
Effect of Environmental Conditions on the Conservation of the Fructooligosaccharide Content of Smallanthus Sonchifolius in the Andes
*Corresponding Author(s):
David Juan Ramos HuallpartupaUniversidad Nacional Jose Maria Arguedas, Facultad De Ingenierías, E.P. Ingeniería Agroindustrial, Jr. Abancay 789 - Andahuaylas, Apurímac, Peru
Tel:+51 83421992,
Email:davisrh22@gmail.com
Abstract
The storage conditions required by the fresh yacon were determined and the content of fructooligosaccharides was preserved in amounts similar to those harvested. The variety under study was "Ch’ecche Llajum". The storage of the fresh yacon was carried for 60 days, in conditioned chambers of: temperature, relative humidity and light intensity. To obtain responses of the preservation of the content of fructooligosaccharides was analyzed at the beginning and at the end of storage, the results were processed with a 2^3 factorial arrangement, which obtained a response surface that allowed to optimize the storage conditions being at a temperature of 15°C, relative humidity of 70% and light intensity of 200lm. to obtain a variation of the content of fructooligosaccharides of 25.47%, concluding that the management of the factors of temperature, relative humidity and light intensity, in low conditions, in a controlled and combined way, allows minimal variation of the FOS content.
Keywords
Conservation; Fructooligosaccharides; Storage; Variation and yacon
INTRODUCTION
Yacon (Smallanthus sonchifolius) is a perennial that originates in the Andean region of South America [1,2]. According to reports, its cultivation has been spreading in other countries, including the USA, Europe, New Zealand and Brazil [1]; It is a plant that stores in its roots and leaves special properties that are favorable for health. The antioxidants it generates make it very important for the treatment of diabetes and obesity problems [3]. In folk medicine, people suffering from diabetes or various digestive or renal disorders consume tuberous roots of yacon and infusions of dried leaves. Its tuberous roots are consumed fresh or cooked and it has been considered a functional food due to the large amounts of fructans (fructooligosaccharides and inulin) that it has [1].
Yacon tubers are fusiform and can vary considerably in size, shape and flavor, the color of their shell varies from dark brown to opaque purplish, even to orange. Internally the tuber is presented as a transparent fleshy body. Tubers generally weigh 200 to 500grams, but can weigh 2kilograms. In addition, there is no official standard in the market for classifying roots according to size [4]. In order to estimate the type of roots produced and the relative proportion in the harvest, in Cajamarca the roots have been classified into 3 categories: First roots. They are the largest, exceed 20cm long, are between 7 and 10cm of greater diameter and a weight not less than 300g. Roots of second. They are those that are between 12 to 20cm long and 5 to 6cm in diameter with a weight that varies from 120 to 300g. Third roots. They are non-commercial, their length is less than 12cm, their greater diameter is less than 5cm and their weight is less than 120g [5].
The properties of yacon have been attributed to its content of fructooligosaccharides so it is important to know how the chemical composition of the roots changes in its main stages of development by changing its characteristics [5]. After harvesting, a progressive decrease in fructooligosaccharides will occur while that of simple sugars (glucose, fructose and sucrose) will increase. These two processes, the synthesis and degradation of fructooligosaccharides, are under enzymatic control, biochemical changes and storage conditions. During storage, yacon roots are quite susceptible to dehydration when exposed directly to the sun. As a result of dehydration, the roots lose weight and acquire a rough appearance that makes them less attractive to the consumer [6].
With respect to carbohydrates, among the sugars found are: monosaccharides (fructose and glucose), sucrose and oligosaccharides (fructooligosaccharides), traces of starch and inulin [7]. The roots contain between 10 and 14% dry matter, which is composed of approximately 90% carbohydrates [8,9]. The sugar composition varies significantly due to factors such as agriculture, season, harvest, weather and postharvest temperature [5]. Unlike most tubers and roots that store carbohydrates in the form of starch, yacon roots contain essentially Fructooligosaccharides (FOS), sugars that cannot be digested directly by the human body due to the lack of necessary enzymes for the metabolism of these elements and are considered bioactive compounds in food [10]. There is confusion of terms when referring to the predominant type of carbohydrates in yacon roots. In several studies in the literature, it is claimed that yacon roots contain inulin main component. Although many scientific references citing this information, this is not accurate, since, strictly speaking, the yacon contains only fructooligosaccharides. The difference between FOS and Inulin is the number of fructose molecules. In inulin, this number varies between 2 and 60, while in FOS the number ranges from 2 to 10. This means that FOS can be considered as an inulin subgroup, so some authors prefer to use the term inulin-type fructooligosaccharides at more accurately refer to the nature of these sugars [5]. Although the proportion of each may vary, sugar may be considered on the basis of the following dry composition of 40 to 70% FOS, 5 to 15% sucrose, 5 to 15% fructose and less than 5% glucose [11].
