
Effects of Processing Methods on Total Phenolic, Total Flavonoid and Antioxidant Capacities of Moringa Seeds
*Corresponding Author(s):
James SDepartment Of Food Science And Technology, Federal University Of Technology, PMB 65, Minna, Niger State, Nigeria
Email:samaila.james@futminna.edu.ng
Abstract
This study evaluated the effects of fermentation time (48-120 h), roasting temperatures (120-150oC), cooking time (10-40 min) and germination time (2-5 days) on total phenolics, total flavonoids, reducing power and antioxidant potentials of moringa seeds. The results revealed that fermentation time significantly (p < 0.05) decreased the total phenolic and antioxidant capacities with increasing fermentation time. However, there was significant (p < 0.05) increase in total flavonoid where the values peaked at 96 h (7.20 mg/100 g) and 120 h (7.21 mg/100 g) fermentation time. Roasting temperatures had negetive impact where the total phenolic, total flavonoid, reducing power and antioxidant capacity significantly (p < 0.05) reduced with increasing roasting temperatures. There were significant (p < 0.05) and steady increases in total flavonoid, reducing power and antioxidant capacity with increasing cooking time. However, total phenolic exhibited significant reduction. Germination length exhibited increase in all the parameters measured. However, total phenolic and flavonoid contents peaked at the fourth day of germination 31.96 mg/100 g and 5.62 mg/100 g, respectively. The reducing power was highest at the second germination day (26.96 mmol/100 g) while antioxidant capacity was highest at the fifth day (39.42 mmol/100 g). Therefore, cooking and germiantion are most preferred in enhacing the bioactive compounds in moringa seeds.
Keywords
Antioxidants; Moringa seeds; Phytonutrients; Treatments
Introduction
Moringa oleifera is commonly known all over the world by different names "Moringa", "The Drumstick Tree", or "The Tree of Life" [1,2]. It is a cherished and highly priced underutilized vegetable tree that is widely cultivated in India and many countries in the tropics and the most cultivated plant from Moringaceae family [2-5]. The tree is increasingly being used for nutritional supplementation. The flowers, leaves, stem and roots are used in teas and other herbal preparations. The plant produces seeds, which contains high level of vitamin C and moderate amount of B vitamins, dietary minerals and are good source of protein, vitamins, beta- carotene, amino acids and various phenolic compounds [4,6]. Also, the seeds contain 50- 60% oil which is beneficial to the skin as moisturizer and others use it to treat cuts and scrapes.
The phenolic contents of medicinal plants are related to their antioxidant capacity. The scavenging ability of the Moringa oleifera leaf extract could be attributed to the presence of polyphenols which are capable of donating hydrogen atoms to -OH radicals, thus inhibiting the oxidation process. Moringa leaves can be eaten raw but, generally, they are eaten cooked, added to local food preparations or to prepare decoctions [7]. Naturally occurring plant phenolics include several groups of compounds that have health promoting properties. Phenolics may act as antioxidants, thereby reducing the risk of atherosclerosis and coronary heart disease, which can be caused by oxidation of low-density lipoproteins. They also may protect against some forms of cancer [8]. Phenolic compounds play an important role in plant resistance and defense against microbial infections which are intimately connected with Reactive Oxygen Species (ROS) [9]. Many phenolic compounds found in plant tissues in addition to ocopherols are potential antioxidants such as flavonoids, tannins and lignin.
Food processing such as fermentation, sprouting, roasting, cooking among others affect the antioxidant capacity, bioactive availability, nutritional composition and functionality of food materials [10-14]. Excessively high temperature treatment is the main cause of bioactive loss in foods due to their susceptibility to high temperature. However, for non-polar ones such as carotenoids, lycopene which are abundant in fruit and vegetables, their concentrations might remain stable or increase upon application of high temperature treatment [15,16]. Also, mechanical processing liberates phenolic compounds, carotenoids and other phytonutrients from cell vacuoles leading to increase in their availability [16,17]. In an attempt to make foods edible, raw foods are subjected to different processing technologies which help in predigesting, eliminating/reducing phytotoxins or the so-called ‘antinutrients’, altering the sensory and textural characteristics of the food among others. This explains the rationale for the application of local processing methods such as soaking, cooking, roasting and fermentation in converting non edible raw foods into nutritious, safe and edible forms [13,18].
The application of foods most especially underutilised legumes such Moringa into food systems depends largely on the knowledge of their nutritional composition, functional properties and their phytonutrient potentials. Research efforts are geared toward presenting underutilised crops; their suitability for incoporation in different food systems as well as their bioactive potentials. Several studies have been conducted on the bioactive components of conventional legumes; however, there is dearth of information on nonconventional ones. Therefore, it is important to assess the effects of processing methods on the availability of total phenolics, total flavonoids as well as evaluate the effects of those processing methods on antioxidant potentials of Morinag seeds.
Materials and Methods
Materials
Moringa seed were purchased from Kure Ultramodern Market, Minna, Niger State. All other chemicals and reagents used were of analytical grades.
