaeus vannamei; Shrimp+Artemia, Litopneaus vannamei+Artemia salina; Artemia, Artemia salina.
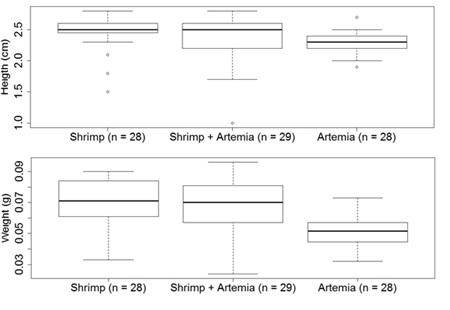
Figure 3: Experiment 2MD: Height and weight of seahorses and magnitude of variation in the three treatments on day 30 (Shrimp, Litopnaeus vannamei; Shrimp+Artemia, Litopneaus vannamei+Artemia; Artemia, Artemia salina).
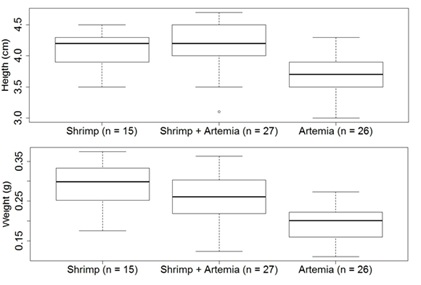
Figure 4: Experiment 2MD: Height and weight of seahorses and magnitude of variation in the three treatments on day 60 (Shrimp, Litopnaeus vannamei; Shrimp+Artemia, Litopneaus vannamei+Artemia salina; Artemia, Artemia salina).
At the end of the first month, only the Artemia treatment showed a significant difference in comparison with other treatments, in the height and weight of the seahorses (p<0.01). At the end of the second month, the Artemia treatment showed little development in height as well as weight (p<0.01), even though the final survival rate was 83%.
The maximum and minimum measurements obtained for the size of the mouth opening of the newborn seahorses was 375-500 µm, 150-170 µm for length of rotifers and 420-540 µm for the length of marine shrimp nauplii (without considering the bristles, which increased the size to 730-750 µm). The newborn seahorses had great difficulty in feeding on the marine shrimp nauplii during the first 10 days of life because of their large size (1- to 2-day-old nauplii).
DISCUSSION
In the short-term experiment, both treatments (A & B) had similar and positive results when considering the survival rates (over 70%) of H.reidi on the sixth day of life (which is the time that fish farmers usually use rotifers). Murugan et al. [17] cultivated Hippocampus trimaculatus, which had a 29% survival rate when fed a diet of B.plicatilis until the sixth day. The authors comment that the rotifers were preferentially consumed by seahorses in the first two days old and that the nauplii of copepods promoted greater development, providing a survival rate of 73% on the sixth day. Hora and Joyeux (2009) [18], obtained high survival rates by using wild zooplankton as food until H.reidi was six days old. Similarly, Silveira and Santos [19] achieved 84% survival using wild zooplankton as the first feed of H.erectus until the 10th day of life. Melo et al. [20], achieved a survival rate of 76% by cultivating H.reidi from birth to the 15th day of life on a diet of copepods and Artemia enriched with seahorses kept in green water (2.02±0.44 9 106 cells mL-1 Nannochloropsis oculata) against 25% survival of treatment maintained without microalgae.
Using a diet of rotifers and enriched Artemia franciscana for the newborn H.abdominalis, Thompson [21] did not observe growth or survival benefit with this diet, but this species is more than twice the average size at birth than H.reidi (15 mm and 7 mm, respectively). Seahorse species that produce small juveniles with fine snouts such as H.kuda and H.subelongatus are believed to be restricted to smaller prey as the first food (such as rotifers and small copepods) to increase growth and survival rates [22]. It would be the same case of H.reidi in this study, which obtained a good survival rate predating B.plicatilis and A.salina enriched until the sixth day. For seahorses that were fed rotifers only, after the fifth day the drop in survival rate can be seen as a reflection of nutritional deficiency or disinterest in a small food item that has become unattractive for the age and size of 6 days of life.
