
Effect of Conjugated Linoleic Acids, Free and Esterified Linoleic Acids, and Trans-Vaccenic Acid on Rat Pulmonary 15-Lipoxygenase-1 Activity
*Corresponding Author(s):
Saari Csallany ADepartment Of Food Science And Nutrition, University Of Minnesota, 1334 Eckles Avenue, St Paul, Minnesota, United States
Tel:+1 6126243683,
Fax:+1 6126255272
Email:ascsalla@umn.edu
Abstract
In the present study, the inhibition of rat pulmonary 15-Lipoxygenase-1 (15-LOX-1) enzyme activity using Linoleic Acid (LA) as substrate, was investigated by two of the major Conjugated Linoleic Acid (CLA) isomers, cis-9, trans-11 (c9,t11) and trans-10, cis-12 (t10,c12). Activity was measured by the production of 13(S)-Hydroxyoctadecadienoic acid [13(S)-HODE], an LA metabolite. Both CLA isomers c9,t11 and t10,c12 were found to inhibit 15-LOX-1 enzyme activity in a dose-dependent manner. 13(S)-HODE production from added 8?M CLA resulted in 33% and 57% reduction by c9,t11 and t10,c12-CLA, respectively. Interestingly from the addition of 8?M trans-Vaccenic Acid (t-VA) also resulted in 51% reduction of 13(S)-HODE production. It was found that 8?M of c9,t11 or 1?M of t10,c12 or 1?M of t-VA were equivalent to 0.1?M Nordihydroguaiaretic Acid (NDGA) (LOX inhibitor) inhibition of the enzyme 15-LOX-1. The effect of esterified CLA on 15-LOX-1 activity was also investigated. Results showed that c9,t11-Conjugated Linoleic Acid Methyl Ester (c9,t11-CLAME) did not have any effect on 15-LOX-1 activity measured by the 13(S)-HODE production from LA. However using Linoleic Acid Methyl Ester (LAME) as a substrate, c9,t11-CLA was found to increase 13(S) HODE production in a dose dependent manner. The increases of 13(S)-HODE production were 21,63,146,264 and 350%, respectively compared to the control. If LAME was the substrate t10,c12-CLA had no effect. Results show that if LA was a substrate the t10,c12-CLA and t-VA were found to be more effective as pulmonary 15-LOX-1 enzyme inhibitors than the c9,t11-CLA.
Keywords
ABBREVIATIONS
CLA: Conjugated Linoleic Acid
CLAME: Conjugated Linoleic Acid Methyl Ester
c9,t11-CLA: cis-9, trans-11-CLA
FFA: Free Fatty Acid
HPLC: High-Performance Liquid Chromatography
LA: Linoleic Acid
LAME: Linoleic Acid Methyl Ester
LOX: Lipoxygenase
NDGA: Nordihydroguaiaretic acid
TG: Triglyceride
t10,c12-CLA: trans-10, cis-12-CLA
13(S)-HODE: 13(S)-Hydroxy-cis-9, trans-11-Octadecadienoic acid
INTRODUCTION
Fetomaternal hemorrhage was first described by Wiener in 1948 [1]. This condition is considered physiological if a small amount of fetal blood enters the maternal circulation [1] and it occurs in 50 to 75% of all pregnancies. In this case, the volume of the transfusion ranges from 1 to 50 mL. The majority of blood losses are 1 mL or less, 1 in 400 cases are approximately 30 mL and 1 in 2000 the transfusion volume is about 100 mL [2].
Sometimes, besides the rarity of the condition, FMH involves a large amount of fetal blood which can lead to important fetal morbidity and mortality [1]. During pregnancy, clinical manifestations can be subtle and nonspecific which difficult the recognition of this condition. Antenatal suspicion of the diagnosis should occur when absent fetal movements is reported.
We describe this case to alert for this condition and the importance of maternal symptoms and newborn clinical findings. The prompt recognition and intervention is the key to the prognosis of this entity that can be fatal.
CLINICAL CASE REPORT
Conjugated Linoleic Acid (CLA) is a mixture of positional and geometrical isomers of Linoleic Acid, cis-9, cis-12-octadecadienoic acid (LA). Of the CLA isomers, cis-9, trans-11 (c9,t11) and trans-10, cis-12 (t10,c12) are the most biologically active isomers [1,2]. The principal dietary form of CLA is c9,t11, however, the concentrations of c9,t11 and t10,c12 isomers in dairy products or meats from ruminants vary depending on the diet fed to animals [3,4]. CLA is produced by microbial conversion in the rumen from the biohydrogenation of polyunsaturated fatty acid, but also from the ?9 desaturation of trans-Vaccenic Acid (t-VA), the predominant trans monounsaturated fatty acid of milk and animal tissue fat [5]. About 70% of c9,t11 present in milk fat is derived from the conversion of t-VA [6]. Trace of amounts of CLA may be also produced in vivo from free radical-mediated oxidation of LA [7], which consequently could form CLA isomers. However, it has been suggested that CLA in animal tissues may only be derived from dietary sources [8].