Processing Methods
Fermentation of the samples
One thousand (1000) gramme of Morinaga seeds were thoroughly washed and allowed to naturally ferment in tap water in a ratio of 1:3 (w/v) contained in a beaker covered with aluminium foil. The fermentation was done for 0 h, 24 h, 48 h, 72 h and 96 h at room temperature (28 ± 2oC). After each fermentation time, the seeds were drained and oven dried at 80oC for 24 h in a laboratory air convection oven (Gallenkamp, England) and milled in a disc attrition mill (7hp, China). The resultant flours were sieved into a powder of 0.5 mm size, kept in plastic bags and stored at refrigeration temperature (4°C) for further analysis.
Roasting of the legume samples
One thousand (1000) gramme of the sample was roasted in a laboratory oven (Gallenkamp, England) at 120°C, 130°C,140°C and 150°C for 30 min. After 30 min of roasting at set temperatures, roasted samples were milled in a disc attrition mill (7hp, China), sieved into a particle size of 0.50 mm and kept at refrigeration temperature (4°C) until needed for use [13].
Cooking of the sample
One thousand (1000) gramme the sample was cooked in distilled water (1:10 w/v) in a stainless steel pot covered with a lid and at reduced pressure using a domestic pressure cooker (Portable Steam Steriliser, 7500 Series) at 15 psi for 10 min, 20 min, 30 min and 40 min. After each cooking time, the seeds were drained and dried at 80oC for 24 h in a laboratory air convection oven (Gallenkamp, England) and milled in a disc attrition mill (7hp, China). The resultant flours were sieved into a particle size of 0.5 mm and kept in a refrigeration temperature (4oC) until needed for further analysis [13].
Germination of the moringa seeds
Intact seeds (1000 g) were germinated in the dark at room temperature 28 + 2oC for two (2), three (3),four (4) and five (5) days after sterilizing in ethanol (50%, v/v) for 1 min and hydrating the seeds in water (1:3 w/v) for 12 h.
Extract preparation for total phenolic and flavonoid quantification
Ten gramme (10 g) of the sample was transferred to dark-coloured flasks and mixed with 200 mL of solvents with different polarities (water, methanol, ethylacetate, acetone, petroleum ether), respectively and stored at room temperature. After 24 h the infusion was filtered through Whatman No. 1 filter paper and the residue was re-extracted with equal volume of solvents. The process was repeated for 48 h. At the end, supernatants were combined and evaporated to dryness under vacuum at 40oC using rotary evaporator at kept in sterile sample tube and stored in a refrigerator at 4oC.
Quantification of Total Phenolic Compounds (TPC)
Folin-Ciocalteu method as described by [19] was used. An aliquot of 10 μL of the sample solution was mixed with 100 μL of commercial FolinCiocalteu reagent and 1580 μL of water. After a brief incubation at room temperature (5 min), 300 μL of saturated sodium carbonate was added. The colour generated was read after 2 h at room temperature at 760 nm using a UV-Vis spectrophotometer (UV-9200, UK). The correlation between the absorbance and gallic acid concentrations creates a calibration standard curve. The phenolic compounds concentration of the samples was expressed as gallic acid equivalents in mg/L, then the Total Phenolic Compounds yields (TPC) were calculated by transforming milligrams of Gallic Acid Equivalent (GAE) per litre (mg GAE/L) into grams of GAE per 100 g dry matter (g GAE/100 g DM).
Determination of total flavonoids content
A modified method (Chen, 2008) was used: 1 ml of a diluted solution containing flavonoids, 0.7 ml of 5% (w/w) NaNO2 , and 10 ml of 30% (v/v) ethanol were combined and stirred for 5 min, and then 0.7 ml of 10% AlCl3 (w/w) was added and the mixture was stirred up. Six minutes later, 5 ml of 1 mol/l NaOH was added. Subsequently, the solution was diluted to 25 ml with 30% (v/v) ethanol prior to the measurement. After 10 min standing, the absorbance of the solution was measured at 500 nm with a Unico WFJ2000 spectrophotometer (Unico, Shanghai, China). The contents of flavonoids were expressed in mg rutin per gram dry weight basis by comparison with rutin standard curve, and the yield of flavonoids was calculated using the following formula:
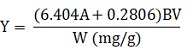
Where: A-absorbance (500 nm) B – dilution factor W – dry weight of cactus skin precisely measured (g) V – volume of the extracting agent (ml).
1,1-Diphenyl-2-picrylhydrazyl method (DPPH?) assay
The radical scavenging capacity of the samples was tested based on the procedure described by [20]. Briefly, the reaction contained 1 mL of extracts, 3 mL of methanol, and 150 µL of DPPH 0.1%. The absorbance was recorded at 517 nm after 30 min. The capacity of radical scavenging was calculated with the following formula:
 Where: Abssample is absorbance of extract solution, and Abscontrol is absorbance of methanol in DPPH.
Where: Abssample is absorbance of extract solution, and Abscontrol is absorbance of methanol in DPPH.
