
Effects of Dietary Supplementation of Zingiber officinale Root-Powder on Growth, Nutrient Utilization and Intestinal Microbes of African Mud Catfish (Clarias gariepinus) Fingerlings
*Corresponding Author(s):
Sherifat Ibidunni AdegbesanDepartment Of Aquaculture And Fisheries Management, College Of Environmental Resources Management, Federal University Of Agriculture, Abeokuta, Nigeria
Tel:+234 8167846123,
Email:ibidunnisherifat@yahoo.co.uk
Abstract
Phytobiotics are valuable materials in promoting growth and reducing pathogenic diseases in cultured fish diets. To this effect, a 12 weeks experiment was conducted in a 40 litres fresh water filled rectangular plastic tanks to evaluating the growth, nutrient utilization potential and gasytointestinal microbes of Clarias gariepinus fed varying levels of Zingiber officinale root powder. 120 C. gariepinus fingerlings (2.33 ± 0.07 g average weight) were fed with 40 % crude protein diets containing three concentrations of Z. officinale roots-powder: ZOP1-1 %; ZOP2-2 %; ZOP3-3 %, and control-0 % ad libitum twice daily for 12 weeks. Significant differences (p < 0.05) occurred in the growth and nutrient utilization parameters except feed conversion ratio and specific growth rate.
Survival rate decreased as concentration of powder increased. The Total Bacterial Counts (TBC) and Total Fungal Counts (TFC) decreased as inclusion levels of the supplements increased which were different (p < 0.05) from the control. The study concluded that 1 % Z. officinale roots-powder dietary supplementation in cultured C. gariepinus could effectively promote growth and reducing the microbial load of the fish.
Keywords
INTRODUCTION
To develop alternative practices for growth promotion and disease management in aquaculture, attention has also been focused in identifying novel drugs, especially from plant sources. Rao et al. [5], reported that these drugs may be delivered to the cultivable organisms either through feed supplementation or oral delivery through diets mode.
(Z. officinale) belongs to the Zingiberaceae plant [6]. It is beneficial to growth and immune systems in aquatic animals [7]. The rhizome of Z. officinale has been reported to possess a broadspectrum of prophylactic and therapeutic activities [8]. It is important in fish diet in that it control infection [9]. The function the plant performed is linked to the active ingredients that are present in the plant. Themajor constituents in ginger rhizomes are carbohydrates (50-70 %), lipids (3-8 %), terpenes, and phenolic compounds [10]. Hence, this study aims at assessing the growth, nutrient utilization and anti-microbial potentials of cultured Clarias gariepinus [11] fed varying inclusion levels of Z. officinale root-powder.
Experimental system
Experimental fish
Experimental diets
• Treatment 1 (Control) - 0 %
• Treatment 2 (ZOP1) - 1 % Z. officinale root-powder
• Treatment 3 (ZOP2) - 2 % Z. officinale root-powder
• Treatment 4 (ZOP3) - 3 % Z. officinale root-powder
Diets formulation and preparation
Preparation and processing of Z. officinale root-powder
Incorporation of Z. officinale root-powder into the diets
|
Ingredients (%) |
Control |
ZOP1 |
ZOP2 |
ZOP3 |
|
Fishmeal |
31.2 |
31.2 |
31.2 |
31.2 |
|
Soybean meal |
15.6 |
15.6 |
15.6 |
15.6 |
|
Groundnut cake |
15.6 |
15.6 |
15.6 |
15.6 |
|
Yellow Maize |
30.5 |
30.5 |
28.75 |
27.75 |
|
Vitamin Premix |
1.0 |
1.0 |
1.0 |
1.0 |
|
Lysine |
0.5 |
0.5 |
0.5 |
0.5 |
|
Salt |
0.5 |
0.5 |
0.5 |
0.5 |
|
Vegetable oil |
4.0 |
4.0 |
4.0 |
4.0 |
|
Methionine |
0.5 |
0.5 |
0.5 |
0.5 |
|
DCP |
0.5 |
0.5 |
0.5 |
0.5 |
|
Z. officinale root-powder |
0.0 |
1.0 |
2.0 |
3.0 |
|
Total |
100 |
100 |
100 |
100 |
PROXIMATE ANALYSIS
|
Parameter |
Amount (%) |
|
Crude protein |
34.13 |
|
Crude fat |
4.02 |
|
Ether extract |
4.07 |
|
Ash content |
7.64 |
|
Moisture content |
13.75 |
|
Vitamin C |
1.036 |
Experimental procedure
Fish feeding
Water quality management
MONITORING OF FISH GROWTH
DETERMINATION OF GASTROINTESTINAL MICROBES OF THE FISH
Estimation of bacterial counts
Microbial identification
The fungal isolates were subjected to morphological characteristics according to Barnett and Hunter [18] and the isolated fungi were identified according to Campbell et al. [19].
