
Effect of Traditional Processing and Storage on Retention of Xanthophyll Antioxidant Carotenoids in Malawi’s Orange Maize
*Corresponding Author(s):
Alex Arves KatolaUniversity Of Malawi, Zomba, Malawi
Email:alexkatola@gmail.com
Abstract
Xanthophyll carotenoids (lutein and zeaxanthin) from pigmented maize offer a good source of dietary antioxidants. However, during storage and processing, carotenoids can degrade, giving rise to smaller compounds without antioxidant properties. Therefore, this study was aimed at investigating the impact of traditional processing techniques on the retention of carotenoids with antioxidant properties in orange maize farmed in Malawi. Standard spectrophotometric methods determined the Total Carotenoid Content (TCC), while High-Pressure Liquid Chromatography (HPLC) identified individual carotenoids within the samples. Independent t-tests were used to compare carotenoid content and concentration between unprocessed maize flours and processed maize products. The analysis of carotenoid content in flour and food products (nsima and porridge) derived from orange maize revealed a substantial retention of carotenoids, even after 10 months of storage under uncontrolled conditions and after undergoing milling and cooking processing techniques. Lutein and zeaxanthin, carotenoids with antioxidant properties, were identified in these samples. Milled flour contained higher levels of lutein (2.9±0.1µg/g) and zeaxanthin (21.4±0.6µg/g) compared to the derived food products (2.6±0.2µg/g and 12.9±1.1µg/g respectively). This study demonstrates the notable retention of antioxidative carotenoids including those with antioxidant properties, in orange maize throughout storage and traditional processing. These findings demonstrate the potential of orange maize as a valuable source of carotenoids, highlighting the importance of preserving these compounds in staple foods. Further research is necessary to explore strategies for improving carotenoid preservation.
Keywords
Antioxidants; Malawi; Orange maize; Retention; Xanthophyll carotenoids
Introduction
Pigmented maize grains are rich dietary sources of bioactive compounds, such as carotenoids [1]. These carotenoids possess antioxidant activities with a wide variety of biological attributes, such as anti-ageing, anti-inflammatory, anti-viral, anti-microbial, and anti-cancer properties [2]. Antioxidant carotenoids scavenge free radicals including Reactive Oxygen Species (ROS) and help to decrease the incidence of oxidative stress-induced damage [3]. One source of free radical formation is aerobic biochemical processes that happen in the mitochondrion of a cell [4].
The inhibitory effect of carotenoids on superoxide is related to the number of conjugate double bonds in the structure of Reactive Oxygen Species (ROS) and free radicals [5]. The carotenoids with more conjugated double bonds provide more antioxidant protection as compared to those with less [6]. The proposed inhibitory mechanisms are through the transfer of excitation energy through electron transfer, donation of hydrogen atoms to radicals, or attachment to radicals total a low concentration to give a triplet-state carotenoid and triplet ground-state oxygen [5,6]. Additionally, carotenoids as antioxidants inhibit the expression of genes of certain types of cancers [5].
Humans cannot synthesize most of the carotenoids as such and it is very important to include them in the daily diet. Therefore, maintenance of carotenoid content in agronomic practices and reduction in post-harvest losses during storage and processing are essential [7]. The contents of carotenoids in carotenoid-containing foods including pigmented maize might be lost due to unsuitable storage conditions before consumption, and temperature during processing [7]. It has been shown that changes in the temperature during dry milling of orange maize can cause isomerization and degradation of carotenoids. Thermal degradation destroys as much as 10% of carotenoid content when the pericarp and germ are removed and an additional 15% when maize flour fractions are separated Ortiz et al. [8].
Research in Malawi has determined that producing orange maize has the potential as a sustainable food-based approach to improve the intake of carotenoids for optimal nutrition and overall health of the population [9-11]. Preliminary results have shown that orange maize produced in Malawi has high levels of naturally occurring carotenoids that include lutein, zeaxanthin, beta-carotene and beta-cryptoxanthin [9]. However, studies on the effect of storage and processing on the retention of carotenoids with antioxidant properties are limited. This study aimed to investigate the effects of uncontrolled storage conditions on orange maize and traditional processing techniques on the retention of carotenoids with antioxidant properties, particularly lutein and zeaxanthin.
Methodology
The methodology used in this study followed the research approach outlined by Katola et al. [11].