CLA isomers have been reported to have beneficial effects on cancer [9-12], atherosclerosis [13-15], immune function [16,17] inflammation [18-20] and body composition [21,22], and c9,t11 and t10,c12 isomers of CLA together might exhibit synergistic actions. Of the CLA isomers, t10,c12 was reported to be specially effective on body composition in mice. It should be noted that most of the studies were performed using the Free Fatty Acid (FFA) type of CLA, whereas most dietary CLA is in the Triglyceride (TG) form [23].
Dietary TG is degraded in vivo by lipase, and decomposed into 2-monoglyceride and two molecules of FFAs. Approximately 40 to 50% of TG is completely hydrolyzed to glycerol and FFAs prior to absorption, however, about 40 to 50% of fat is only partially hydrolyzed to monoglycerides, and about 10% of the ingested lipid remains as TG or degraded to diglyceride [23].
Lipoxygenases (LOXs) are a class of non-heme iron dioxygenases found in plants, animals and microorganisms that insert molecular oxygen into free and/or esterified polyunsaturated fatty acids with regional specificity, and are designated 5-,8-,12-, and 15-LOX accordingly [24]. Two different 15-LOXs have been identified that differ in substrate preference and in tissue distribution. The major substrate for 15-LOX-1 is LA in human and animal tissues and its primary oxidation product is 13(S)-Hydroxy-cis-9, trans-11-Octadecadienoic acid [13(S)-HODE] [25]. 15-LOX-1 is mainly expressed in reticulocytes, eosinophils, monocytes/macrophages, airway epithelial cells, atherosclerotic lesions, colorectal carcinomas, and prostate adenocarcinomas [26,27]. In numerous biological systems, 13(S)-HODE is involved in the regulation of cell proliferation and differentiation, and is suggested to be related to carcinogenesis [28]. Consequently the inhibition of 13(S)-HODE production from LA, by the inhibition of 15-LOX-1, may lead to lower cell proliferation and tumor growth in biological systems. The purpose of this study was to (a) investigate the inhibition of c9,t11 and t10,c12-CLA on 15-LOX-1 activity when LA is used as a substrate, (b) determine whether c9,t11 methyl ester inhibits 15-LOX-1 activity using LA as substrate, and (c) determine the effect of c9,t11 and t10,c12-CLA on 15-LOX-1 activity using linoleic acid methyl ester as a substrate. Inhibition of c9,t11 and t10,c12-CLA, and t-VA on 15-LOX-1 activity were compared to Nordihydroguaiaretic Acid (NDGA) LOX inhibitor.
MATERIALS AND METHODS
Materials
LA, LAME, c9,t11-CLA, t10,c12-CLA, and c9,t11-CLAME were stored as 200mM stock solutions in absolute ethanol at -20ºC. The compound 13(S)-HODE was stored as 100mM stock solutions in absolute ethanol at -20ºC. The LA, LAME and CLA isomers, CLAME, and 13(S)-HODE working solutions were prepared by diluting the stock solution with appropriate amounts of absolute ethanol before each experiment.
Lipoxygenase enzyme assay
Measurement of free radical type of oxidation of LA, LAME, c9,t11 and t10,c12-CLA, and c9,t11-CLAME
5-LOX-1 enzyme induced oxidation of substrates measured by the net production of 13(S)-HODE
Inhibitory effect of c9,t11 or t10,c12-CLA on 15-LOX-1 activity using LA or LAME as substrate
Inhibitory effect of c9,t11-CLAME on 15-LOX-1 activity using LA or LAME as substrate
Inhibition of 15-LOX-1 activity by NDGA using LAME or c9,t11-CLAME as substrate
Effect of LA and LAME on 15-LOX-1 activity using c9,t11-CLA as substrate
Inhibitory effect of trans-vaccenic acid on 15-LOX-1 activity using LA as substrate
STATISTICAL ANALYSIS
Results are presented as mean ± Standard Error of the Mean (SEM) of at least three replications of 13(S)-HODE production. Statistical analyses were conducted using SigmaStat statistical software package version 3.0 (Jandel Corp., San Rafael, CA). Statistical differences among treatments were tested by one-way Analysis of Variance (ANOVA) and the Student’s t-test (p<0.05) where appropriate.