In medium-term Experiment 1MD, there was a high mortality (50%) during the first 10 days of cultivation, which was attributed to the larger size of the white shrimp nauplii (420-540 µm long) in relation to the size of the mouth opening (375-500 µm) of the newborn H.reidi. The width between the lateral appendages with bristles of shrimp nauplii (750 µm) also represented an impediment, because the bristles although flexible gave the animals a larger appearance, making them look intimidating and precluding attack by the seahorse newborn. The variable size of the prey and the small size of the mouth opening of the seahorses resulted in 50% mortality during the first 10 days of the experiment, but the survival rate stabilized between day 11 and day 22. Seahorses that achieved mechanical ability or showed a larger oral perimeter were able to establish themselves in the aquarium. Seven days after the food type was changed (after day 22) from white shrimp nauplii to young A.salina, seahorses began to die again. The seahorses that adapted to this new food type (18.35%, nine fishes) remained alive until the end of the experiment (60 days). When cultivating H.reidi, Hora and Joyeux (2009) [18], always observed mortality after changing the food type, being essential that the substitutions happen gradually with a period of supply of both foods simultaneously, before the complete substitution, in order to reduce the food stress. Silveira and Silva [19] used the marine shrimp nauplii L.vannamei and A.salina for the cultivation of H.erectus, from the 11th day of life, obtaining 84% survival until adulthood, but in the first days of life used plankton as food until the 17th day.
In medium-term experiment 2MD, whose diet combined B.plicatilis with L.vannamei, the survival rate was 89% at the end of 20 days. From then on, these animals arranged in the three treatments (CAM; CAM+AERT; ART) and cultivated up to 60 days of life had survival rates of 50, 86 and 83%, respectively. Job et al. [23], achieved a 92% survival rate for H.kuda at 42 days of life, using a species of enriched Artemia. Bustamante and Salas [24], obtained an 84% survival rate for H.igens until the 63 days of life, while Woods [25], obtained a survival rate of 68 to 82% using the same species.
When we look at the height and weight performance provided by the treatments of the 2MD, we see that while the ART treatment had a good survival rate (83%), CAM and CAM+ART were significantly superior and similar to each other in height and weight at the end of the first month and the second month. These facts are possibly attributes to the use of L.vannamei, though extensive comparisons with the available literature become difficult, since the cultivation of seahorses with L.vannamei nauplii was seen only in Silveira and Silva [19], cultivating H.erectus. Although they presented a similar height at birth of 7 mm (H.erectus, Silveira and Silva [19] and H.reidi, in this experiment), the first reached 53 days with 4.36±0.31 cm and weight of 0.377±0.074 g, while H.reidi reached 60 days (seven more days) with 4.17±0.413 cm and 0.265±0.064 g. Both used the same type of food (CAM+ART) and the same temperature range, suggesting that perhaps the first species might have a faster development. Animals whose adult body size is smaller often have a faster metabolism than larger ones [26,27]. In this case, H.erectus has a maximum observed size of 12 cm in height, while for H.reidi, the maximum observed size was 18.7 cm in height, both in Brazilian waters [4].
Captive growth data are diverse for different species, and dependent on the variables to be considered in crops such, as type and quantity of feed, temperature, photoperiod, tank sanitation, water quality, and biological variables such as specific growth rate [8,24,28-33].
In Experiment 2MD’ treatments (CAM; CAM+ART and ART), the survival rate at the end of 60 days was 50% for the CAM treatment. For CAM+ART and ART treatments, rates were similar, between 86 and 83%, respectively, resulting in a significant difference only in CAM treatment (p-value=0.0147, figure 4).
In the 2MD, the three treatments proceeded in a similar way until the seahorses were 30 days old. We expected the Shrimp treatment to have the highest seahorse survival rate because of the nutritional value of marine shrimp [34]; however, it had the lowest. In searching for an explanation for this situation, we talked to the technicians of Netuno (supplier of marine shrimp nauplii) and found out that shrimp mortality in their tanks was attributed to rod-shaped bacteria, which could to be related to the mortality we observed in the treatment with only marine shrimp nauplii. Balcázar et al. [35] identified two species of Vibrio, which killed individuals of Hippocampus guttulatus that were being cultivated in Spain. Symptoms reported by these authors and Planas et al. [36], included lethargy, loss of appetite, white spots on the skin and necrosis of the tail; this was similar to what we observed in the medium-term Experiment 2MD. Although it was not possible to categorically determine the cause of death of the seahorses, we observed that poor nutrition (only A.salina) and the size of the prey caused food shortages, growth deficit, which probably made the seahorses more vulnerable to diseases.