Preparation of Maize Flour Samples
All the maize used in this study was cultivated in Dedza district (central region of Malawi) by farmers of the Pro Farmer project during the 2017-2018 season and was harvested in April 2018. The orange maize was stored indoors in white polythene sacks under conditions that replicated those used by the local population. No attempt was made to control temperature, light or oxygen exposure. The maize was processed in February 2019 after 10 months of storage. Some of the grains of maize were dehulled and some were not dehulled before being dry-milled into flours of both the grand mill (dehulled) and whole grain. The whole grain orange maize flour was used to prepare porridge and the grand mill flour was used to prepare nsima. After milling, the flours were kept for 24 hours at room temperature before being used to produce the orange maize foods. These flours together with the prepared food products were also sampled for carotenoid analysis.
Preparation of the Food Products
Food products from maize were freshly prepared by local women at both the Dedza and Thyolo sites. The women were requested to prepare the orange maize products as they would at home. All the women were given: 3kg of the grand mill and 2kg of whole grain flour (orange maize) and bottled water (4.5 litres). Three different recipes were used to prepare the orange maize products. They included: (1) orange maize porridge with no added sugar (2) orange maize porridge with added sugar (3) orange maize nsima. In the sweetened recipe, sugar, approximately 6 table spoons per 4.5 litre, was used.
Porridge and nsima were prepared by cleaning a pot and filling it with bottled water. The water was heated on an open flame and orange flour or white flour was added to make porridge. Additional flour was added to the porridge to achieve the appropriate consistency. Nsima was prepared similarly to the porridge but using grand mill flours. When the porridge/nsima had cooked for an average of 30 min it was ready to be served.
Carotenoid Analyses
Immediately following food preparation, samples of the porridge and nsima were collected using zip lock bags and put in tightly closed dark containers and placed in a mobile cooler box. Samples were transported from the field to Chancellor College Chemistry Laboratory and stored at (-80°C). Before analysis, all food and flour samples were freeze-dried for 48 hours in containers covered with tin foil to avoid exposure to light. In a dark room, dried samples were then ground using a mortar and pestle.
Carotenoids were extracted according to the method of Ndolo and Beta [12], with some modifications. Briefly, 500mg samples (flour and foods) were prepared by freeze-drying, powdering and sifting (250-micron sieve). Samples were then mixed with 10ml of water-saturated butanol in tubes covered with black caps and aluminium foil in a fume hood. Each sample was then homogenized for 5 min using a 10mm diameter vortex. Tubes stood in the fume hood at room temperature for 60 min. Samples were then homogenized for a second time and let stand for another 60 min. About 10mL of extract was centrifuged (5,000g, 25°C, 5 min) (Labtech, Mumbai, India). All the procedures were done in the dark and each tube was wrapped in aluminium foil to avoid degradation of the carotenoids during the extraction.
Determination of Total Carotenoid Content (TCC)
Total carotenoid content was analysed using a spectrophotometric method. Supernatants of extracted samples were transferred from the centrifuge tubes into a semi-micro quartz cuvette and absorbance was measured at 450 nm using a PG instrument T90+UV/Visible spectrophotometer (Alma Park, Wibtoft Leicestershire, England). Total Carotenoid Content (TCC) was calculated using the following equation and expressed as µg lutein equivalent/g sample.
C=(10 x A)/S x W(µg/g)
Where C=lutein content, µg/g; A=absorbance reading, S=regression coefficient (the number that expressed the relationship which is created based on the concentration of lutein standard solutions in µg/mL and the absorbance); 10=dilution factor (the dilution factor of 10 is based on the total extracted volume of 10ml) and W=sample weight, g [12].
For calorimetric determination, a standard curve based on lutein concentrations was used in figure 1.
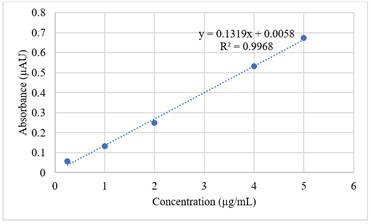 Figure 1: Lutein standard curve.
Figure 1: Lutein standard curve.