RESULTS AND DISCUSSION
One of two known biologically active CLA isomers, c9,t11 has been associated with anticarcinogenic activity in various cancers, especially in mammary gland [9], skin [10], and colon [11,12]. The other isomer, t10,c12, recently has been shown to have a wide range of biological functions [15,24-31]. T-VA is a major trans monounsaturated fatty acid in dairy and animal tissue fat, and is converted to c9,t11 CLA by ?9-desaturase reaction [5].
Enzymatic oxidation of LA is catalyzed either by lipoxygenase or cyclooxygenase depending on the tissue type [32]. The major 15-LOX-1 product from LA oxidation, 13(S)-HODE is involved in regulation of cell proliferation and differentiation in numerous biological systems [21-28]. This suggests that it may be directly or indirectly related to carcinogenesis. The inhibition of 13(S)-HODE production from LA by 15-LOX-1 may lead consequently to lower cell proliferation and tumor growth. In early studies, some trans fatty acids have been shown to be competitive inhibitors for soybean LOX-induced LA oxidation [33,34]. Since c9,t11 and t10,c12 CLA, and t-VA contain trans configuration, the possible inhibition of 15-LOX-1 activity and the consequent production of 13(S)-HODE was investigated in the present study. Since the major polyunsaturated fatty acid in the human diet is LA [35], inhibition of 15-LOX-1 activity is a matter of interest.
Isolated rat lung 15-LOX-1 was selected for studying the possible inhibition of the enzyme activity by CLA isomers since high level of 15-LOX-1 activity was reported in lung epithelial cells [36,37]. To verify the enzymatic oxidation of LA by isolated rat pulmonary 15-LOX-1, it was incubated with various concentrations of NDGA, a well established LOX inhibitor. It was found previously in this laboratory that NDGA inhibited enzymatic oxidation and 13(S)-HODE production in a dose-dependent manner. The reduction of 13(S)-HODE production from LA in the presence of 0.1, 0.5 and 1µM NDGA was 32, 52 and 71%, respectively [30].
The free radical type of oxidation was measured before and after 45 min incubation without the addition of 15-LOX-1 enzyme to LA, LAME, c9,t11-CLA, t10,c12-CLA, and c9,t11-CLAME. Contents of 13(S)-HODE was measured by peak area from HPLC analyses for LA; 9, 050, 719, LAME; 24, 741, c9,t11-CLA; 139, 734, t10,c12-CLA; 122, 108 and c9,t11-CLAME; 55, 549. Incubation had no further effect on 13(S)-HODE concentrations.
Comparison of the relative activities of rat pulmonary 15-LOX-1 is demonstrated in figure 1, using substrates as LA, LAME, c9,t11-CLA, c9,t11-CLAME and t10,c12-CLA. The relative levels of enzymatic oxidation were measured by 13(S)-HODE production. It can be seen that esterified LA and c9,t11-CLA are very limitedly used as substrate by 15-LOX-1. The relative activity of c9,t11-CLA as substrate is only 25% of the activity of LA and t10,c12-CLA is inactive. Compared to that of LA the activity of LAME and c9,t11-CLAME as substrates are only 7% and 1%, respectively. Again the nonenzymatic oxidations of LA, LAME, c9,t11-CLA, t10,c12-CLA, and c9,t11-CLAME were subtracted from the 13(S)-HODE production.
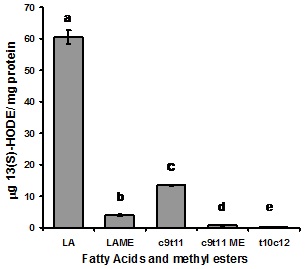 Figure 1: Comparison of 15-LOX-1 activity measured by 13(S)-HODE production when LA, LAME, c9,t11-CLA, c9,t11-CLAME, and t10,c12-CLA used as a substrate under identical condition. The levels of 13(S)-HODE from non-enzymatic oxidation were subtracted from data. Values are expressed as mean ± SEM (n=6, collected from three independent experiments). Means with different letters are significantly different at p<0.01.
Figure 1: Comparison of 15-LOX-1 activity measured by 13(S)-HODE production when LA, LAME, c9,t11-CLA, c9,t11-CLAME, and t10,c12-CLA used as a substrate under identical condition. The levels of 13(S)-HODE from non-enzymatic oxidation were subtracted from data. Values are expressed as mean ± SEM (n=6, collected from three independent experiments). Means with different letters are significantly different at p<0.01.