Reducing power assay
For the assay of reducing power, the protocol was used and described as follows. One millilitre of the filtrate was mixed with 2.5 mL of phosphate buffer (pH 6.6) and 2.5 mL of K3[Fe(CN)6] (1%), which was followed by incubation at 50oC for 20 min. The reaction was then stopped by adding 2.5 mL of trichloroacetic acid (10%), followed by centrifuging at 3000 rpm (1000 x g) for 10 min. The supernatant (2.5 mL) was mixed with distilled water (2.5 mL) and 5 mL of FeCl3 solution (1%) and the absorbance was measured at 700 nm. In the reducing power assay, the more the absorbance of the reaction increased, the more reducing power was obtained. The percentage of the reducing power was calculated based on the following formula:
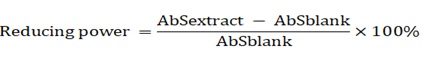 Where: Absextract is absorbance of extracts, and Absblank is absorbance of water.
Where: Absextract is absorbance of extracts, and Absblank is absorbance of water.
Statistical Analysis
The data obtained from the analysis were subjected to one-way analysis of variance (ANOVA) and separation of the mean values was carried out using Duncan Multiply Range Test at 5%.
Results And Discussion
Effect of fermentation length on total phenolic, flavonoid and antioxidant property of moringa seeds
The result on Table 1 shows the total phenolic, total flavonoid, reducing power and antioxidant properties of fermented moringa seeds. The results of the study revealed that fermentation time significantly (p < 0.05) reduced the total phenolics of fermented Moringa seeds with increasing fermentation time. The values were found to be 19.65 mg/100 g, 19.56 mg/100 g, 19.32 mg/100 g, 18.65 mg/100 g and 18.44 mg/100 g for 0 h, 48 h, 72 h, 96 h and 120 h fermentation time, respectively. The results of this finding imply that fermentation has the capacity to significantly (p < 0.05) decrease TPC with increasing fermentation time. The results of this finding agree with the reports of [11] in fermented wild beans where they reported significant loss in TPC with increasing fermentation time. The mechanism of the loss is explained by different reseachers [10]. Attributed the decrease to the activities of polyphenol oxidases which are responsible for catalyzing polyphenols to low molecular weight condensed polyphenols. In similar vein [12,21], also revealed that the loss could be linked to leaching of some of the components of lipophilic polyphenols in the fermentation medium and their possible oxidation as a result of favourable condition. Furthermore, activities of polyphenol oxidase and proteolytic enzymes facilitate the conversion of simple phenolics and de-polymerization of high molecular weight phenolic compounds. This results into formation of phenolic complexes with other molecules which make their quantification difficult. However, the results of this finding contrast the report of [12,22] who revealed decreased TPC in rice bran and Nigeria's undrutilised legumes with increasing fermentation time.
|
Fermentation Time (h) |
TPC (mg/100 g) |
TFC (mg/100 g) |
FRAP (mmol/100 g) |
DPPH (mmol /100 g). |
|
0 |
19.65a ± 0.00 |
6.34d ± 0.14 |
42.12a ± 0.07 |
45.67a ± 0.07 |
|
48 |
19.56b± 0.14 |
6.73c ± 0.07 |
38.65b ± 0.00 |
38.63e ± 0.07 |
|
72 |
19.32c ± 0.07 |
7.10b ± 0.00 |
37.16c ± 0.07 |
38.95d ± 0.00 |
|
96 |
18.65d ± 0.00 |
7.20a ± 0.00 |
36.35d ± 0.07 |
39.77c ± 0.07 |
|
120 |
18.45e ± 0.07 |
7.21a ± 0.14 |
36.03e ± 0.07 |
40.16b ± 0.07 |
Table 1: Total phenolic, total flavonoid, FRAP and DPPH free-scavenging properties of fermented moringa seeds.
The total flavonoid of fermented Moringa seeds were 6.34 mg/100 g, 6.73 mg/100 g, 7.10 mg/100 g, 7.20 mg/100 g and 7.21 mg/100 g at the end of 0 h, 48 h, 72 h, 96 h and 120 h fermentation time, respectively. The results of this finding imply that there were significant (p < 0.0) increase in total flavonoid up to 72 h of fermentation but at 98 h and 120 h of fermentation time, the increase in TFC was not significant (p>0.05). Fermentation process has been shown to activate and mobilize microbial enzymes such as glucosidase, amylase, cellulase, tannase, esterase, invatase or lipases. This enzyme pool catalyze the hydrolysis of plant materials. Their activities lead to the disintegration of plant matrix and consequently facilitating the extraction of flavonoids [12,23,24]. Also, [26] revealed that β. glucosidase of microbial origin most especially the activities of L. plantarum could hydrolyze phytonutrients notably phenolics and flavonoids. Consequently, activities of this microbial species can result into either increase or decrease in flavonoids. Furthermore, rise in acidity during fermentation which might liberate bound flavonoids with resultant increase their bioavailability [26].