Determination of Carotenoid Composition
High-Performance Liquid Chromatography (HPLC) was used to separate and identify the individual carotenoids. Supernatants from the calorimetric determination of total carotenoids were used for HPLC analyses. Samples were put in brown vials and kept at -18°C for analysis on the following day. Stored supernatants were filtered through a 0.45µm nylon disc filter and underwent analysis. The determination of carotenoid composition was done according to the method described by Ndolo and Beta [12], with some modifications. Briefly, the chromatographic separation and quantification of carotenoids was carried out by HPLC (Agilent,1200 Infinity Series) equipped with a Diode Array detector, Mumbai, India) and autosampler (Waters 717 Plus, Waters, Milford, MA) using YMCTM carotenoid S-3, 3µm packing, 4.6×100mm column (Waters, Milford, MA). The column was operated at 35°C. Twenty µL of the sample was injected by the autosampler and eluted with a gradient system consisting of (A) methanol/methyl tert-butyl ether/Milli-Q water (81:15:4, v/v/v) and (B) methyl tert-butyl ether/methanol (90:10, v/v). The flow rate was set at 1mL/min. The gradient was programmed as follows: 0-9min, 100-75% A; 10-12 min 0% A; 12-13 min, 0-100% A; and 13-15 min, 100% A. The separated carotenoids were detected and measured at 450nm. The carotenoids under study were identified based on the similar retention time of commercial standards which included beta-cryptoxanthin and beta-carotene.
Carotenoid retention was calculated using the formula labelled Equation 1 below [13]. Carotenoid concentration retention after 10 months of storage to that found during the harvest period [9], was compared using the formula labelled Equation 2 below.
Equation 1
Total TCC/Concentration Retention = (Processed products TCC/concentrations) / (Unprocessed flours TCC/concentrations) ×100
Equation 2
Carotenoid retention after storage = (Carotenoid concentration at 10 months) / (Carotenoid concentration at harvest) ×100
A mixed plot of standard lutein and zeaxanthin against their peak areas was used in figure 2.
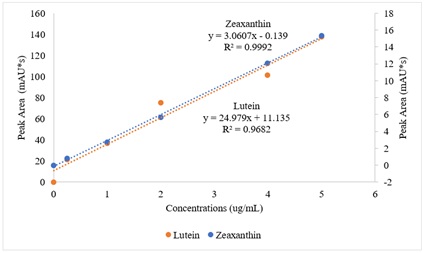 Figure 2: A mixed plot of standard lutein and zeaxanthin carotenoids against their peak areas.
Figure 2: A mixed plot of standard lutein and zeaxanthin carotenoids against their peak areas.
Statistical Analysis
Analysis was carried out using IBM SPSS statistics for Windows, version 25.0 (Armonk, New York, 2017). Descriptive statistics were expressed as Mean ± Standard Deviation. Independent t-test was used to compare means for significant differences in HPLC between the cooked samples and raw samples; and between the two districts.
Results
Findings in table 1 show that orange maize products had measurable amounts of xanthophyll carotenoids. The orange maize products from Dedza had overall significantly higher xanthophyll carotenoid concentrations than the orange maize products from Thyolo district. Overall, orange maize Nsima from Dedza District had significant amounts of lutein (3.4±0.2µg/g) and zeaxanthin (24.9±0.5µg/g).
|
Product |
Carotenoids |
District |
Concentrations (µg/g) |
|
Plain Porridge |
Lutein |
Dedza |
2.8±0.1a |
|
Thyolo |
2.7±0.2b |
||
|
Zeaxanthin |
Dedza |
22.9±1.7a |
|
|
Thyolo |
20.5±1.1b |
||
|
Sugar Porridge |
Lutein |
Dedza |
2.2±0.7a |
|
Thyolo |
2.1±0.9b |
||
|
Zeaxanthin |
Dedza |
17.7±4.2a |
|
|
Thyolo |
16.7±5.2b |
||
|
Nsima |
Lutein |
Dedza |
3.4±0.2a |
|
Thyolo |
3.1±0.6b |
||
|
Zeaxanthin |
Dedza |
24.9±0.5a |
|
|
Thyolo |
22.3±3.2b |
Table 1: Xanthophyll carotenoid concentrations in orange maize products by district.
Note: Each value is expressed as mean ± SD of triplicate samples. a, b, = Same column values with different superscript letters are significantly different at p ≤ 0.05. n = 3
Results in table 2, show that unprocessed orange maize and grand mill flour had the highest concentrations of carotenoids with antioxidant properties (lutein, 3.6±0.0µg/g and zeaxanthin 25.3±0.0µg/g). Furthermore, a reduction in carotenoid concentration was observed for both lutein and zeaxanthin in all processed products except for plain porridge’s zeaxanthin concentration which increased. However, despite this reduction, there was an average of 73.4% retention of lutein and an average of 83.4% retention of Zeaxanthin. Overall, the orange maize and orange maize products were found to have retained significant amounts of carotenoids with antioxidant properties despite long-term storage, milling and cooking.
|
Products |
Lutein |
Zeaxanthin |
|
Whole Flour |
2.9±0.1a |
21.4±0.6 |
|
Plain Porridge |
2.6±0.2b |
22.0±2.6 |
|
Whole Flour |
2.9±0.1a |
21.4±0.6a |
|
Sugar Porridge |
1.4±0.1b |
12.9±1.1b |
|
Grand Mill Flour |
3.6±0.0a |
25.3±0.0a |
|
Nsima |
2.9±0.4b |
21.9±2.8b |
Table 2: Xanthophyll carotenoid concentrations in flours and products.