When rat pulmonary 15-LOX-1 enzyme homogenates were incubated with LA in the presence of c9,t11-, t10,c12-CLA and t-VA, both CLA isomers and t-VA inhibited the enzyme activity, and 13(S)-HODE production was suppressed in a dose-dependent manner (Figure 2). When 0.5, 1, 2, 4 and 8µM of c9,t11-CLA was incubated with LA; 8, 20, 22, 20 and 33% reduction of 13(S)-HODE production, respectively was observed. However, when t10,c12-CLA at 0.5, 1, 2, 4 and 8µM was incubated with LA; 26, 30, 43, 44 and 57% reduction of 13(S)-HODE production, respectively was observed. When t-VA at 0.5, 1, 2, 4 and 8µM was incubated with LA; 13, 31, 37, 50 and 51% reduction of 13(S)-HODE production, respectively was observed.
 Figure 2: Comparison of the dose-dependent inhibition of 15-LOX-1 enzyme activity using LA as a substrate, by c9,t11-CLA, t10,c12-CLA, or trans-Vaccenic Acid (t-VA) measured by the production of 13(S)-HODE. The levels of 13(S)-HODE from non-enzymatic oxidation were subtracted from data. Values are expressed as mean ± SEM (n=8, collected from four independent experiments). Means with different letters are significantly different at p
Figure 2: Comparison of the dose-dependent inhibition of 15-LOX-1 enzyme activity using LA as a substrate, by c9,t11-CLA, t10,c12-CLA, or trans-Vaccenic Acid (t-VA) measured by the production of 13(S)-HODE. The levels of 13(S)-HODE from non-enzymatic oxidation were subtracted from data. Values are expressed as mean ± SEM (n=8, collected from four independent experiments). Means with different letters are significantly different at p
These results demonstrate that t10,c12-CLA and t-VA inhibit 15-LOX-1 activity more than does c9,t11-CLA. It was found that 8µM of c9,t11, 1µM of t10,c12-CLA or 1µM of t-VA were equivalent to 0.1µM NDGA in inhibition of the enzyme measured by 13(S)-HODE. Furthermore, it was found that 8µM t10,c12 and 8µM t-VA demonstrated the same amount of enzyme inhibition as did 0.5µM NDGA. Thus t10,c12 and t-VA are 8 times more effective in 15-LOX-1 inhibition than is c9,t11- CLA.
In food, t10,c12 isomer is present in minor quantities; commercial butter contains about 1.1% t10,c12 compared to 76.5% c9,t11 [38] and beef contains about 2.6% t10,c12 compared to 72.0% c9,t11 of total CLA [39]. Besides c9,t11 CLA, all the other isomers are creating only about 10% of the total CLA (t7,c9>c11,t13>c8,t10>t10,c12) [40]. Partially hydrogenated oils such as shortenings and margarines are the main sources of t10,c12 isomer. It was also reported that a minor isomer t9,t11 may be more potent inhibitor of tumorigenesis than t10,c12 [41]. It is interesting to note that the average intake of total CLA isomers in the US population is reported to be <500mg/day [42]. However, ruminant products contain about two times or more t-VA than c9,t11 CLA. Based on clinical studies, humans can convert t-VA to c9,t11 CLA. It was reported that ~20% of t-VA can be converted endogenously to c9,t11 CLA, therefore, the effective physiological dose of c9,t11 CLA is 1.4 times the c9,t11 CLA actual intake [43].
It should also be noted that most studies on CLA were reported using the unesterified FFA forms of CLA, whereas most dietary CLA is in the esterified TG form [22]. Dietary TG is degraded by lipase and decomposed into 2-monoglyceride and two molecules of FFA, and it was reported that c9,t11-CLA was esterified mainly in the sn-1 and/or sn-3 position (74%), and only 26% esterified in the sn-2 position. This indicates that the major part of c9,t11 isomer was released as FFA from the TG in the intestine and was absorbed as FFA from food such as butter and cheese [37]. Rumenic acid (c9,t11) was shown by Chardigny et al., [44] to be better absorbed, oxidized and incorporated into rat carcass from the external position (sn-1) than the internal position (sn-2) from dietary synthetic dioleyl monorumeryl glycerol. Although some investigators have reported no significant differences between the physiological effects of free and esterified forms of CLA [45-48], our interest was to see the possible differences in two forms of CLA relating to 15-LOX-1 activity and, therefore, the production of 13(S)-HODE, which have been shown to relate to cell proliferation.
When various concentrations (0.5, 1, 2, 4 and 8µM) of c9,t11-CLAME were incubated with 15-LOX-1 using LA as substrate, 13(S)-HODE production was not affected. No significant differences were found in 13(S)-HODE production from LA with or without the incubation with c9,t11-CLAME.