DATA ANALYSIS
Analysis of fish growth performance

Analysis of feed conversion and nutrient utilization
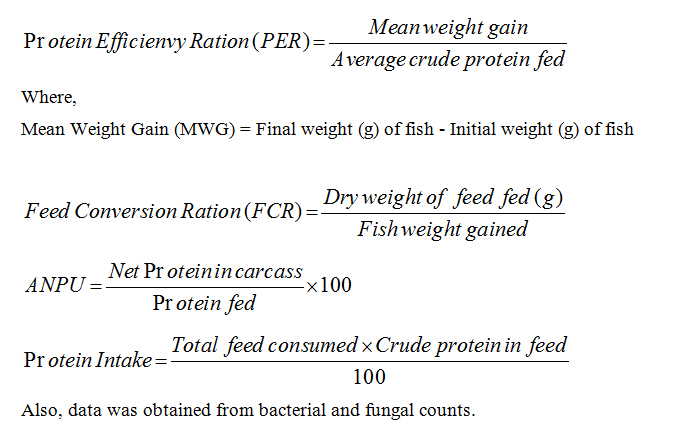
STATISTICAL ANALYSIS
RESULTS
Proximate composition of experimental diets
|
Proximate components (%) |
Control (0 %) |
ZOP1 (1 %) |
ZOP2 (2 %) |
ZOP3 (3 %) |
|
Moisture |
10.50 |
10.98 |
9.86 |
9.56 |
|
Crude protein |
40.01 |
40.00 |
40.04 |
39.98 |
|
Fibre content |
3.10 |
3.12 |
3.04 |
3.42 |
|
Ash |
5.20 |
4.45 |
3.95 |
3.74 |
|
Ether extract |
5.42 |
5.20 |
4.98 |
4.70 |
|
Nitrogen free extract |
35.77 |
36.25 |
38.13 |
38.60 |
Carcass compositions of experimental fish
|
Proximate components (%) |
Initial |
Control (0 %) |
ZOP1 (1 %) |
ZOP2 (2 %) |
ZOP3 (3 %) |
|
Moisture |
11.54 |
11.84 ± 0.23b |
11.92 ± 0.02a |
11.85 ± 0.03a |
11.97 ± 0.04a |
|
Crude protein |
43.50 |
47.37 ± 0.55c |
52.48 ± 0.65a |
50.83 ± 0.17b |
50.19 ± 0.38b |
|
Fibre content |
0.90 |
1.23 ± 0.02c |
1.34 ± 0.05b |
1.60 ± 0.12a |
1.39 ± 0.04b |
|
Ash |
0.98 |
4.18 ± 0.31a |
3.83 ± 0.13b |
3.49 ± 0.01b |
3.57 ± 0.04b |
|
Ether extract |
8.50 |
12.44 ± 0.08a |
9.85 ± 0.20b |
10.01 ± 0.28b |
9.58 ± 0.06b |
|
Nitrogen free extract |
34.58 |
22.95 ± 0.09a |
19.58 ± 0.38c |
21.22 ± 0.60b |
23.30 ± 0.36a |
Physicochemical parameters of the water
|
Week |
pH |
Dissolved Oxygen (Mg / L) |
Temperature (°C) |
|
0 |
6.00 |
6.10 |
25.00 |
|
1 |
6.90 |
6.20 |
25.41 |
|
2 |
7.15 |
6.18 |
25.60 |
|
3 |
7.03 |
6.24 |
25.78 |
|
4 |
7.34 |
6.35 |
25.83 |
|
5 |
7.41 |
6.50 |
25.61 |
|
6 |
7.48 |
7.21 |
26.13 |
|
7 |
7.31 |
7.25 |
25.53 |
|
8 |
7.22 |
7.40 |
26.20 |
|
9 |
7.28 |
7.56 |
26.13 |
|
10 |
7.50 |
7.45 |
26.30 |
|
11 |
7.55 |
7.61 |
26.05 |
|
12 |
7.56 |
7.70 |
26.02 |
GROWTH PERFORMANCE
|
Parameters |
Control (0 %) |
ZOP1 (1 %) |
ZOP2 (2 %) |
ZOP3 (3 %) |
|
Initial weight (g) |
2.30 ± 0.06a |
2.33 ± 0.09a |
2.33 ± 0.03a |
2.33 ± 0.09a |
|
Final weight (g) |
14.22 ± 1.12c |
20.27 ± 0.92a |
18.49 ± 1.09b |
20.14 ± 1.15a |
|
Weight gain (g) |
11.92 ± 1.16c |
17.95 ± 0.90a |
16.15 ± 1.06b |
17.82 ± 1.07a |
|
Percentage weight gain (%) |
518.3 ± 10.21c |
840.8 ± 7.70a |
691.4 ± 10.63cb |
762.3 ± 17.69b |
|
Feed intake (g / fish) |
19.17 ± 2.29c |
27.11 ± 1.49a |
25.2 ± 1.65b |
25.64 ± 1.96b |
|
Feed conversion ratio |
1.40 ± 0.07b |
1.57 ± 0.04a |
1.56 ± 0.02a |
1.55 ± 0.02a |
|
Specific growth rate (% / day) |
2.31 ± 0.19b |
2.57 ± 0.10a |
2.46 ± 0.06a |
2.56 ± 0.02a |
|
Protein efficiency ratio |
1.80 ± 0.09a |
1.65 ± 0.04b |
1.60 ± 0.02b |
1.61 ± 0.03b |
|
Apparent net protein utilization (%) |
50.29 ± 1.38c |
83.04 ± 3.92a |
68.270 ± 5.12b |