Note: Each value is expressed as mean ± SD of triplicate samples. a, b, = Same column values with different superscript letters are significantly different at p ≤ 0.05. n = 3
Discussion
The study has demonstrated a substantial retention of carotenoids with antioxidant properties even after subjecting the orange maize to 10 months of uncontrolled storage conditions, followed by milling and cooking. A comparative analysis with the investigation conducted by Hwang et al. [9], on orange maize carotenoid content and concentration conducted close to the time of harvest followed by milling and cooking, revealed reduced content and concentrations in maize stored for an extended 10-month period [9,14]. This degradation may be influenced by various factors that were not measured in the current study. These potential factors include exposure to light, heat, and oxygen Schieber and Weber [15]. Notably, there was a location effect on concentrations of carotenoids with antioxidant properties in cooked food products made from orange maize. Products prepared in Dedza had higher concentrations than the orange maize products processed in Thyolo. This could be attributed to differences in cooking methods and thermal exposure period of the products [16].
Despite the susceptibility of antioxidant carotenoids in orange maize to degradation during storage and processing, the individual carotenoids present in orange maize showed an average retention of 73% for lutein and 83% for zeaxanthin. These findings were found to be higher than those reported by other studies [17,18]. This discrepancy is likely attributable to variations in the maize varieties and agricultural practices as suggested in other studies [19,20], where varied agronomic practices and plant genotypes influenced the content of phytochemicals. It could also be because of the diversity in accuracy and sensitivity of the analytical methods utilized in this study, a concern similarly raised in the previous study [11].
Depending on the type of product and how it was prepared, this study reported varied retention rates for concentrations of carotenoids with antioxidant properties. For instance, the sugar-added porridge demonstrated retention concentrations of 48% for lutein and 60% for zeaxanthin. Nsima mirrored this pattern, showing lutein and zeaxanthin retention of 60% and 86%, respectively. Plain porridge had a lutein retention concentration of 90%. Other studies have also reported significant retention of lutein and zeaxanthin [17,18]. In the current study, the most abundant and retained antioxidant carotenoid was zeaxanthin despite both lutein and zeaxanthin belonging to the xanthophylls group of carotenoids.
Lutein and zeaxanthin are the only dietary carotenoids that accumulate in the retina, specifically the macula, and are called macular pigments [21]. Maintaining macular health is critical to maintaining normal visual function. [21]. Lutein, zeaxanthin, and are absorbers of blue visible light and thus protect the eye structures from dangerous doses of this radiation [22]. The abundance of zeaxanthin reported in the current study is significant because the zeaxanthin is highly concentrated in the mid-peripheral and at the epic entre of the macula and it is more efficient in eliminating ROS that could affect the health of the macular than lutein leading to an improvement in vision for both normal and abnormal retinas [23].
Contrary to sugar-added porridge and Nsima in which lutein and Zeaxanthin concentrations degraded to considerable amounts, plain porridge's concentrations for zeaxanthin increased when compared to the whole flour. This could be because plain porridge took a short period to cook when compared to sugar-added porridge and Nsima which had high exposure to heat and led to degradation as suggested by a similar study done on the same maize Katola et al. [11]. The suggested mechanism observed on plain porridge is that short-period cooking results in higher extraction efficiency since the heat treatment inactivates oxidative enzymes and denatures the complex between carotenoid and protein that exists in plant cells Moreira et al. [24]. The current study's findings mirror what was reported by Drini? et al. [25], where after cooking there was an increase in the total carotenoid content, lutein and zeaxanthin concentrations that was observed in all hybrids of sweet corn of their study. Similarly, there was an observed increase of total carotenoids in all samples of varieties of Cucurbita moschata Duch fruits after being subjected to a short period of cooking Ma?ová et al. [26].