Overall, the results of this study suggest that the breeding of seahorses using rotifers for the first 5 days and L.vannamei nauplii afterwards is promising for this type of aquaculture. This could be attributed to the higher nutritional value of white shrimp compared to A.salina nauplii [34,37]. Out of curiosity, after the end of the experiment (60 days of life of the seahorse), cultivation continued successfully using the other life stages of L.vannamei and adult brine shrimp, but with different objectives than those described here. For large maricultures, it would even be possible to cultivate L.vannamei and seahorses at the same time. It is necessary to conduct a study of economic viability recommending (or not) an experimental plot of seahorses for commercial purposes using the various stages of white shrimp as food. The findings of this study suggest a new alternative food source for farmed seahorses with the possibility of a type of consortium or simultaneous cultivation with L.vannamei and optimization techniques, considering commercial and conservation proposals.
ACKNOWLEDGMENT
We thank Maricultura Netuno for providing the larval and post-larval white shrimp, IBAMA (Brazilian Environmental Agency) for the collection and aquaculture permits, and Petrobras for their financial support of Projeto Hippocampus. Dr. A. Leyva helped with English editing of the manuscript.
REFERENCES
- Lourie S, Vincent ACJ, Hall HJ (1999) Seahorses: An identification guide to the word’s species and their conservation. Project Seahorse, London, England.
- Ginsburg I (1933) Descriptions of five new species of seahorses. J Washingt Acad Sci 23: 560-563.
- Piacentino G, Luzzatto D (2004) Hippocampus patagonicus sp. nov., nuevocaballito de mar para la Argentina (Pisces, Syngnathiformes). Rev Del Mus Argentino Ciencias Nat 6: 339-349.
- Silveira RB, Siccha-Ramirez R, Silva JRS, Oliveira C (2014) Morphological and molecular evidence for the occurrence of three Hippocampus species (Teleostei: Syngnathidae) in Brazil. Zootaxa 3861: 317-332.
- MMA (2014) Portaria no 445, de 17 de Dezembro de 2014. Lista Nacional Oficial de Espécies da Fauna Ameaçadas de Extinção - Peixes e InvertebradosAquáticos. DiárioOficial da União, Brasília, Brazil.
- Vincent ACJ (1996) The international trade in Seahorses. TRAFFIC International, Cambridge. England.
- Herald E, Rakowicz M (1951) Stable requirements for raising sea horses. Aquarium J 3: 234-242.
- Koldewey HJ, Martin-Smith KM (2010) A global review of seahorse aquaculture. Aquaculture 302: 131-152.
- Baum JK, Vincent ACJ (2005) Magnitude and inferred impacts of the seahorse trade in Latin America. Environ Conserv 32: 305-19.
- Gasparini JL, Floeter SR, Ferreira CEL, Sazima I (2005) Marine ornamental trade in Brazil. Biodivers Conserv 14: 2883-2899.
- Alves RRN, Rosa IL (2006) From cnidarians to mammals: The use of animals as remedies in fishing communities in NE Brazil. J Ethnopharmacol 107: 259-276.
- MMA (2008) Instrução Normativa no 202, de 22 de Outubro de 2008: Dispõesobrenormas, critérios e padrões para aexplotação com finalidade ornamental e de aquariofilia de peixesnativosouexóticos de águasmarinhas e estuarinas. Diário Oficial da União, Brasília, Brazil.
- Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53: 457-481.
- Wilcoxon F (1945) Individual comparisons by ranking methods. Biometrics Bull 1: 80-83.
- Tukey JW (1949) Comparing individual means in the analysis of variance. Biometrics 5: 99-114.
- The R Core Team (2019). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria.
- Murugan A, Dhanya S, Sreepada RA, Rajagopal S, Balasubramanian T (2009) Breeding and mass-scale rearing of three spotted seahorse, Hippocampus trimaculatus Leach under captive conditions. Aquaculture 290: 87-96.
- Hora MSC, Joyeux JC (2009) Closing the reproductive cycle: Growth of the seahorse Hippocampus reidi (Teleostei, Syngnathidae) from birth to adulthood under experimental conditions. Aquaculture 292: 37-41.