Fructans are natural carbohydrates from reserves found in numerous plants, particularly in the composite family. Fructans are formed by fructose polymers derived from the sucrose molecule. Fructans have different chain structures and lengths and a wide variety of glycosidic bonds and fructosyl residues; They are soluble in water and are not reducing sugars. There is no single way to classify fructans, which has created some confusion [12]. Fructans are carbohydrate reserves that contain up to 70 fructose units attached or not to a terminal sucrose molecule, they can have a linear or branched structure linked by fructose bonds [13,14]. Fructans are fructose polymers, structurally and metabolically related to sucrose. They consist of homologous series of oligo and non-reducing polysaccharides, each containing a residue more than fructose than the previous member of the series, so that the simplest fructane is a trisaccharide [15]. Fructans are oligo or polysaccharides, consisting of a molecule of sucrose, to which fructose residues are linked by beta (2-1) and beta (2-6) glucosidic bonds, which can be linear or branched [16], the oligosaccharides present in the yacon have fructose residues linked by beta glucosidic bonds (2-1) with a terminal sucrose unit and carrying an inulin type [17]. The fluctuation of the content of fructans in the tuberous roots of yacon during its development and storage. The average degree of polymerization of these fructans increased linearly during development, after harvest decreased, while the free fructose, sucrose and glucose contents increased [18].
In behavioral studies of reserve carbohydrates in yacon tuberous roots, Vilhena [19], I observe an expressive decrease in the content of fructans of 101.3mg/g in the newly exposed root, at 84.31mg/g on the second day of sun exposure, stabilizing for this period. This is due to enzymatic degradation of these compounds, which is the same when the root is stored at low temperatures. Approximately 15% of flowering plants store fructans as a reserve carbohydrate. But the most studied and sold worldwide is the Inulin Type Fructan (ITF) [20].
Fructooligosaccharides (FOS), are fructan-type carbohydrates, fructose polymers, linked by β-type glycosidic bonds (2-1), are also known as oligofructose, oligosaccharides, have a lower degree of polymerization, approximately between 3 and 7 units [21]. These fructans are considered prebiotics, since they are not digestible by the human digestive tract, they have bifidogenic character (they stimulate the growth of bifidobacteria) and also, when consumed frequently, they favor the absorption of minerals such as calcium, they contribute to health and well-being of the colon through the strengthening of its epithelium and prevent colorectal pathologies such as cancer [22-24].
FOS are prospective prebiotics as they are fermented by beneficial species of intestinal bacteria [25-27]. They are also used as a source of natural sweeteners and syrups suitable for people suffering from digestive problems. [28]. Oral treatment with yacon syrup significantly accelerated colonic transit time in healthy individuals [29] and increased the frequency of defecation and the feeling of satiety in premenopausal women obese and with dyslipidemia [30].
During post-harvest storage and exposure of the jicama roots to the sun, bioenzymatic processes of transformation of fructooligosaccharides into simple or common sugars (fructose, glucose and sucrose) are generated, by the action of the enzyme fructanohydrolase, which determines a decrease in up to 39% [8,31].
Yacon roots are an important contribution to medicine since it acts as a natural rehydrator because of its high content of 3.73% minerals and 22% sugars and a low calorie content, they are composed of fructooligosaccharides, which are sugars the same as not they are metabolized by the human organism, being ideal for people suffering from diabetes problems since consuming these sugars does not raise the glycemic index [31].
MATERIALS AND METHODS
The research was developed in the laboratories of chemistry and Agro industrial processes, of the National University José María Arguedas, located in the District of Talavera de la Reyna, Province of Andahuaylas and Department of Apurímac, at 2815masl, Latitude 13° 39´13´ ´ South and Longitude 73° 25´40´´ West. Talavera has an average annual temperature of 14.7°C and rainfall of 881mm per year; The hottest month is October with 16.2°C and the coldest is July with 12.9°C. Fructooligosaccharide content analyzes were analyzed in the laboratories of the National University San Antonio Abad of Cusco and the National University of the Altiplano.