65.60 ± 5.05b |
|
Survival rate (%) |
86.67 ± 8.82a |
80.00 ± 5.77b |
63.33 ± 3.33c |
63.33 ± 3.33c |
INTESTINAL MICROBES OF THE FISH
Microbial count of fish
|
Parameters |
Control 0 % |
ZOP1 1 % |
ZOP2 2 % |
ZOP3 3 % |
|
Total Bacteria Count (CFU / ml) × 105 |
23.67 ± 0.88a |
17.33 ± 4.76b |
7.4 ± 5.31c |
8.84 ± 7.58c |
|
Total Fungal Count (CFU / ml) × 105 |
7.67 ± 0.44a |
2.83 ± 1.36d |
2.00 ± 0.00e |
2.50 ± 0.00e |
Morphological characteristics of bacteria colonies isolates
|
Isolates Details |
A1 |
B1 |
C1 |
D1 |
|
Colour |
Creamy |
Dull Cream |
Dull Cream |
White |
|
Shape / Margin |
Irregular |
Entire |
Entire |
Entire |
|
Arrangement |
Cocci in long chain |
Baccilli |
Bacilli |
Cocci in pair |
|
Surface Appearance |
Smooth and Glistening |
Smooth |
Smooth |
Smooth and Glistening |
|
Elevation |
Raised |
Raised |
Raised |
Raised |
|
Texture |
Dry |
Moist |
Moist |
Dry |
|
Opacity |
Translucent |
Translucent |
Translucent |
Translucent |
A = Treatment one (Control)
B = Treatment five (1 % of Z. officinale root-powder)
C= Treatment six (2 % of Z. officinale root-powder)
D = Treatment seven (3 % of Z. officinale root-powder)
|
Isolates Details |
A2 |
B2 |
C2 |
D2 |
|
Colour |
Creamy |
Creamy |
Creamy |
White |
|
Shape / Margin |
Irregular |
Entire |
Entire |
Entire |
|
Arrangement |
Cocci in chain |
Baccilli |
Baccilli |
Cocci in pair |
|
Surface Appearance |
Smooth and Glisteningv |
Smooth and Glistening |
Smooth and Glistening |
Smooth and Glistening |
|
Elevation |
Raised |
Raised |
Raised |
Raised |
|
Texture |
Mucoid |
Moist |
Moist |
Dry |
|
Opacity |
Translucent |
Translucent |
Translucent |
Translucent |
Biochemical characterization of the bacteria isolate
|
Isolate |
Gram |
Spores |
Motility |
Catalase |
Starch hydrolysis |
Gelatin liquefaction |
Oxidase |
Mannitol |
Sucrose |
Lactose |
Glucose |
Suspected organism |
|
A1 |
+ |
- |
- |
- |
- |
- |
- |
- |
+ |
- |
+ |
Streptococcus agalactiae |
|
A2 |
+ |
- |
- |
- |
- |
- |
- |
- |
+ |
- |
+ |
S. agalactiae |
|
B1 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
+ |
Baccillus subtilis |
|
B2 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
+ |
B. subtilis |
|
C1 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
+ |
B. subtilis |
|
C2 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
- |
- |
+ |
B. subtilis |
|
D1 |
+ |
- |
- |
- |
+ |
- |
- |
+ |
- |
+ |
+ |
E. faecialis |
|
D2 |
+ |
- |
- |
- |
+ |
- |
- |
+ |
- |
+ |
+ |
E. faecialis |
Colonial characteristics of fungal isolate
|
Treatment |
Colour of spore |
Appearance of mycelia |
Type of spores |
Arrangement of spores |