The reducing power (FRAP) is an important indicator of potential antioxidant activity. The reducing powers of the extracts were assessed based on their ability to reduce and to stabilize reactive compounds. The reducing power of the fermented seeds at the end of 0 h, 48 h, 72 h, 96 h and 120 h fermentation time were found to be 42.12 mmol/100 g, 38.65 mmol/100 g, 37.16 mmol/100 g, 36.35 mmol/100 g and 36.03 mmol/100 g, respectively. The reducing power of the control was significantly (p < 0.05) higher than the fermented seeds. The reducing power of the fermented seeds positively correlates with the total phenolic contents of the seeds. This relationship is envisaged due to the fact that antioxidant/reducing power is dependant on phenolic content. The DPPH radical scavenging activity exhibited similar trend as found in FRAB, where it showed significant (p ≥ 0.05) decrease with increasing fermentation time. The values were found to be 45.67 mmol/100 g, 38.63 mmol/100 g, 38.95 mmol/100 g, 39.77 mmol/100 g and 40.16 mmol/100 g, at the end of 0 h, 48 h, 72 h, 96 h and 120 h, respectively. The trend of reduction in the reducing/oxidising power with increasing fermentation time in this study agrees with the finding of [27] who reported similar result in different fermented genotypes of Brassica oleraceaenecephala.
Effect of roasting temperatures on total phenolic, flavonoid and antioxidant property of moringa Seeds
The result on Table 2 shows the total phenolic content, total flavonoid content and antioxidant properties of roasted moringa seeds. Roasting temperatures adopted in this study significantly (p < 0.05) reduced all the parameters quantified. The dietary intake of phenolics differs considerably among countries and regions. It is estimated that the daily intake of total free phenolics ranged from 20 mg-1 g [28]. Phenolic compounds have been found to confer physiological benefits such as antioxidant, antimicrobial, antimutagenic, therapeutic and pharmaceutical properties. Therefore, the need to harness its availability in treated Moringa. Dry heat processing (roasting) has been reported to significantly reduce the anti-nutrient content of food samples, however, the level of reduction is usually low compared with cooking [29]. This attributed to the fact that dry heat exact more labile effect on food nutrients than moisture heat treatment [13]. The values of total phenolic content were 19.66 mg/100 g, 12.46 mg/100 g, 11.25 mg/100 g, 11.25 mg/100 g and 11.10 mg/100 g at the end of 0oC, 120oC, 130oC, 140oC and 150oC roasting temperatures, respectively. The decrease in the total phenolics is in line with the report of [30,31] who revealed that phenolic compounds are highly thermo-labile and are early decomposed with high temperature (above 80oC). The results of this study also agree with the report of [12] who reported significant (p<0.05) losses in TPC of African breadfruit, pigeonpea and African yam bean seeds at different roasting temperatures.
|
Roasting Temp. (oC) |
TPC (mg/100 g) |
TFC (mg/100 g) |
FRAP (mmol/100 g) |
DPPH (mmol /100 g) |
|
0 |
19.66a ±0.07 |
6.34a ± 0.10 |
42.16a ± 0.07 |
45.67a ± 0.00 |
|
120 |
12.46b ± 0.07 |
4.64b ± 0.21 |
33.15b ± 0.00 |
41.53e ± 0.07 |
|
130 |
11.76c ± 0.14 |
4.65b± 0.00 |
32.62c ± 0.14 |
42.62d ± 0.07 |
|
140 |
11.25d ± 0.00 |
4.00c ± 0.00 |
31.05d ± 0.00 |
42.86c ± 0.07 |
|
150 |
11.10e ± 0.00 |
3.65d ± 0.00 |
31.00e ± 0.00 |
43.16b ± 0.14 |
Table 2: Total phenolic, total flavonoid, FRAP and DPPH free-scavenging properties of roasted moringa seeds.
[12,32] revealed that flavonoids are wide spread in fruits, vegetables and legumes and are heat sensitive phenolic compounds. As in other forms of high temperature processing such as cooking, roasting too has been shown to results into either increase or decrease in flavonoid. [33,34] have revealed that high temperature processing such as cooking and roasting have destructive effects on flavonoids and phenolic compounds as a result of their high instability. The values of total flavanoids were quantified to be 6.34 mg/100 g, 6.64 mg/100 g, 4.65 mg/100g, 4.00 mg/100 g and 3.65 mg/100 g at the end of 0oC, 120oC, 130oC, 140oC and 150oC roasting temperatures, respectively. The results showed significant decrease in total flavonoid at higher roasting temperatures 140oC (4.00 mg/100 g) and 150oC (3.65 mg/100 g) however, at 120oC and 130oC the losses were minimal and not significant from each other. This implies that, to ensure minimal losses in total flavonoid in foods, roasting temperatures during processing should not exceed 130oC at 30 min. The decrease in the flavonoid content with increasing roasting temperatures can also be attributed to possible Maillard reaction [35,36].
The reducing power and antioxidant capacity of roasted moringa seeds showed marked decrease with increasing roasting temperature. This implies that roasting temperatures adopted have negative influence on the antioxidant capacity of moringa seeds. The result of this finding is in contrast with [37] who reported that high temperature treatment results into production of stronger radical scavenging antioxidant compounds via series of thermal chemical reactions. This leads to the liberation of high amount of antioxidant compounds as a result of thermal destruction of cell walls and sub cellular components with consequent increase in the antioxidant capacity. The percentage decrease in reducing power were 21.37%, 22.62%, 26.35% and 26.47% at 120oC, 130oC, 140oC and 150oC roasting temperatures, respectively; while, 9.07%, 6.69%, 6.15% and 5.50% losses were recorded at 120oC, 130oC, 140oC and 150oC roasting temperatures, respectively [38]. Revealed that loss in antioxidant capacity as a result of thermal treatment could be due to the Mailard reaction of the antioxidant pigments.