The effect of CLA isomers on 15-LOX-1 activity was investigated if LAME is used as substrate (Figure 3). When LAME was incubated with 0.5, 1, 2, 4, 8µM c9,t11-CLA, the production of 13(S)-HODE was significantly increased (p<0.01). The increases of 13(S)-HODE production were 21, 63, 146, 264 and 350%, respectively compared to the control. However, when the same concentrations of t10,c12-CLA was incubated with 15-LOX-1 using LAME as substrate, no change in 13(S)-HODE production was observed. The results demonstrate that 15-LOX-1 can use LAME as a substrate but only in a very limited way. Small concentrations of c9,t11-CLA significantly increased the enzyme activity, however, t10,c12 did not increase the production of 13(S)-HODE from LAME.
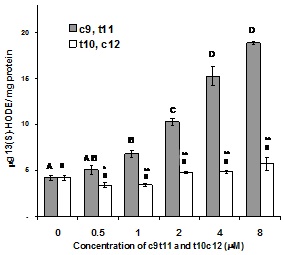 Figure 3: Dose dependent effect of c9,t11 and t10,c12-CLA on 15-LOX-1 activity measured by the production of 13(S)-HODE using LAME as substrate. The non-enzymatic oxidation of 13(S)-HODE for LAME, and the added CLA isomers were subtracted from data. Values are expressed as mean ± SEM (n=8, collected from four independent experiments). Means with different letters are significantly different at p
Figure 3: Dose dependent effect of c9,t11 and t10,c12-CLA on 15-LOX-1 activity measured by the production of 13(S)-HODE using LAME as substrate. The non-enzymatic oxidation of 13(S)-HODE for LAME, and the added CLA isomers were subtracted from data. Values are expressed as mean ± SEM (n=8, collected from four independent experiments). Means with different letters are significantly different at p
*indicates statistical differences between c9,t11 and t10,c12 addition to substrate at the same level (*p<0.05, **p<0.005)
Since 15-LOX-1 activity is only about 7% if LAME is used as substrate compared to LA, it is not surprising that the addition of c9,t11 increases enzyme activity, therefore, 13(S)-HODE production. Since t10,c12 was found previously not to be a substrate for 15-LOX-1 [23], addition of this CLA isomer did not change the limited enzyme activity of LAME.
To verify the enzymatic activity of the isolated pulmonary 15-LOX-1 when LAME was used as substrate, 0.1, 0.5 and 1µM NDGA, a LOX inhibitor, was used. Although the enzyme activity is relatively low when LAME is used as a substrate, NDGA significantly inhibited the enzyme activity dose-dependently by 70, 91 and 93%, respectively (p<0.001) (Figure 4). When the inhibition of NDGA was measured with the same concentrations using c9,t11-CLAME as substrate, the enzyme activity was decreased by 20, 48 and 61%, respectively (p<0.05). The enzyme activity measured by the production of 13(S)-HODE from c9,t11-CLAME was about 20% of LAME without the addition of enzyme inhibitor NDGA. The enzyme activity for c9,t11-CLA was as mentioned earlier to be 25% of LA if used as substrate (Figure 1). However, when c9,t11-CLAME used as substrate, only 1.2% was the enzyme activity compared to LA. Nevertheless, this small activity seems to be enzymatic since it was significantly suppressed by 0.5µM NDGA.
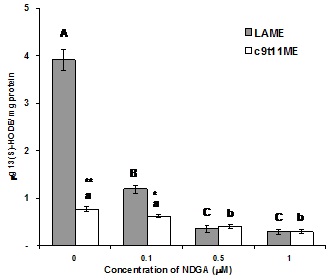
Figure 4: Dose dependent inhibition of 15-LOX-1 activity by NDGA measured by 13(S)-HODE production using LAME or c9,t11-CLAME as substrate. Values are expressed as mean ± SEM (n=6, duplicate determinations in three independent experiments). Means with different letters are significantly different. Capital letters indicate LAME at p
*indicates statistical differences between LAME and c9,t11-CLAME group at the same level (*p<0.01, **p<0.001)
Incremental additions of LA when c9,t11-CLA was used as a substrate very effectively increased the enzyme activity however the incremental additions of LAME was very different (Figure 5). Due to 0.5, 1, 2, 4 and 8µM of LA incubation with c9,t11 as substrate, 37, 60, 88, 109 and 137% increases in 13(S)-HODE productions were observed, respectively (p<0.05). However, the same amounts of LAME were incubated with the substrate, only 17, 34, 55, 79 and 105% increase of 13(S)-HODE productions were observed, respectively (p<0.05). This is not surprising since the primary substrate for the enzyme is LA, therefore, increased addiction to a lower activity substrate would substantially increase. In all cases of the present experiments, the 13(S)-HODE levels were the net result of oxidation by 15-LOX-1 enzyme.