To determine the variation of the content of Fructooligosaccharides and the selection of temperature, relative humidity, and intensity of storage light that manifests a high content of fructooligosaccharides in the stored yacon root, the 2^3 factorial design with 03 repetitions was developed, with the combination of factors and variables (Table 1):
|
Temperature (Ti) (ºC) |
15 |
25 |
||||||
|
Relative Humidity |
50 |
70 |
50 |
70 |
||||
|
Light Intensity (ILk) |
200 |
300 |
200 |
300 |
200 |
300 |
200 |
300 |
|
Cameras |
C1 |
C2 |
C3 |
C4 |
C5 |
C6 |
C7 |
C8 |
|
Rep. 1 |
Y1111 |
Y1121 |
Y1211 |
Y1221 |
Y2111 |
Y2121 |
Y2211 |
Y2221 |
|
Rep. 2 |
Y1112 |
Y1122 |
Y1212 |
Y1222 |
Y2112 |
Y2122 |
Y2212 |
Y2222 |
|
Rep. 3 |
Y1113 |
Y1123 |
Y1213 |
Y1223 |
Y2113 |
Y2123 |
Y2213 |
Y2223 |
Table 1: Structure for the experimental design, elaborated in Andahuaylas, 2017.
Note: Legend: Tºi=Temperature; HRj=Relative Humidity; ILk=Intensity of Light; Yijkl=Dependent variable (Fructooligosaccharides); Rep.= Repetition.
Depending on the variables proposed, the harvested yacon followed step by step the following flow chart, which leads to the experimental design, which is detailed in figure 1.
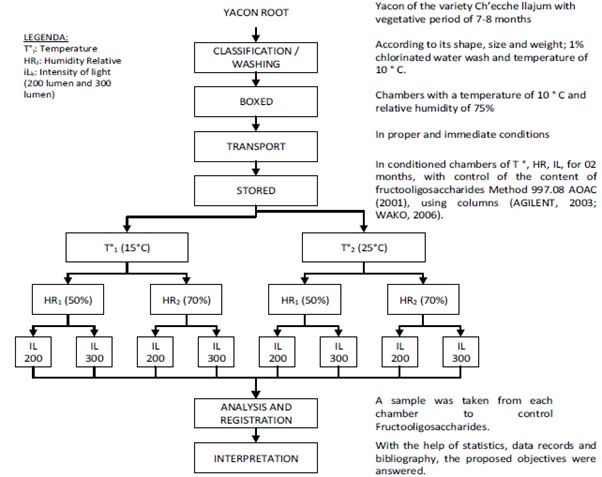 Figure 1: Flowchart to determine the storage conditions required by the fresh yacon in order to preserve the fructooligosaccharides in amounts similar to that harvested; Elaborated in Andahuaylas, 2017.
Figure 1: Flowchart to determine the storage conditions required by the fresh yacon in order to preserve the fructooligosaccharides in amounts similar to that harvested; Elaborated in Andahuaylas, 2017.
Determination of fructooligosaccharides: It was performed using Method 997.08 AOAC [32], using columns and modified methods of [33-35]; at the beginning and end (0 days and 60 days) of storage.
RESULTS AND DISCUSSION
Variation of Fructooligosaccharide (FOS) content in stored fresh yacon roots
From the results obtained, we observe that, after 60 days of storage of yacon roots, all samples suffer a decrease in the content of Fructooligosaccharides. Likewise, the yacon stored in chamber eight (C8: Tº=25; HR=70; IL=300) has the highest percentage of variation of the content of fructooligosaccharides (43.19%) and the yacon stored in chamber three (C3: Tº=15; HR=70; IL=200) presents less variation (25.48%), all with respect to the initial content of fructooligosaccharides of each stored yacon (Table 2).