Effect of cooking time on total phenolic, flavonoid and antioxidant property of moringa seeds
The total phenolic, total flavonoid and antioxidant properties of cooked moringa seeds are shown in Table 3. The results showed significant (p < 0.05) decrease in TPC of cooked moringa with increasing cooking time; while, total flavonoid, reducing power and antioxidant capacity exhibited significant (p < 0.05) increases as cooking time increased.
|
Cooked (min) |
TPC (mg/100 g) |
TFC (mg/100 g) |
FRAP (mmol /100 g) |
DPPH (mmol/100 g) |
|
Uncooked |
26.36a ± 0.07 |
3.86e ± 0.14 |
23.54e ± 0.21 |
32.25e ± 0.00 |
|
10 |
25.65b ± 0.14 |
6.33d ± 0.07 |
24.16d ± 0.00 |
33.63d ± 0.07 |
|
20 |
24.80c ± 0.14 |
7.13c ± 0.07 |
24.79c ± 0.07 |
36.17c ± 0.07 |
|
30 |
24.32d ± 0.14 |
7.56b ± 0.14 |
25.19b ± 0.14 |
39.16b± 0.07 |
|
40 |
22.67e ± 0.14 |
8.13a ± 0.14 |
27.33a ± 0.07 |
41.14a ± 0.21 |
Table 3: Total phenolic content, total flavonoid content and antioxidant properties of cooked moringa seed.
The total phenolics were quantified to be 26.36 mg/100 g, 25.65 mg/100 g, 24.80 mg/100 g, 24.32 mg/100 g and 22.67 mg/100 g at the end of 0 min, 10 min, 20 min, 40 min and 50 min cooking time, respectively. The reduction in total phenolics of cooked moringa indicates it susceptibility to cooking time [39]. Revealed that loss in TPC of food samples during processing depends on plant species and cooking method adopted. They reported that steaming of banana blossom and cauliflower floret caused an increase in TPC while, microwaving and boiling resulted in to significant reduction. The results of this study also agree with [40] who reported that blanching of spinach, swamp cabbage, kale, shallots and cabbage for 1 min in boiling water reduced the total polyphenols in these vegetables. In the same vein, [12,34] indicated that application of heat during cooking involves changes in the structural integrity and food cellular matric. These lead to either loss or gain in the phytochemical profiles. Furthermore, high temperature treatments have destructive effect on some TPC with resultant reduction in their physiological benefits. Therefore, it can deduced that some phenolic compounds are heat labile and their decrease is as a result of thermal impact on their profile in cell wall and other subcellular structures. However, the results of this finding contradict [34,40,41] who revealed that application of high temperature treatments breaks down the chemical bond between phenolic-macromolecule complexes. This leads to the formation of phenolic aglycon which has high affinity to Folin-Ciocalteu regents, hence, increasing total phenolic quantification. [42,43] also reported increased total phenolics during thermal food processing and they attributed the increase to the liberation of polyphenols embedded in the plant matrix, disruption of protein-polyphenol complexes, and the inactivation of endogenous polyphenol oxidase which favour efficient recovery of phenolic compounds in plant materials.
The total flavonoid content of cooked moringa seeds showed significant (p < 0.05) increase with increasing cooking time. Total flavonoids were quantified to be 3.86 mg/100 g, 6.33 mg/100 g, 7.13 mg/100 g, 7.56 mg/100 g and 8.13 mg/100 g at the end of 0 min, 10 min, 20 min, 30 min and 40 min cooking time, respectively. The results of this study imply that cooking time has significant positive influence on total flavonoid release. Similar increase in the yield of total flavonoid in boiled broccoli and spinach was reported previously by [44]. The increase in total flavonoid with increasing cooking time might be hinged on its release as a result of thermal disruption of intracellular complexes between macro molecules and flavonoid compounds [13,34]. Also, the increase after subsequent boiling might be related to their enhanced availability for extraction and a more efficient release from intracellular proteins and altered cell wall structures [45].
There were marked increases in the reducing and antioxidant powers of cooked moringa seeds with increasing cooking time. The reducing power significantly (p < 0.05) increased by 2.57%, 5.04%, 6.55% and 13.87% at the end of 10 min, 20 min, 30 min and 40 min cooking time, respetively. The results of this study is at variance with the finding of [46,47] who reported losses of 40% and 9% in antioxidant activity in boiled green beans, respectively. The increase in the reducing power and antioxidant capacity is due to suppression of the oxidation capacity of antioxidants by thermal inactivation of oxidative enzymes. Furthermore, the increase can be attributed to the production of stronger radical-scavenging antioxidants by thermal chemical reaction, the liberation of high amounts of antioxidant components due to the thermal destruction of cell walls and sub cellular compartments [13,45].