 Figure 5: Dose dependent effect of added LA or LAME of 15-LOX-1 activity measured by the production of 13(S)-HODE in the presence of c9,t11-CLA used as substrate. Values are expressed as mean ± SEM (n=6, duplicate determinations in three independent experiments). Means with different letters are significantly different at p<0.05. Capital letters indicate LA and lower letters indicate LAME addition to c9,t11-CLA substrate.
Figure 5: Dose dependent effect of added LA or LAME of 15-LOX-1 activity measured by the production of 13(S)-HODE in the presence of c9,t11-CLA used as substrate. Values are expressed as mean ± SEM (n=6, duplicate determinations in three independent experiments). Means with different letters are significantly different at p<0.05. Capital letters indicate LA and lower letters indicate LAME addition to c9,t11-CLA substrate.
*indicates statistical differences between LA and LAME addition to substrate at the same level (*p<0.05, **p<0.01)
In conclusion, the present study demonstrates that both CLA isomers, c9,t11 and t10,c12, partial inhibitors for rat pulmonary 15-LOX-1 enzyme activities measured by the primary LA oxidation product 13(S)-HODE. We find that t10,c12-CLA and t-VA are more effective inhibitors of 15-LOX-1 than is the c9,t11-CLA isomer and that the methyl esters of LA and CLA have differing effects on 15-LOX-1 activity compared to the free acids.
In vitro studies of CLA have led to their consideration as therapeutic agents or as “Functional Foods” [49]. It is clear that dietary supplementation can increase CLA serum levels [50] but the therapeutic benefits thereof are less clear. A recent meta analysis of 8 studies with nine trials “did not support overall favorable effect of CLA supplementation BP regulation” [51]. Similarly Slojis [52] reported that a trial of CLA in overweight or obese individuals “did not support an antiatherosclerotic effect”. It is tempting to speculate that the inhibitory effects described in the present study could contribute these therapeutic failures by reducing the endogenous production of 13(S) HODE from LA.
REFERENCES
- Pariza MW, Park Y, Cook ME (2000) Mechanisms of action of conjugated linoleic acid: evidence and speculation. Proc Soc Exp Biol Med 223: 8-13.
- Wang T, Lee HG (2015) Advances in research on cis-9, trans-11 conjugated linoleic acid: a major functional conjugated linoleic acid isomer. Crit Rev Food Sci Nutr 55: 720-731.
- Chin SF, Liu W, Storkson JM, Ha YL, Pariza MW (1992) Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. Journal of Food Composition and Analysis 5: 185-197.
- Dhiman TR, Helmink ED, McMahon DJ, Fife RL, Pariza MW (1999) Conjugated linoleic acid content of milk and cheese from cows fed extruded oilseeds. J Dairy Sci 82: 412-419.
- Pollard MR, Gunstone FD, James AT, Morris LJ (1980) Desaturation of positional and geometric isomers of monoenoic fatty acids by microsomal preparations from rat liver. Lipids 15: 306-314.
- Griinari JM, Bauman DE (1999) Biosynthesis of conjugated linoleic acid and its incorporation into meat and milk in ruminants. In: Yurawecz MP, Mossoba MM, Kramer JKG, Pariza MW, Nelson GJ (eds.). Advances in conjugated linoleic acid research. (Volume 1), AOCS Press, Champaign, IL, USA. Pg no: 180-200.
- Dormandy TL, Wickens DG (1987) The experimental and clinical pathology of diene conjugation. Chem Phys Lipids 45: 353-364.
- Britton M, Fong C, Wickens D, Yudkin J (1992) Diet as a source of phospholipid esterified 9,11-octadecadienoic acid in humans. Clin Sci (Lond) 83: 97-101.
- Ip C, Dong Y, Ip MM, Banni S, Carta G, et al. (2002) Conjugated linoleic acid isomers and mammary cancer prevention. Nutr Cancer 43: 52-58.
- Belury MA, Nickel KP, Bird CE, Wu Y (1996) Dietary conjugated linoleic acid modulation of phorbol ester skin tumor promotion. Nutr Cancer 26: 149-157.
- Liew C, Schut HA, Chin SF, Pariza MW, Dashwood RH (1995) Protection of conjugated linoleic acid against 2-amino-3-methylimidazo[4,5-f]quinone-induced colon carcinogenesis in the F344 rat: a study of inhibitory mechanisms. Carcinogenesis 16: 3037-3043.