|
Temperature (ºC) |
15 |
25 |
|||||||
|
Relative Humidity (%) |
50 |
70 |
50 |
70 |
|||||
|
Light intensity (Im) |
200 |
300 |
200 |
300 |
200 |
300 |
200 |
300 |
|
|
Almac./Día |
C1 |
C2 |
C3 |
C4 |
C5 |
C6 |
C7 |
C8 |
|
|
FOS (mg/10g) |
0 |
120.74 |
100.33 |
104.42 |
116.23 |
121.34 |
117.33 |
106.51 |
99,85 |
|
60 |
84.78 |
69.91 |
77.82 |
83.55 |
77.83 |
72.98 |
66.89 |
56,73 |
|
|
FOS variation (mg/10g) |
35.96 |
30.41 |
26.60 |
32.68 |
43.51 |
44.35 |
39.62 |
43.12 |
|
|
Percentage (%) |
29.78 |
30.31 |
25.48 |
28.11 |
35.86 |
37.79 |
37.20 |
43.19 |
|
Table 2: Results of the variation of the content of the fresh fructooligosaccharides of the yacon root (ch’ecche llajum) stored in the 08 chambers, obtained in Andahuaylas, April to June 2018.
The decrease of the content of fructooligosaccharides is an effect of the storage conditions, mainly to the temperature and relative humidity of the medium to which the yacon roots were subjected, which accelerate the synthesis or hydrolysis of the FOS in simple sugars, this effect It is generated by the presence of the enzyme Fructane Hydrolase (FH), which acts by successively releasing the fructose molecules that are in the terminal position within the chain of fructooligosaccharides, this enzymatic mechanism is “ignited” inside the plant with the purpose of using fructans as a source of energy for regrowth [6]; Shortly after the start of the harvest, a rapid process of change in the chemical composition of its sugars begins at the roots: the FOS are hydrolyzed to sugars by the simple action of Fructan Hydrolase (FH) that converts it into fructose, sucrose and glucose. Then, the invertase breaks the sucrose molecule resulting in glucose and free fructose [5,36,37]. After a week of storage at room temperature, 30 to 40% of the FOS will be converted into simple sugars. However, the speed of this conversion is slower if the yacon is stored at cooling temperatures [8,36,38].
We can affirm that when storing yacon roots at the temperature (15ºC) considered low, and high relative humidity (70%) and a light intensity of 200 lumen, the loss of FOS is lower, reaching 77, 82mg /10g at 60 days of storage of 104.42mg/10g which was at the beginning (0 days of storage), making a variation of 26.60 mg / 10g and representing a loss of 25.48% of FOS, which is similar to what indicate [8,36,38]. This indicates that by storing yacon at a low temperature, with high humidity and low light intensity, minor variations in the FOS content of the fresh yacon stored for 60 days are obtained.
Selection of temperature, relative humidity, and intensity of storage light that manifests a high content of fructooligosaccharides in the stored yacon root The analysis of variance (ANOVA) of the effect of the factors on the variation of the FOS, allowed to observe the factors under study that influence the variation of the content of the fructooligosaccharides, as well as the statistical significance of each effect by comparing its mean square against a estimated experimental error. In this case, seven of the effects have p-values lower than 0.05 (Temperature, light intensity, temperature-relative humidity interaction, light-intensity temperature interaction, moisture-light intensity interaction, Temperature interaction -moisture-intensity of light and blocks), indicating that they are significantly different from zero to 95.0% confidence level, these factors being the ones that significantly influence the variation of Fructooligosacaridos of fresh stored yacon, to a statistician R-Square of 99.9349%, only the relative humidity does not significantly influence the variation of the FOS (Table 3).
|
Source |
Sum of Squares |
Gl |
Middle Square |
Reason-F |
P-Value |
|
A: Temperature |
61,095,000 |
1 |
61,095,000 |
17572,33 |
0,0000 |
|
B: Relative Humidity |
0,01927 |
1 |
0,01927 |
0,55 |
0,4689 |
|
C: Light Intensity |
4,616,490 |
1 |
4,616,490 |
1327,81 |
0,0000 |
|
AB |
6,574,320 |
1 |
6,574,320 |
1890,93 |
0,0000 |
|
AC |
847,757 |
1 |
847,757 |
243,83 |
0,0000 |
|
BC |
1,418,960 |
1 |
1,418,960 |
408,13 |
0,0000 |
|
ABC |
141,135 |
1 |
141,135 |
40,59 |
0,0000 |
|
Blocks |
0,50455 |
2 |
0,25227 |
7,26 |
0,0069 |
|
Total error |
0,48675 |
14 |
0,03477 |
||
|
Total (corr.) |
74,794,700 |
23 |
Table 3: Analysis of variance and the most influential effects on the variation of the content of fructooligosacaridos of the fresh stored yacon (ch’ecche llajum), carried out in Andahuaylas, July 2018.