Effect of germination on total phenolic, flavonoid and antioxidant property of moringa seeds
The total phenolic, total flavonoid and antioxidant properties of germinated moringa seeds are shown in Table 4. Germination significantly (p < 0.05) increased the total phenolics; total flavonoids, reducing power and antioxidant capacity with increasing germination days.
|
Germination ( days) |
TPC (mg/100 g)
|
TFC (mg/100 g)
|
FRAP (mmol/100 g) |
DPPH (mmol /100 g) |
|
0 |
26.36c ± 0.07 |
3.86e ± 0.14 |
23.54d ± 0.21 |
33.63e ± 0.07 |
|
2 |
30.41b ± 0.07 |
4.63d ± 0.07 |
26.96a ± 0.07 |
34.65d ± 0.00 |
|
3 |
30.40b ± 0.14 |
4.76c ± 0.00 |
25.87b ± 0.14 |
36.25c ± 0.00 |
|
4 |
31.96a ± 0.07 |
5.62a ± 0.07 |
25.15c ± 0.14 |
37.85b ± 0.14 |
|
5 |
30.41b ± 0.14 |
5.21b ± 0.00 |
23.53d ± 0.07 |
39.42a ± 0.07 |
Table 4: Total phenolic content, total flavonoid content and antioxidant properties of germinated moringa seeds.
There were significant (p < 0.05) increases in total phenolics with increasing germination days where it peaked at day 4 (31.96 mg/100 g) while at day five the value significantly reduced. This implies that germination has the capacity to significantly increase the total phenolics of moringa seeds up to 4 days [12,48,49]. Explained that during plants' growth and development, they naturally produce phenolic compounds to confer them resistant against biotic and abiotic factors. Therefore, structural changes in phytochemical profile during germination process is considered a natural phenomenon in plants. The results of this finding agree with [12,25,49,50] who showed that germination days significantly increased the total phenolics of chickpeas, lupin seeds, peanut and eight underutilized legumes, respectively [51]. Also, revealed that germination modifies phenolic compounds both qualitatively and quantitatively and the degree of changes depends on seed type and germination conditions employed. These changes have consequential effects on textural characteristics, nutritional composition, functional properties and antioxidant capacity of the resultant flour. Furthermore, the increase in total phenolics could be attributed to solubilization of condensed tannins during seeds soaking in water prior to germination and the migration of phenolic compounds to the outer layer as a result of germination as indicated by the browning of the germinated seeds [52,53].
The total flavonoid contents of germinated moringa seeds were significantly (p < 0.05) higher than the control. This implies that there were increases in total flavonoid content as the number of germination days increased and it peaked at the fourth day of germination (5.62 mg/100 g). However, at the end of fifth day of germination, the total flavonoid significantly (p < 0.05) reduced. This suggests that extended days of germianation,5 days and above might negetively affect total flavonoids. The results here is in line with the findings of [54,55] who reported increase of 8.40%, 13.88% and 41.46% at the end of second, third and fourth days of African oil bean seed germination, respectively. The mechanism of loss/gain in total flavonoids during germination is largely unknown.
The reducing powers of the germinated moringa seeds were found to be 23.54 mmol/100 g, 26.96 mmol/100 g, 25.87 mmol/100 g, 25.15 mmol/100 g and 23.53 mmol/100 g at the end of 0 day, 2 days, 3 days, 4 days and 5 days germination. It can be deduced that the reducing power had the highest percent increase at the end of two days germination (12.96%), while it reduced by 9.00% and 6.40% at the end of 3 and 4 days germination, respectively. Therefore, for high reducing power benifit in germinated moringa seeds, 2 days germination is the best. There were significant (p < 0.05) increases in the antioxidant power by 2.94%, 7.23%, 11.15% and 14.69%, at the end of 2, 3 and 4 days germination respectively. It can be deduced that successive germination up to 5 days gives significant (0.05) and study increase in antioxidant capacity of germinated moringa seeds.
Conclusion
This study demonstrated significant (p < 0.05) decrease in total phenolic contents, reducing power (FRAP) and antioxidant capacity of moringa seeds with increasing fermentation days; however, increasing fermentation days exhibited increase in total flavonoids. There were significant (p < 0.05) decreases in total phenolic, total flavonoid, reducing power and antioxidant capacity with increasing roasting temperatures (120oC to 150oC). However, cooking time adopted in this study (10 min to 30 min) exhibited significant (p < 0.05) increases in total flavonoid, reducing power and antioxidant capacity. Germination days followed a similar pattern where they were increases in total phenolic, flavonoid, reducing power and antioxidant capacity with increasing germiantion days.
References
- Olson ME (2010) Flora of North America Committee (Ed.). Eflora summary: Moringaceae: Drumstick family. Flora of North America; North of mexico 7. New York and Oxford 167-169.
- Oloyede OO, James S, Ocheme OB, Chinma CE, Akpa VE (2016) Effects of fermentation time on the functional and pasting properties of defatted Moringa oleifera seed flour. Food Science and Nutrition 4: 89-95.
- Morton JF (1991) The horse reddish tree, Moringa pterygosperma (Moringaceae)-A boon to Arid Lands? Econ Bot 45: 318-333.
- Anjorin TS, Ikokoh P, Okolo S (2010) Mineral composition of Moringa Oleifera leaves, pods and seeds from two regions in Abuja, Nigeria. International Journal of Agriculture and Biology 12: 431-434.