The current study findings are important since porridge and nsima are staples widely consumed across both rural and urban settings in Malawi [27]. The observed retention of carotenoids with antioxidant properties in sugar porridge and nsima and an increase in carotenoids observed in plain porridge presents a compelling opportunity for popularizing orange maize as a product that could play an important role in mitigating an increasing prevalence of chronic diseases in Malawi. These are diabetes, cancer and cardiovascular diseases which might be caused by oxidative stress [28]. These products would also serve as complementary food in school feeding programs across Malawi, aiming to protect students' eyes from harmful high-energy light waves such as ultraviolet rays in sunlight, which could lead to Macular Degeneration (AMD) at a later age. Di Carlo and Augustin [29].
In addressing the challenge of carotenoid degradation in orange maize, it is important to promote the adoption of improved storage and processing techniques. Ekpa et al. [18], affirm that the use of improved storage materials and practices plays an important role in retaining the total carotenoid content and concentrations. They reported that biofortified maize stored in laminated paper bags, characterized by the highest oxygen permeability, exhibited the lowest retention of total carotenoid content when compared to maize stored in double-layered polyethene bags.
Conclusion
In conclusion, the study highlights the substantial retention of carotenoids with antioxidant properties in orange maize, even after long-term uncontrolled storage conditions, milling, and cooking. Despite some degradation, the overall retention rates, particularly for lutein and zeaxanthin, remained significant. This study suggests that variations in agricultural practices, storage techniques, maize varieties, and cooking methods significantly influence carotenoid retention. The observed retention of carotenoids in staple foods like porridge and Nsima, widely consumed in both rural and urban settings in Malawi, presents an opportunity to promote orange maize as a product that could contribute to mitigating varied health problems including eye-related problems like macular degeneration in later years.
Authors Contribution
Alex Arves Katola, Alizah Hannah Stark and Mangani Chilala Katundu were involved in the study conceptualisation and writing of the original manuscript draft. Alizah Hannah Stark and Mangani Chilala Katundu were responsible for funding acquisition for the study. All the authors were involved in developing data collection tools, data collection, data analysis, reviewing and editing, and approving the final manuscript.
Acknowledgement
The authors would like to thank the Pears Foundation and the International School of Agricultural Sciences at The Hebrew University of Jerusalem for the scholarship that enabled the carrying out of this research. We also appreciate the Open Society Initiative for Southern Africa (OSISA) for providing support [grant number GA8139] through the University of Malawi, Pro-Farmer Project for the fieldwork. Special thanks go to the lab technicians (Mr Timothy Mguntha and Mr Idris Mtewa) from the University of Malawi who supported the sample analysis work.
Ethical Statements
Conflict of Interest: The authors declare that they do not have any conflict of interest.
Ethical Review: This study does not involve any human or animal testing.
Informed Consent: Written informed consent was not obtained since the study did not involve humans or animals.
Availability of Data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Consent to Participate
Corresponding and all the co-authors are willing to participate in this manuscript.
Consent for Publication
All authors are willing to publish this manuscript.
References
- Navarro A, Torres A, Fernández-Aulis F, Peña C (2018) Bioactive compounds in pigmented maize. Corn-Production and Human Health in Changing Climate 69-91.
- Samtiya M, Aluko RE, Dhewa T, Moreno-Rojas JM (2021) Potential health benefits of plant food-derived bioactive components: An overview. Foods10:
- Sen S, Chakraborty R (2011) The role of antioxidants in human health. In: Oxidative stress: Diagnostics, prevention, and therapy. American Chemical Society, Washington, D.C, USA.
- Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial Reactive Oxygen Species (ROS) and ROS-induced ROS release. Physiol Rev94: 909-950.
- Merhan O (2017) The biochemistry and antioxidant properties of carotenoids. Carotenoids5:
- Mordi RC, Ademosun OT, Ajanaku CO, Olanrewaju IO, Walton JC (2020) Free radical mediated oxidative degradation of carotenes and xanthophylls. Molecules25:
- Trono D (2019) Carotenoids in cereal food crops: Composition and retention throughout grain storage and food processing. Plants8:
- Ortiz D, Ponrajan A, Bonnet JP, Rocheford T, Ferruzzi MG (2018) Carotenoid stability during dry milling, storage, and extrusion processing of biofortified maize genotypes. J Agric Food Chem66: 4683-4691.
- Hwang T, Ndolo VU, Katundu M, Nyirenda B, Bezner-Kerr R, et al. (2016) Provitamin A potential of landrace orange maize variety (Zea mays L.) grown in different geographical locations of central Malawi. Food Chem196: 1315-1324.
- Murayama D, Yamazawa T, Munthali C, Ephantus NB, Rodney LG, et al. (2017) Superiority of Malawian orange local maize variety in nutrients, cook ability and storability. African Journal of Agricultural Research12: 1618-1628.