Shape of spore |
Type of hyphae |
Identified organism |
|
A11 |
Black |
Grainy |
Conidiosphore |
In masses |
Cylindrical |
Septate |
Aspergillus niger |
|
A12 |
Yellow black |
Fluffy |
Sporangiospore |
In masses |
Globuse |
Aseptate |
Mucor mucedo |
|
B1 |
Black |
Grainy |
Conidiosphore |
In masses |
Cylindrical |
Septate |
A. niger |
|
C1 |
Yellow black |
Fluffy |
Sporangiospore |
In masses |
Globuse |
aseptate |
Mucor mucedo |
|
D1 |
Black |
Grainy |
Conidiosphore |
In masses |
Cylindrical |
septate |
A. niger |
|
Treatment |
Colour of spore |
Appearance of mycelia |
Type of Spores |
Arrangement of spores |
Shape of spore |
Type of hyphae |
Identified organism |
|
A21 |
Black |
Grainy |
Conidiosphore |
In masses |
Cylindrical |
Septate |
A. niger |
|
A22 |
Black |
Grainy |
Conidiosphore |
In masses |
Cylindrical |
Septate |
A. niger |
|
B2 |
Black |
Grainy |
Conidiosphore |
In masses |
Cylindrical |
Septate |
A. niger |
|
C2 |
Black |
Grainy |
Conidiosphore |
In masses |
Cylindrical |
Septate |
A. niger |
|
D2 |
Black |
Grainy |
Conidiosphore |
In masses |
Cylindrical |
Septate |
A. niger |
DISCUSSION
There was a general increase in weight gain with the highest growth performance observed in fish fed 1 % and 3 % Z. officinale root-powder. Fish fed 1 % Z. officinale root-powder diet recorded the highest and showing best growth responses in terms of Percentage Weight Gained (PWG), Specific Growth Rate (SGR) and Apparent Net Protein Utilization (ANPU). The fish fed the control diet recorded the lowest values of PWG and ANPU. This is in agreement with the work of Bello et al. [24], who recorded similar increase in weight gain of fish when fed diets supplemented with Walnut leaf and Onion bulb residues. There was a rapid growth rate of C. gariepinus in the first few weeks of culture in the study; this could probably due to initial starvation of the fish which made them more metabolically active. This is similar to report of Obasa and Faturoti [25] who recorded similar observation in the growth of juvenile Heterotis niloticus. They recorded an increase in growth of the fish as they were subjected to delay in feed distribution.
The remarkable performances of fish fed the supplemented diets in PWG, SGR and ANPU over control diet could be due to the presence of growth promoters: zingiberene, glycosides and terpenoids in 1 % Z. officinale root-powder. This is in agreement with the result of Onibi et al. [26], suggested that ginger and garlic supplements collectively or individually improved growth performance of broilers. This was corroborated by Haghighi and Rohani [13].