- Ogunsina BS, Radha C, Govardhan SRS (2010) Physicochemical properties and functional properties of fullfat and defatted Moringa (Moringa oleifera) kernel flour. International Journal of Food Science and Technology 45: 2433-2439.
- Anwar F, Latif S, Ashraf M, Gilani AH (2007) Moringa oleifera: A food plant with multiple medicinal uses. Phytotherapy Research 21: 17-25.
- Leone A, Spada A, Battezzati A, Schiraldi A, Aristil J, et al. (2015) Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: An overview. International Journal Molecular Science 16: 12791-12835.
- Emmons C, Peterson DM (2001) Antioxidant activity and phenolic content of oat as affected by cultivar and location. Crop Science 41: 1676-1681.
- Grassmann J, Hippeli S, Elstner EF (2002) Plant’s defence and its benefits for animals and medicine: Role of phenolics and terpenoids in avoiding oxygen stress. Plant Physiology and Biochemistry 40: 471-478.
- Weisburger JH (2001) Chemo-preventive effects of cocoa polyphenols on chronic diseases. Expository Biol Medicine 226: 891-897.
- Alvarez LC, Alvarez NC, García PG, Salazar JCS (2017) Effect of fermentation time onphenolic content and antioxidant potential in Cupuassu (Theobroma grandiflorum (Willd. Ex Spreng.) K.Schum.) beans. Acta Agron 66: 473-479.
- James S, Nwabueze TU, Ndife J, Onwuka GI, Usman MA (2020a) Influence of fermentation and germination on some bioactive components of selected lesser legumes indigenous to Nigeria. Journal of Agriculture and Food Research 2: 100086.
- James S, Nwabueze TU, Ndife J, Onwuka GI, Usman MA, et al. (2020b) Effects of treatments on some bioactive components of selected lesser known legumes indigenous to Nigeria. Journal of Food Chemistry and Nanotechnology 6: 197-206.
- James S, Nwabueze TU, Onwuka GI, Ndife J, Amuga JS (2020c) Effect of extraction variables on total phenol yield in some selected legumes indigenous to Nigeria. Croatian Journal of Food Science and Technology 12: 1-10.
- Dewanto V, Wu X, Adom KK, Liu RH (2002) Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem 50: 3010-3014.
- Palermo M, Pellegrini N, Fogliano V (2014) The effect of cooking on the phytochemical content of vegetables. J Sci Food Agric 94: 1057-1070.
- Hotz C, Gibson RS (2007) Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based J Nutr 137: 1097-1100.
- FAO (2002) World Agriculture: Towards 2015/2030. Summary report,
- Rajha HN, Boussetta N, Louka N, Maroun RG, Vorobieva E (2014) A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res Int 65: 462-468.
- Siddiqua A, Premakumari KB, Sultana R, Savitha VA (2010) Antioxidant activity and estimation of total phenolic content of Muntingia calabura by colorimetry. International Journal Chemical Technology Research 2: 205-208.
- Wollgast J, Anklam E (2000) Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res Int 33: 423-447.
- Singh AK, Rehal J, Kaur A, Jyot G (2013) Enhancement of attributes of cereals by germination and fermentation: A review. Critical Reviews in Food Science and Nutrition 55: 1575-1589.
- Hur SJ, Lee SY, Kim YC, Choi I, Kim GB (2014) Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem 160: 346-356.
- Nazarni R, Purnama D, Umar S, Eni H (2016) The effect of fermentation on total phenolic, flavonoid and tannin content and its relation to antibacterial activity in Jaruk tigarun-crataeva nurvala, Buch HAM. Inter. Food Res J 23: 309-315.
- Duenas M, Hernandez T, Estrella I, Fernandez D (2009) Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinusangu stifolius L.). Food Chem 117: 599-607.
- Moktan B, Saha J, Sarka PK (2008) Antioxidant activities of soybean as affected by Bacillus- fermentation to kinema. Food Research International 41: 586-593.
- Ahmed S, Beigh SH (2009) Ascorbic acid, carotenoids, total phenolic content and antioxidant activity of various genotypes of Brassica oleracea Encephala. Journal of Medical and Biological Sciences 3: 1-8.
- Vadivel V, Biesalski HK (2012) Effect of certain indigenous processing methods on the bioactive compounds of ten different wild type legume grains. Journal of Food Science and Technology 49: 673-684.
- Osunbitan SO, Taiwo KA, Gbadamosi SO (2015) Effects of different processing methods on the anti-nutrient contents in two improved varieties of cowpea. American Journal of Research Communication 3: 74-87.
- Larrauri JA, Ruperez P, Saura-Calixto F (1997) Effect of drying temperature on the stability of polyphenols and antioxidant activity of red grape pomace peels. J Agric Food Chem 45: 1390-1393.
- Katsube T, Tsurunaga Y, Sugiyama M, Furuno T, Yamasaki Y (2009) Effect of air-drying temperature on antioxidant capacity and stability of polyphenolic compounds in mulberry (Morus alba L.) leaves. Food Chem 113: 964-969.