- Silveira RB, Silva JRS (2016) Growing the threatened seahorse Hippocampus erectus Perry 1810 in the laboratory. Int J Oceanogr Mar Biol 3: 93-101.
- Mélo RCS, Santos LPS, Brito APM, Gouveia AA, Marçal C, et al. (2016) Use of the microalga Nannochloropsis occulata in the rearing of newborn longsnout seahorse Hippocampus reidi (Syngnathidae) juveniles. Aquac Res 47: 3934-3941.
- Thompson SJ (2002) The effect of diet enrichment on the growth and survival of pot-bellied seahorse (Hippocampus abdominalis) juveniles, with particular emphasis on fatty acid content. University of Otago, New Zealand.
- Woods CMC (2007) Aquaculture of the big-bellied seahorse Hippocampus abdominalis lesson 1827 (Teleostei: Syngnathidae). Biological Sciences, Victoria University of Wellington, Footscray, Australia.
- Job SD, Do HH, Meeuwig JJ, Hall HJ (2002) Culturing the oceanic seahorse, Hippocampus Aquaculture 214: 333-341.
- Reyes-Bustamante H, Ortega-Salas A (1999) Cultivo delcaballito de mar, Hippocampus ingens (Pisces: Syngnathidae) en condiciones artificiales. Rev Biol Trop 47: 1045-1049.
- Woods CM (2000) Improving initial survival in cultured seahorses, Hippocampus abdominalis Lesson, 1827 (Teleostei: Syngnathidae). Aquaculture 190: 377-388.
- Randall D, Burggren W, French K (1997) Fisiologia Animal: Mecanismos e adaptações. Guanabara Koogan, Rio de Janeiro, Brazil.
- Speakman JR (2005) Body size, energy metabolism and lifespan. J Exp Biol 208: 1717-1730.
- Bingsheng L (1992) Research on the culture of Hippocampus. J Ocean Univ Qingdao 22: 39-44.
- Correa MKSC, Manrique R (1989) Experimental culture of the seahorse, Hippocampus Bol Do Inst Oceanogr DaVenez 28: 191-196.
- Filleul MA (1996) Optimizing growth of juvenile big bellied seahorse Hippocampus abdominalis Lesson. University of Tasmania, Launceston, Australia.
- Ortega-Salas A, Reyes-Bustamante H (2006) Fecundity, survival, and growth of the seahorse Hippocampus ingens (Pisces: Syngnathidae) under semi-controlled conditions. Rev Biol Trop 54: 1099-1102.
- Shapawi R, Purser GJ (2003) The value of enriched artemia in supporting growth and survival of Juvenile pot?bellied seahorses hippocampus J World AquacSoc 34: 533-541.
- Wilson MJ, Vincent ACJ (2000) Preliminary success in closing the life cycle of exploited seahorse species, Hippocampus, in captivity. Aquarium SciConserv 2: 179-196.
- Araujo DFS, Silvestre DD, Damasceno KSFSC, Pedrosa FCL, Seabra LMAJ (2012) Proximate composition and cholesterol content of the Pacific white shrimp. Cienc Rural 42: 1130-1133.
- Balcázar JL, Pintado J, Planas M (2010) Bacillus galliciensis sp. nov., isolated from faeces of wild seahorses (Hippocampus guttulatus). Int J Syst Evol Microbiol 60: 892-895.
- Planas M, Chamorro A, Quintas P, Vilar A (2008) Establishment and maintenance of threatened long-snouted seahorse, Hippocampus guttulatus, broodstock in captivity. Aquaculture 283: 19-28.
- Blanco LT, Tacon AGJ (1989) La producción de alimento vivo y su importancia en Acuicultura. Documento de campo 12. In: Apoyo a lasactividadesregionales de acuicultura para a America Latina y el Caribe. Organizacion de lasNacionesUnidas para la Agricultura Y Alimentacion-FAO, Rome, Italy.
Citation: Silveira RB, Silva JRS (2020) Early Success Using Larval Stages of White Shrimp Litopenaeus vannamei as Seahorse Feed: Indicative for a Simultaneous Mariculture? J Aquac Fisheries 4: 025.
Copyright: © 2020 Rosana Beatriz Silveira, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