Note: R-square = 99.9 percent; R-square (adjusted by g.l.) = 99 percent
The regression analysis for the content data of the fructooligosaccharides allowed to obtain the regression equation that has been adjusted to the data. Being the equation of the adjusted model that allows to obtain a variation of the content of the fructooligosaccharides the following:
varFOS=51,7567−0,11635∗T−0,558658∗HR+0,00432167∗IL+0,00885167∗T∗HR−0,00344267∗T∗IL−0,000402167∗HR∗IL+0,000097∗T∗HR∗IL
Where the values of the variables are specified in their original units; which allowed to evaluate this function and make the response surface graph, which indicates that the blue zone shows the least variation of fructooligosaccharides at 60 days of storage, that is, under the conditions that intersect the factors that mark in this Fructooligosaccharides are conserved in greater amounts, resulting in up to 26% in vation of the FOS content (Figure 2).
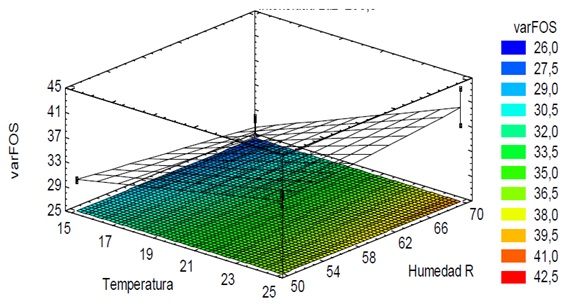 Figure 2: Response surface for the variation of Fructooligosaccharides. of the fresh stored yacon (ch’ecche llajum), made in Andahuaylas, July 2018.
Figure 2: Response surface for the variation of Fructooligosaccharides. of the fresh stored yacon (ch’ecche llajum), made in Andahuaylas, July 2018.
To better confirm what was shown by the response surface, we proceeded to optimize and observe what are the factors where the smallest variation of FOS can be obtained or also referred to as the conservation of FOS in greater quantity. Thus, at a temperature of 15°C, a relative humidity of 70% and a light intensity of 200 lumen allow an optimal variation of the fructooligosaccharide content of 25.48% (Table 4).
|
Factor |
Low |
Tall |
Optimum |
|
Temperature (ºC) |
15.0 |
25.0 |
15.0 |
|
Relative Humidity (%) |
50.0 |
70.0 |
70.0 |
|
Light Intensity (lm) |
200.0 |
300.0 |
200.0 |
Table 4: Optimization of the response for the smallest variation of the fresh stored Yacon (ch’ecche llajum) fructooligosaccharides, carried out in Andahuaylas, July 2018.
Note: Goal: minimize var FOS; Optimum value=25.48%
This optimal storage range is similar to what it indicates [31], "The post-harvest storage of jicama must be carried out at refrigeration temperatures and a relative humidity between 60 and 70%" for the use of Fructooligosaccharides (FOS), the rate of deterioration and moisture loss must be reduced and the root rot ”, likewise Jicama tubers are highly susceptible to cold damage and should be stored between 12.5ºC to 15ºC, and moderate relative humidity (70-80%). Under these conditions, the tubers can withstand 2 to 4 months [39], On the other hand, the content of oligofructans after one week in storage at room temperature may decrease by 30 to 40%; our case being much lower (25.48%) and in 60 days, this is because the storage system was controlled both in the temperature, relative humidity and light intensity established [5,40].
We can affirm that when storing yacon fresh at the temperature (15ºC) considered low, relative humidity considered high (70%) and a light intensity of 200lumen, the loss of FOS reaches a 25.48% variation compared at the beginning, that is to say that the fresh yacon can be preserved in Chamber three (C3), the concentrations of Fructooligosaccharides (FOS) being at the beginning (0 days of storage) of 104.42 mg/10g and final (60 days of storage) 77.82mg/10g, which implies a variation of 25.48%, at this time, thus ensuring durability of the product with respect to its content of fructooligosaccharides as obtained with the optimization results shown in the table 4, the one that allows us to accept the hypothesis that indicates that the temperature below 15°C, the relative humidity above 50% and the intensity of storage light allows to preserve a high content of fructooligosaccharides in the stored yacon root, assure ndonos conditions the storage in a controlled yacon can preserve FOS in amounts similar to the crop.