- Viita H, Pacholoska A, Ahmad F, Tietäväinen J, Naarala J (2012) 15- Lipoxygenase-1 induces lipid peroxidation and apoptosis, and improves survival in rat malignant glioma. In Vivo 26: 1-8.
- Kritchevsky D, Tepper SA, Wright S, Tso P, Czarnecki SK (2000) Influence of Conjugated Linoleic Acid (CLA) on establishment and progression of atherosclerosis in rabbits. J Am Coll Nutr 19: 472-477.
- Mitchell PL, Karakach TK, Currie DL, McLeod RS (2012) t-10, c-12 CLA dietary supplementation inhibits atherosclerotic lesion development despite adverse cardiovascular and hepatic metabolic marker profiles. PLoS One 7: 52634.
- Cathcart MK, Folcik VA (2000) Lipoxygenases and atherosclerosis: protection versus pathogenesis. Free Radic Biol Med 28: 1726-1734.
- Hayek MG, Han SN, Wu D, Watkins BA, Meydani M, et al. (1999) Dietary conjugated linoleic acid influences the immune response of young and old C57BL/6NCrlBR mice. J Nutr 129: 32-38.
- Cook ME, Butz D, Li G, Pariza MW, Whigham L, et al. (2003) Conjugated linoleic acid enhances immune responses but protects against the collateral damage of immune events. In: Christie WW, Sébédio JL, Adlof R (eds.). Advances in Conjugated Linoleic Acid Research. (Volume 2), AOCS Press, USA. Pg no: 283-291.
- Park NY, Valacchi G, Lim Y (2010) Effect of dietary conjugated linoleic acid supplementation on early inflammatory responses during cutaneous wound healing. Mediators Inflamm 2010: 342328.
- Penedo LA, Nunes JC, Gama MA, Leite PE, Quirico-Santos TF, et al. (2013) Intake of butter naturally enriched with cis-9, trans-11 conjugated linoleic acid reduces systemic inflammatory mediators in healthy young adults. J Nutr Biochem 24: 2144-2151.
- Jaudszus A, Foerster M, Kroegel C, Wolf I, Jahreis G (2005) Cis-9, trans-11-CLA exerts anti-inflammatory effects in human bronchial epithelial cells and eosinophils: comparison to trans-10, cis-12-CLA and to linoleic acid. Biochim Biophys Acta 1737: 111-118.
- Park Y, Storkson JM, Albright KJ, Liu W, Pariza MW (1999) Evidence that the trans-10, cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids 34: 235-241.
- Garcia HS, Keough KJ, Arcos JA, Hill CG Jr (2000) Interesterification (acidolysis) of butterfat with conjugated linoleic acid in a batch reactor. J Dairy Sci 83: 371-377.
- Mattson FH, Volpenhein RA (1962) Rearrangement of glyceride fatty acids during digestion and absorption. J Biol Chem 237: 53-55.
- Funk CD (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294: 1871-1875.
- Giovannucci E (1995) Epidemiologic characteristics of prostate cancer. Cancer 75: 1766-1777.
- Conrad DJ (1999) The arachidonate 12/15 lipoxygenases. A review of tissue expression and biologic function. Clin Rev Allergy Immunol 17: 71-89.
- Wuest SJ, Crucet M, Gemperle C, Loretz C, Hersberger M (2012) Expression and regulation of 12/15-lipoxygenases in human primary macrophages. Atherosclerosis 225: 121-127.
- Kuhn H, Walther M, Kuban RJ (2002) Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat 68-69: 263-290.
- Cho H, Gallaher DD, Csallany AS (2003) Nonradiometric HPLC measurement of 13(S)-hydroxyoctadecadienoic acid from rat tissues. Anal Biochem 318: 47-51.
- Cho H, Gallaher DD, Csallany AS (2005) Conjugated linoleic acid, cis-9, trans-11, is a substrate for pulmonary 15-lipoxygenase-1 in rat. J Agric Food Chem 53: 7262-7266.
- Pariza MW, Park Y, Cook ME (2001) The biologically active isomers of conjugated linoleic acid. Prog Lipid Res 40: 283-298.
- Funk CD, Powell WS (1983) Metabolism of linoleic acid by prostaglandin endoperoxide synthase from adult and fetal blood vessels. Biochim Biophys Acta 754: 57-71.
- BergstrÖm S, Holman RT (1948) Lipoxidase and the autoxidation of unsaturated fatty acids. In: Advances in Enzymology and Related Areas of Molecular Biology. (Volume 8), Wiley Online, Germany.