- Katola AA, Stark AH, Ndolo VU, Tembo DT, Katundu MC (2023) Provitamin A retention and sensory acceptability of landrace orange maize (MW5021) food products among school-aged children living in rural Malawi. Food Production, Processing and Nutrition5:
- Ndolo VU, Beta T (2013) Distribution of carotenoids in endosperm, germ, and aleurone fractions of cereal grain kernels. Food Chem139: 663-671.
- Taleon V, Mugode L, Cabrera-Soto L, Palacios-Rojas N (2017) Carotenoid retention in biofortified maize using different post-harvest storage and packaging methods. Food Chem232: 60-66.
- Beta T, Hwang T (2018) Influence of heat and moisture treatment on carotenoids, phenolic content, and antioxidant capacity of orange maize flour. Food Chem246: 58-64.
- Schieber A, Weber F (2016) Carotenoids: In: Handbook on Natural Pigments in Food and Beverages. Woodhead Publishing, Sawston, UK.
- Pillay K, Siwela M, Derera J, Veldman FJ (2014) Provitamin A carotenoids in biofortified maize and their retention during processing and preparation of South African maize foods. J Food Sci Technol51: 634-644.
- Sowa M, Yu J, Palacios-Rojas N, Goltz SR, Howe JA, et al. (2017) Retention of carotenoids in biofortified maize flour and β-cryptoxanthin-enhanced eggs after household cooking. ACS Omega2: 7320-7328.
- Ekpa O, Fogliano V, Linnemann A (2021) Carotenoid stability and aroma retention during the post?harvest storage of biofortified maize. J Sci Food Agric101: 4042-4049.
- Mesarovi? J, Srdi? J, Mladenovi?-Drini? S, Dragi?evi? V, Simi? MR, et al. (2018) Antioxidant status of the different sweet maize hybrids under herbicide and foliar fertilizer application. Genetika50: 1023-1033.
- Mesarovi? J, Srdi? J, Mladenovi?-Drini? S, Dragi?evi? V, Simi? M, et al. (2019) Evaluation of the nutritional profile of sweet maize after herbicide and foliar fertilizer application. Journal of Cereal Science87: 132-137.
- Mrowicka M, Mrowicki J, Kucharska E, Majsterek I (2022) Lutein and zeaxanthin and their roles in age-related macular degeneration-neurodegenerative disease. Nutrients14:
- Colijn JM, Buitendijk GH, Prokofyeva E, Alves D, Cachulo ML, et al. (2017) Prevalence of age-related macular degeneration in Europe: The past and the future. Ophthalmology124: 1753-1763.
- Lima VC, Rosen RB, Farah M (2016) Macular pigment in retinal health and disease. Int J Retina Vitreous2: 1-9.
- Moreira LDAS, Carvalho LMJD, Cardoso FDS, Neves S, Ortiz GMD, et al. (2019) Different cooking styles enhance antioxidant properties and carotenoids of biofortified pumpkin (Cucurbita moschata Duch) genotypes. Food Science and Technology40: 302-306.
- Drini? SM, Vukadinovi? J, Srdi? J, Šeremeši? MM, Andjelkovic V (2021) Effect of cooking on the content of carotenoids and tocopherols in sweet corn. Food and Feed Research48: 119-129.
- Ma?ová A, Heged?sová A, Andrejiová A, Heged?s O, Golian M, et al. (2021) Evaluation of storage and freezing, baking, and boiling treatments on total carotenoids content in the fruits of selected cucurbita moschata Duch varieties. Journal of Food Quality2021: 1-9.
- Ekpa O, Palacios-Rojas N, Kruseman G, Fogliano V, Linnemann AR (2019) Sub-Saharan African maize-based foods-processing practices, challenges and opportunities. Food Reviews International35: 609-639.
- Gowshall M, Taylor-Robinson SD (2018) The increasing prevalence of non-communicable diseases in low-middle income countries: The view from Malawi. Int J Gen Med 11: 255-264.
- Di Carlo E, Augustin AJ (2021) Prevention of the onset of age-related macular degeneration. J Clin Med10:
Citation: Katola AA, Stark AH, Ndolo VU, Tembo DT, Katundu MC (2024) Effect of Traditional Processing and Storage on Retention of Xanthophyll Antioxidant Carotenoids in Malawi’s Orange Maize. J Food Sci Nutr 10: 176.
Copyright: © 2024 Alex Arves Katola, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