There was a reduction in the total feed intake at higher levels of (2 % and 3 %) of Z. officinale root-powder; this could probably due to lower palatability of the two diets as a result of the presence of tannin in the Z. officinale root-powder. The work of Fasasi et al. [12], stated that tannins interfere with digestion by displaying anti-trypsin and anti- amylase activity, forming complexes with vitamin B12 and interfering with the bioavailability of proteins. Also, authors such as Azaza et al. [27], are of opinion that the presence of 2.4 % tannin in faba beans (Vicia faba L. var. minuta) might be responsible for low palatability and consequently low feed intake in Nile tilapia. The increased FCR as revealed in fish fed Z. officinaleroot-powder and higher than the fish fed control diet is similar to the report of Bello et al. [24], who revealed that inclusion of 1.5 % walnut leaf increased FCR in the supplemented groups than the control. This was also corroborated by the work of Abd El - Rahman [28] which indicated that the inclusion of Propolis - ethanolic extract and crude propolis increased the FCR, FER and PER in the supplemented groups when compared with the control. Also the findings of Zomrawi et al. [29], who showed that were no significant differences (p > 0.05) in FCR among all dietary Z. officinale root-powder treatments which corroborated with the result obtained in this present study.
The better SGR recorded in the supplemented diets is in correlation with the result of Abou-Zeid [30] which showed that Allium sativum supplementation positively affected O. niloticus biomass and Specific Growth Rate (SGR).
There is a reduction in survival rate in fish fed the Z. officinale root-powder diets as seen in this experiment could be as a result of some phytochemicals present in the phytobiotic. Fish fed 2 % and 3 % Z. officinale root-powder recorded the highest mortality rate. The findings of Ashade et al. [31], also revealed that the mortality rate of fish fed untreated ginger peel increased with respect to the different concentrations and the highest concentration having more mortality rate.
The bacteria flora of the intestine of C. gariepinus consisted of Bacillus substilis, Streptococcus agalactiaeand Enterococcus faecialis. This could indicate that Z. officinale root-powder favours or promote the growth of gram positive bacteria in the intestine of C. gariepinus. This is in the agreement with the work of Olojo et al. [32] who observed similar bacteria flora in the intestine of C. gariepinus. This is corroborated by Nwabueze [11] who observed similar bacteria flora from the epidermal mucus of C. gariepinus fed ginger powder. Pandy et al. [33], also reported that all medicinal plants are able to stimulate only non-specific immune responses and suggested that the medicinal plants could be used to treat diseases. This gastrointestinal microbes could also be advantageous in that they help in digestive processes of fish such as microbial breakdown of chitin, collagen, cellulose and they may also supply fatty acids and other vitamins to the host and hence promoting growth of the fish [34]. These might have taken place in the fish fed the dietary supplement. The gastrointestinal microbes also prevent colonization of the fish by other microbes that might otherwise be pathogenic. B. substilis has been reported to promote performance and immune responses which might have taken place in fish fed ZOP1 and ZOP3. Tannins and alkaloid are present in ginger roots powder. Tannins and saponins are responsible for antibacterial activity, and able to permeate cells without destroying cell morphology. Tannins inhibit microbial proliferation by denaturation of enzymes of involved in microbial metabolism [35]. This might have occurred in the intestine of fish fed the dietary supplement because majorly the bacteria flora in the intestine of C. gariepinus are mostly gram positive bacteria and thus these phytochemicals probably inhibited the growth of gram negative bacteria and promoted the growth of gram positive bacteria.
There were differences (p < 0.05) in the reductions in the total bacteria count of fish fed varying levels of Z. officinale root-powder than the control. This is in agreement with the report of Bello et al. [24], who observed that there was a decrease in values of the bacterial load of the supplemented groups (onion bulb and walnut leaves) as the level of inclusion (0.5 %, 1.0 % and 2.0 %) increased and as the months increased. The enterobacteriaceae load in the intestine of C. gariepinus fed Z. officinale root-powder were lower than the control with significant decrease (p < 0.05) as recorded in this study. This decrease in bacteria load in fish as observed in this study has been linked to the presence of antimicrobial properties in Z. officinale root-powder. This study suggests that Z. officinale root-powder is more effective as an antibacterial. Tannins also have shown potential antiviral, antibacterial properties [36].
The fungi flora of the intestine of C. gariepinus fed diets containing varying levels of Z. officinale root-powder revealed the presence of Aspergillus niger and Mucor mucedo. A. niger and M. mucedo are associated to food spoilage. Their presence in the fish intestine could indicate that some of the experimental feeds might have been rancid and support the growth of these microbes.
Also, there were differences (p < 0.05) in the reductions in the total fungal count of fish fed diets supplemented with varying levels of Z. officinale root-powder than the control diet. Also, this study indicated that Z. officinale root-powder could act effectively as an antifungal. This is in line with the work of Idris et al. [37], which worked on the effect of different concentration of ginger on smoke-dried C. gariepinus, and found that ginger reduced the free fatty acid values, tri-methylamine values and reduced the fungi load of the processed fish.