CONCLUSION
Under the eight storage conditions, there is a decrease in the content of Fructooligosaccharides; the handling of the factors temperature, relative humidity and light intensity, in low conditions, in a controlled and combined way allow variations of the FOS content of up to 25.47% in 60 days of storage; being these of 15ºC of temperature, 70% of relative humidity and 200 lumen of luminous intensity.
REFERENCES
- Valentová K, Lebeda A, Doležalová I, Jirovský D, Simonovska B, et al. (2006) The biological and chemical variability of yacon. J Agric Food Chem 54: 1347-1352.
- Zardini E (1991) Ethnobotanical notes on “Yacon,” polymnia sonchifolia (Asteraceae). Economic Botany 45: 72-85.
- Puerta MFP, García MA (2013) Caracterización morfológica y molecular de materiales de yacón (Smallanthus sonchifolius (Poep. & Endl.) H. Robinson) colectados en la eco región eje cafetero de Colombia. Revista De Investigación Agraria Y Ambiental4: 97-116.
- Council NR (1989) Lost crop of the Incas: Little Known Plants of the Andes with Promise for Worldwide Cultivation.
- Seminario J, y Valderrama M (2003) El Yacon: Fundamentos para el aprovechamiento de un recurso promisorio. Lima-Perú: Centro Internacional de la Papa (CIP), Universidad Nacional de Cajamarca, Agencia Suiza para el Desarrollo y la Cooperación (COSUDE).
- Fukai K, Ohno S, Goto K, Nanjo F, y Hara Y (1997) Seasonal fluctuations in fructan content and related enzyme activities in yacon (Polymnia sonchifolia). Soil Science and Plant Nutrition 43: 171-177.
- Grau A, y Rea J (1997) Yacon Smallanthus sonchifolius Andean Root and Tubers: Ahipa, Arracacha, Maca and Yacon. Rome, Italy.
- Manrique I, Párraga A, Hermann M (2005) Conservación y uso de la biodiversidad de raíces y tubérculos Andinos: Una década de investigación para el desarrollo (1993-2003).
- Manrique I, Párraga A, Hermann M (2005) Yacon - Fact Sheet. Lima, Peru: International Potato Center (CIP) Capturado em 22 dez 2005.
- Miguel QFE, Alfaroy QFC, Melgarejo SAV(2005) El yacón Una nueva alternativa en la prevención y el tratamiento de la salud.
- Manrique I, Parraga A, Hermann M (2005) Jarabe de yacón: Principios y procesamiento. Lima-Peru: Centro Internacional de la Papa, Universidad Nacional Daniel Alcides Carrion, Fundacion Erbacher, Agencia Suiza para el Desarrollo y la Cooperacion.
- Book G (2014) Compendium of chemical terminology. International Union of Pure and Applied Chemistry 528.
- Pedreschi R, Campos D, Noratto G, Chirinos R, Cisneros-Zevallos L (2003) Andean Yacon Root (Smallanthus sonchifolius Poepp. Endl) Fructooligosaccharides as a Potential Novel Source of Prebiotics. Journal of Agricultural and Food Chemistry 51: 5278-5284.
- Roberfroid I (1999) Concepts in functional foods: The case of inulin and oligofructose. J Nutr 129: 1398-1401.
- Ribeiro FRCL (1993) Distribuição, aspectos estruturais e funcionais dos frutanos, com ênfase em plantas herbáceas do cerrado. Revista Brasileira de Fisiologia Vegetal 5: 203-208.
- Capito S (2001) Raiz tuberosa de yacón (Polyminia sonchifolia): caracterização química e métodos de determinação de frutanos (CG e CLAE-DPA). Universidade Estadual de São Paulo.
- Goto K, Fukai K, Hikida J, Nanjo F, Hara Y (1995) Isolation and structural analysis of oligosaccharides from yacon (Polymnia sonchifolia). Bioscience, Biotechnology and Biochemistry 59: 2346-2347.
- Asami T, Minamisawa K, Tsuchiya T, Kano K, Hori I, et al. (1991) Fluctuation of oligofructan contents in tubers of yacon (Polymnia sonchifolia) during growth and storage. Japanese Journal of Soil Science and Plant Nutrition (Japan).