- Prasanna KDP, Gunathilake P, Somathilak KKD, Vasantha HP (2018) Effect of different cooking methods on polyphenols, carotenoids and antioxidant activities of selected edible leaves. Antioxidants (Basel) 7: 2-12.
- Ismail A, Marjan Z, Foong CW (2004) Total antioxidant activity and phenolic content in selected vegetables. Food Chemistry 87: 581-586.
- Saikia K, Mahanta CL (2012) Effect of steaming, boiling and microwave cooking on the total phenolics, flavonoids and antioxidant properties of different vegetables of Assam, India. International Journal of Food and Nutritional Sciences 2: 47-53.
- Piga A, Del Caro A, Corda G (2003) From plums to prunes: Influence of drying parameters on polyphenols and antioxidant activity. J of Agric and Food Chem 51: 3675-3681.
- Durmaz G, Alpaslan M (2007) Antioxidant properties of roasted apricot (Prunus armeniaca) kernel. Food Chemistry 100: 1177-1181.
- Nicoli MC, Anese M, Parpinel M (1999) Influence of processing on the antioxidant properties of fruit and vegetables. Trends in Food Science Technology 10: 94-100.
- Surveswaran S, Cai YZ, Corke H, Sun M (2007) Systematic evaluation of natural phenolic antioxidant from 133 Indian medicinal plants. Food Chemistry 102: 938-953.
- Boori F, Cefola M, Gioia FD, Pace B, Serio F (2013) Effect of cooking methods on antioxidant activity and nitrate content of selected wild Mediterranean plants. Int J Food Sci Nutr 64: 870-876.
- Turkmen N, Sari F, Velioglu YS (2005) The effects of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chemistry 93: 713-718.
- Singleton VL, Orthofer R, Lamuela-Raventos R (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of FC reagent. Methods in Enzymolog 299: 152-178.
- Ferracane R, Pellegrini N, Visconti A, Graziani G, Chiavaro E (2018) Effects of different cooking methods on the antioxidant profile, antioxidant capacity, and physical characteristics of artichoke. J Agric Food Chem 56: 8601-8608.
- Gunathilake KDPP, Rupasinghe HPV (2014) Optimization of Water Based-extraction Methods for the Preparation of Bioactive-rich Ginger Extract Using Response Surface Methodology. European Journal Medical Plants 4: 893.
- Mazzeo T, Ndri D, Chiavaro E, Viscconti A, Fogliano V, et al. (2011) Effect of two cooking procedure in phytochemical compounds, total antioxidant capacity and color of selected frozen vegetables. Food chemistry 128: 627-633.
- Galor SW, Wong KW, Benzie IFF (2008) Effects of cooking on brassica vegetables. Food Chemistry 110: 706-710.
- Baardseth P, Bjerke F, Karoline B, Martinsen B, Skrede G (2010) Vitamin C, total phenolics and antioxidant activity in tip-cut green beans (Phaseolus vulgaris) and swede rods (Brassica napus var. napobrassica) processed by methods used in catering. Journal of the Science of Food and Agriculture 90: 1245-1255.
- Jiratanan T, Liu RH (2004) Antioxidant activity of processed table beets (Beta vulgaris var. conditiva) and green beans (Phaseolus vulgaris L.). Journal of Agriculture and Food Chemistry 52: 2659-2670.
- Nderitu AM, Dykes L, Awika JM, Minaar A, Duodu KG (2013) Phenolic composition and inhibitory effect against oxidative DNA damage of cooked cowpeas as affected by simulated invitro gastrointestinal digestion. Food Chem 141: 1763-1771.
- Khang DT, Dung TN, Elzaawely AA, Xuan TD (2016) Phenolic profiles and antioxidant activity of germinated legumes. Foods 5: 27-55.
- Khattak AB, Zeb A, Bibi N, Khalil SA, Khattak MS (2007) Influence of germination techniques on phytic acid and polyphenols content of chickpea (Cicerarietinum ) sprouts. Food Chemistry 104: 1074-1079.
- L_opez-Amor_os M, Hern_ande ZT, Estrella I (2006) Effect of germination on legume phenolic compounds and their antioxidant activity. J Food Compos Anal 19: 277-283.
- Hec?imovic´ I, Belšc?ak-Cvitanovic A, Horz?ic D, Komes D (2011) Comparative study of polyphenols and caffeine in different coffee varieties affected by the degree of roasting. Food Chem 129: 991-1000.
- Benzie IFF, Strain JJ (1996) The Ferric Reducing Ability Of Plasma (FRAP) as a measure of “anti- oxidant power”: The FRAP assay. Anal Biochem 239: 70-76.
- https://www.cabdirect.org/cabdirect/abstract/19810721475.
- Stewart AJ, Bozonnet S, Mullen W, Jenkins GI, Lean MEJ (2000) Occurrence of flavonols in tomatoes and tomato-based products. J Agric Food Chem 48: 2663-2669.
Citation: James S, Yakubu CM, Maxwell YMO, Audu Y, Bankole BM, et al. (2022) Effects of Processing Methods on Total Phenolic, Total Flavonoid and Antioxidant Capacities of Moringa Seeds. J Food Sci Nutr 8: 138.
Copyright: © 2022 James S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