CONCLUSION
AUTHORS’ CONTRIBUTIONS
REFERENCES
- Sayed SH, Zakaria A, Mohamed GA, Mohamed KK (2011) Use of probiotics as growth promoter, anti-bacterial and their effects on the physiological parameters and immune response of Oreochromis niloticus Lin. fingerlings. Journal of the Arabian Aquaculture Society 6: 201-222.
- Sivaram V, Babu MM, Citarasu T, Immanuel G, Murugadass S, et al. (2004) Growth and immune response of juvenile greasy groupers (Epinephelus tauvina) fed with herbal antibacterial active principle supplemented diets against Vibrio harveyi infections. Aquaculture 237: 9-20.
- Adedeji OS, Farimi GO, Ameen SA, Olayemi JB (2006) Effects of bitter kola (Garcinia kola) as growth promoter in Broiler Chicks from day old to four weeks old. Journal of Animal and Veterinary Advances 5: 191-193.
- Subramanian S, Mackinnon SL, Ross NW (2007) A comparative study on innate immune parameters in the epidermal mucus of various fish species. Comp Biochem Physiol B Biochem Mol Biol 148: 256-263.
- Rao YV, Das J, Pradhana BK, Chakrabarthi R (2006) Effect of Achyranthes aspera on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. Shell fish immunology 20: 263-273.
- Akram M, Shah MI, Usmanghan K, Mohiuddinn E, Sami A (2011) Zingiber officinale Roscoe (A medicinal plant). Pakistan Journal of Nutrition 10: 399-400.
- desouky H, Asely AE, Shaheem A, Abass A (2012) Effects of Zingiber officinalis and Cyanodon dactylon on the Growth Performance and Immune Parameters of Macrobrachium rosenbergii. World Journal of Fish and Marine Science 4: 301-307.
- Ernst E (2000) Adverse effects of herbal drugs in dermatology. Br J Dermatol 143: 923-929.
- Nya EJ, Austin B (2011) Development of immunity in rainbow trout (Oncorhynchus mykiss, Walbaum) to Aeromonas hydrophila after the dietary application of garlic. Fish Shellfish Immunol 30: 845-850.
- Grzanna R, Lindmark L, Frondoza CG (2005) Ginger--an herbal medicinal product with broad anti-inflammatory actions J Med Food 8: 125-132.
- Nwabueze AA (2014) Antimicrobial action of epidermal mucus extract of Clarias gariepinus (Burchell, 1822) juveniles-fed ginger inclusion in diet. International Journal of Biology 6: 42-48.
- Fasasi OS, Adeyemi IA, Fagbenro OA (2005) Proximate composition and multi-enzyme in vitroprotein digestibility of maize-tilapia flour blend. Journal of Food Technology 3: 342-345.
- Haghighi M, Rohani MS (2013) The effect of powered ginger (Zingiber officinale) on the haematological and immunological parameters of rainbow trout Oncorrrhychus mykiss. JMPHTR 1: 8-12.
- AOAC (2011) Association of International Official Analytic Chemists, AOAC International, 18th edition, Arlington, Virginia.
- Latona DF, Oyeleke GO, Olayiwola OA (2012) Chemical Analysis of Ginger Root, Journal of Applied Chemistry 1:47-49.
- Hedges AJ (2002) Estimating the precision of serial dilutions and viable bacterial counts. International Journal of Food Microbiology 76: 207-214.
- Buchanan RE, Gibbson ME (1994) Bergey’s Manual of Systematic Bacteriology. The Williams and Wilkins Company, Baltimore, USA. Pg no: 510-593.
- Barnett HL (1987) Illustrated General of Imperfect Fungi, (2rd Edn). Burgess Publishing Company, Minneapolis, USA. Pg no: 241.
- Campbell CK, Johnson EM, Warnork DW (2013) Identification of Pathogenic Fungi, 2nd Edition. John Wiley & Sons, Inc Pg no: 1-350.
- Agbebi OT, Lawal HB, Odebiyi VC (2012) Aflatoxin effect of moulded gel waste mixed with ginger and its histopathological study on Clarias garepinus. Global Journal of Science Frontier research, 12: 7-15.
- Duncan DB (1955) Multiple range and multiple F tests. Biometrics 11: 1-42.