- Vilhena (1997) Ciclo de cultivo e técnicaspós-colheita de yacon (Polymnia sonchifolia Poep. Endl.) em função do conteúdo de frutose total nos órgãos subterrâneos. São Paulo, Botucatu: Tese (Doutorado em Agronomia) - Universidade Estadual de São Paulo, Botucatu.
- Quinteros ETT (2000) Produção com tratamento enzimático e avaliação do suco de yacon. Universidade Estadual de Campinas. Faculdade de Engenharia de Alimentos.
- Olvera C, Castillo E, López-Munguía A (2007) Fructosiltransferasas, fructanas y fructosa. Biotecnología 14: 327-345.
- Aguilera GC, Barberá MJM, Esperanza DL, Duarte de Prato A, Gálvez PJ, et al. (2008) Alimentos funcionales. In D. G. d. S. P. y. Alimentación (Ed.), Aproximación a una nueva alimentación. Instituto de Nutrición y Transtornos Alimentarios: Madrid.
- PoolZobel B, VanLoo J, Rowland I, Roberfroid MB (2007) Experimental evidences on the potential of prebiotic fructans to reduce the risk of colon cancer. Br Nutr 87: 273-281.
- Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, et al. (2007) Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr 85: 488-496.
- DuPont AW, DuPont HL (2011) The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol 8: 523-531.
- Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr 125: 1401-1412.
- Havenaar R (2011) Intestinal health functions of colonic microbial metabolites: A review. Benef Microbes 2: 103-114.
- Charalampopoulos D, Rastall RA (2012) Prebiotics in foods. Curr Opin Biotechnol 23: 187-191.
- Geyer H, Parr MK, Koehler K, Mareck U, Schänzer W, et al. (2008) Nutritional supplements cross-contaminated and faked with doping substances. J Mass Spectrom 43: 892-902.
- Genta S, Cabrera W, Habib N, Pons J, Carillo IM, et al. (2009) Yacon syrup: beneficial effects on obesity and insulin resistance in humans. Clinical nutrition 28: 182-187.
- Rodas DER (2010) Elaboración de una bebida alcohólica fermentada de jícama (Smallanthus sonchifolius) y manzana (Pyrus malus L.).
- AOAC International (2000) Official Methods of Analysis of AOAC International, Volume 2. AOAC International, USA.
- Agilent TU (2003 ) Typical Performance of ZORBAX Carbohydrate Analysis, Column N° 820629-008c.
- González ACL, Ramírez NJS (2018) Desarrollo y validación de un método para la cuantificación de fructooligosacáridos en un helado prebiótico/ Development and validation of a method for the quantification of fructooligosaccharides in a prebiotic ice cream. Journal of Pharmacy & Pharmacognosy Research 6: 108-116.
- Wako PU (2006) Food Analysis, A Fructooligosaccharide Analysis.
- Santana I, Cardoso MH (2008) Raiz tuberosa de yacon (Smallanthus sonchifolius): Potencialidade de cultivo, aspectos tecnológicos e nutricionais. Ciência Rural 38: 898-905.
- Wei B, Hara M, Yamauchi R, Ueno Y, Kato K (1991) Fructooligosaccharides in the tubers of jerusalem artichoke [Helianthus tuberosus] and yacon [Polymnia sonchifolia]. Research Bulletin of the Faculty of Agriculture-Gifu University (Japan).
- Graefe S, Hermann M, Manrique I, Golombek S, Buerkert A (2004) Effects of post-harvest treatments on the carbohydrate composition of yacon roots in the Peruvian Andes. Field Crops Research 86: 157-165.
- Álvarez G, Sánchez S, Uchuari Y (2012) Manual Técnico para el cultivo de Jícama (Smallanthus sonchifolius) en Loja.
- Chasquibol N, Aguirre R, Bravo M, Lengua R, Ch GT, et al. (2002) Estudio químico y nutricional de las variedades de la raíz de la Polymnia sonchifolia "yacon". Revista Peruana de Química e Ingeniería Química 5: 37-42.
Citation: Huallpartupa DJR, Loayza CL, Vizcarra TA, Huanca BR (2020) Effect of Environmental Conditions on the Conservation of the Fructooligosaccharide Content of Smallanthus Sonchifolius in the Andes. J Food Sci Nutr 6: 077.
Copyright: © 2020 David Juan Ramos Huallpartupa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