- Privett OS, Nickell C, Lundberg WO, Boyer PD (1955) Products of the lipoxidase-catalyzed oxidation of sodium linoleate. Journal of the American Oil Chemists’ Society 32: 505-511.
- Earles SM, Bronstein JC, Winner DL, Bull AW (1991) Metabolism of oxidized linoleic acid: characterization of 13-hydroxyoctadecadienoic acid dehydrogenase activity from rat colonic tissue. Biochim Biophys Acta 1081: 174-180.
- Hunter JA, Finkbeiner WE, Nadel JA, Goetzl EJ, Holtzman MJ (1985) Predominant generation of 15-lipoxygenase metabolites of arachidonic acid by epithelial cells from human trachea. Proc Natl Acad Sci USA 82: 4633-4637.
- Holtzman MJ (1991) Arachidonic acid metabolism. Implications of biological chemistry for lung function and disease. Am Rev Respir Dis 143: 188-203.
- Bauman DE, Barbano DM, Dwyer DA, Griinari JM (2000) Technical note: production of butter with enhanced conjugated linoleic acid for use in biomedical studies with animal models. J Dairy Sci 83: 2422-2425.
- Fritsche J, Fritsche S, Solomon MB, Mossoba MM, Yurawecz MP, et al. (2000) Quantitative determination of conjugated linoleic acid isomers in beef fat. European Journal of Lipid Science and Technology 102: 667-672.
- Lawson RE, Moss AR, Givens DI (2001) The role of dairy products in supplying conjugated linoleic acid to man’s diet: a review. Nutr Res Rev 14: 153-172.
- Kelley NS, Hubbard NE, Erickson KL (2007) Conjugated linoleic acid isomers and cancer. J Nutr 137: 2599-2607.
- Ritzenthaler KL, McGuire MK, Falen R, Shultz TD, Dasgupta N, et al. (2001) Estimation of conjugated linoleic acid intake by written dietary assessment methodologies underestimates actual intake evaluated by food duplicate methodology. J Nutr 131: 1548-1554.
- Turpeinen AM, Mutanen M, Aro A, Salminen I, Basu S, et al. (2002) Bioconversion of vaccenic acid to conjugated linoleic acid in humans. Am J Clin Nutr 76: 504-510.
- Chardigny JM, Masson E, Sergiel JP, Darbois M, Loreau O, et al. (2003) The position of rumenic acid on triacylglycerols alters its bioavailability in rats. J Nutr 133: 4212-4214.
- Terpstra AH, Javadi M, Beynen AC, Kocsis S, Lankhorst AE, et al. (2003) Dietary conjugated linoleic acids as free fatty acids and triacylglycerols similarly affect body composition and energy balance in mice. J Nutr 133: 3181-3186.
- Ip C, Scimeca JA, Thompson H (1995) Effect of timing and duration of dietary conjugated linoleic acid on mammary cancer prevention. Nutr Cancer 24: 241-247.
- Wang YM, Rahman SM, Nagao K, Arao K, Inoue N, et al. (2003) Comparison of the effects of triacylglycerol-CLA and free fatty acid-CLA on hepatic lipid metabolism in OLETF obese rats. Journal of Oleo Science 52: 121-128.
- Yamasaki M, Kitagawa T, Chujo H, Koyanagi N, Nishida E, et al. (2004) Physiological difference between free and triglyceride-type conjugated linoleic acid on the immune function of C57BL/6N mice. J Agric Food Chem 52: 3644-3648.
- Benjamin S, Spener F (2009) Conjugated linoleic acids as functional food: an insight into their health benefits. Nutr Metab (Lond) 6: 36.
- Salminen I, Mutanen M, Jauhianen M, Aro A (1998) Dietary trans fatty acids increase conjugated linoleic acid levels in human serum. J Nutr Biochem 9: 93-98.
- Yang J, Wang HP, Zhou LM, Zhou L, Chen T, et al. (2015) Effect of conjugated linoleic acid on blood pressure: a meta-analysis of randomized, double-blind placebo-controlled trials. Lipids Health Dis 14: 11.
- Sluijs I, Plantinga Y, de Roos B, Mennen LI, Bots ML (2010) Dietary supplementation with cis-9, trans-11 conjugated linoleic acid and aortic stiffness in overweight and obese adults. Am J Clin Nutr 91: 175-183.
Citation: Cho H, Shoeman DW, Csallany AS (2015) Effect of Conjugated Linoleic Acids, Free and Esterified Linoleic Acids, and Trans-Vaccenic Acid on Rat Pulmonary 15-Lipoxygenase-1 Activity. J Food Sci Nutr 1: 004.
Copyright: © 2015 Hyejung Cho, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