- Adekoya BB, Olunuga OA, Ayansanwo TO, Omoyinmi GAK (2004) Manual of the second annual seminar and training workshop held at Ogun State Agricultural Development Programme, OGADEP, Olabisi Onabanjo way, Idi-Aba, Abeokuta. Publisher: Fisheries Society of Nigeria (Ogun State Chapter). Pg no: 52.
- Omotayo AM Akegbejo-Samson Y, Olaoye OJ (2006) Fish Production, preservation, processing and storage, Training manual of the 2006 Joint training of fish farmers in Epe, Lagos State Agricultural media Resources and Extension Centre (AMREC), Federal University of Agriculture Abeokuta, Ogun State and BATN Foundation, Victoria Island, Lagos, Canada. Pg no: 19-20.
- Bello OS, Emikpe BO, Olaifa FE (2012) The body weight changes and gut morphometry of Clarias gariepinus juveniles on feeds supplemented with Walnut (Tetracarpidium conophorum) Leaf and Onion (Allium cepa) Bub Residues. International Journal of Morphology 30: 253-251.
- Obasa SO, Faturoti EO (2001) Growth response and serum component and yield of the African bony tongue (Heterotis niloticus) fed varying dietary crude protein level. ASSET Series A 1: 97-104.
- Onibi GE, Adebisi O E, Fajemisin AN, Adetunji AV (2009) Response of broiler chickens in terms of performance and meat quality to garlic (Allium sativum) supplementation. African Journal of Agricultural Research 4: 511-517.
- Azaza MS, Wassim K, Mensi F, Abdelmouleh A, Brini B, Kraiem MM (2009) Evaluation of faba beans (Vicia faba L. var. minuta) as a replacement for soybean meal in practical diets of juvenile Nile tilapia Oreochromis niloticus.Aquaculture 287: 174-179.
- Abdel-Rahman AM (2009) Antagonism of Aeromonas hydrophila by propolis and its effect on the performance of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 27: 454-459.
- Zomrawi WB, Abdel Atti KHA, Mahala AG (2011) Effect of ginger root powder supplementation on broiler chicks performance, blood and serum constituents. Online Journal of Animal and Feed Research 1: 457-460.
- Abou-zeid SM (2002) The effect of some medical plant on reproductive and productive performance of Nile tilapia, Oreochronis niloticus. Ph.D Thesis Cairo, Faculty of Agriculture, Cairo University, Giza, Egypt. Pg no: 212.
- Ashade OO, Adelusi OE, Ligali NA (2014) Histopathological effects of untreated ginger peel (Zingiber officinale) fish meal on the intestine tissue profiling of African catfish (Clarias gariepinus) International Journal of Fisheries and Aquatic studies 2: 95-98.
- Olojo EAA, Amusa NA, Osho A, Badejo VO (2010) Commensal bacterial flora of Synodontis nigritaand Clarias gariepinus from River Osun, Southwest Nigeria, Nigeria. Research Journal of Applied Sciences 5: 231-235.
- Pandy G, Madhuri S, Mandloi AK (2012) Medicinal plants useful in fish diseases. International Research Journal of Pharmacy 12: 1-4.
- Ringo E, Strom E, Tabachek JA (1995) Intestinal microflora of Salmonids: A review. Aquaculture Research 26: 1365-2109.
- Awosika F (1991) Local medicinal plants and health of consumer. Clinical Pharmacognosis Herbal Medicine. 9: 28-29.
- Akinyama H, Kazuyasu F, Yamasaki O, Oono T, Iwatsuki K (2001) Antibacterial action of several tannins against Staphylococcus aureus. Journal of Antimicrobial Chemotherapy 48: 487-491.
- Idris GL, Omojowo FS, Omojasola PF, Adetunju CO, Ngwu EO (2010) The effect of different concentrations of ginger on the quality of smoked dried catfish (Clarias gariepinus). Nature and Science 84: 59-63.
Citation: Adegbesan SI, Obasa SO, Akintokun AK, Abdulraheem I (2019) Effects of Dietary Supplementation of Zingiber officinale Root-Powder on Growth, Nutrient Utilization and Intestinal Microbes of African Mud Catfish (Clarias gariepinus) Fingerlings. J Aquac Fisheries 3: 016.
Copyright: © 2019 Sherifat Ibidunni Adegbesan, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

