
Ergothioneine Rich Agaricus Bisporus Extracts Decreases Lipid Accumulation Induced by Oleic Acid in Hepg2 Cells: Possible Implications in the Treatment of Nonalcoholic Liver Fatty Disease
*Corresponding Author(s):
Juan BautistaDepartamento De Bioquimica Y Biologia Molecular, Facultad De Farmacia, Universidad De Sevilla, Sevilla, Spain
Tel:+34954556854,
Email:jdbaut@us.es
Abstract
Nonalcoholic Fatty Liver Disease (NAFLD)is characterized by the excessive triglycerides accumulation as lipid dropletsin the cytoplasm of hepatocytes, which result from an imbalance between uptake, synthesis, export, and oxidation of fatty acids.In this study, we have evaluated the effect of a natural extracts from mushroom (Agaricusbisporus)enriched in the antioxidant ergothioneine,on the reduction of lipid accumulation in HepG2 cells. Ergothioneine Rich A.bisporusExtracts (ERAbE)decreased the intracellular concentration of lipids, lipids droplets size and intracellular TG content through down regulation of SREBP1c, PPARγ and ACAT1together withPPARαupregulation. These extracts alsodownregulatedhepatic lipogenesis through SREBP1cactivation. Moreover,increased lipolysis was found to be induced through PPARα.
Therefore, we concluded that EEAbEhas the ability to reduce significantly the intracellular lipid content in an in vitro model induced by oleic acid. EEAbEdownregulatedSREBP1cexpression,leading to an inhibition of hepatic lipogenesis. The activation of PPARα induced lipolysis being responsible for lowering hepatic fat content together with the reduction of lipogenesis. EEAbEhas a regulatory effecton lipid accumulation in HepG2 cells.
Keywords
Agaricus bisporus;Ergothioneine; Mushroom;NAFLD; Steatosis
INTRODUCTION
Nonalcoholic Fatty Liver Disease (NAFLD)is one of the most common causes of chronic liver disease in developed countries [1]and is characterized by the excessive accumulation of fat in the hepatocytes of the liver parenchyma in the absence of excessive drinking of alcohol[2].NAFLD is particularly prevalent in patients with metabolic abnormalities such as obesity, type 2 diabetes, arterial hypertension, and hypertriglyceridemia[3,4]and no relationship between NAFLD and race, age or gender has been described to date.
The exact pathogenesis of NAFLD remains poorly understood. Initially, a “twohit” hypothesis was proposed with an initial accumulation of lipids in the liver (simple steatosis), followed be events that propagate oxidative stress, lipid peroxidation, and inflammation possibly leading to Nonalcoholic Steatohepatitis (NASH), liver fibrosis, cirrhosis, or in some cases hepatocellular carcinoma[5,6]. However,a “multiple parallel hits” hypothesis has been recently proposed which suggest that gut and adipose tissuederived inflammatory mediators drive hepatic inflammation which is paralleled by multiple events in the liver including lipid synthesis and retention, and fibrosis [2].
Over the last decade, several research initiatives have been focusedon possible therapies to ameliorate the hepatic damage that accompanies NAFLD, including synthetic and natural products. The majority of the strategies include the use of antioxidant and insulin sensitizers, and also drugs that reduce the effect of dietary carbohydrate and fats by the inhibition of cholesterol micellization, pancreatic lipase and alpha glucosidase[7-9].Recently, the interest for natural phytochemicals as antiNAFLD agents has grown significantly [10,11].
In this context, natural products from edible mushrooms, e.i.Agaricusbisporus (A. bisporus), with hepatoprotective properties are of special interest[12,13].Ergothioneine (ERG), a unique naturally occurring thiol derivative of histidine produced exclusively by certain fungi, mushroom and microorganisms, has been suggested to accumulate in cells and some tissuesundergoing high oxidative stress, such as the liver in NAFLD, where ERGcould play a protective effect [14].
It is known that one of the main environmental factors that favors the development of NAFLD are fat rich diets, and that this type of diets are very common today in our society. Therefore, the development of functional foods that could reduce the accumulation of liver fat, and are easily incorporated into the diet, is a topic of great interest today, since this would allow the treatment of this pathology through nutrition [15-17]. In this context, it has been postulated that products capable of inhibiting "de novo" lipid synthesis and lipid uptake could play a very important role in the effective treatment of NASH [18]. Therefore, the objective of this work was the study of the effect of a mushroom (A. bisporus) extract rich in ERG on the accumulation of fats (lipid droplets) in HepG2 cells grown in a high fat medium (1mMOA), as initial phase of a larger study in mice and humans as a possible nutritional intervention of NAFLD.
EXPERIMENTAL SECTIONS
Preparation of aqueous extracts
White button mushrooms (A.bisporus) were used as raw material after cultivation in a pilot plant at the University of Seville (Spain), according to standard procedures. All chemicals used were of analytical grade. A. bisporus aqueous extract were obtained by an enzymatic procedure based on the protocol described by Cremades et al.,[19]. Briefly, after A. bisporushomogenization (10g+10ml distilled water) and enzymatic digestion with a mixture of glucanase and chitinase enzymes (Novo Nordisk®) at pH=5, temperature 55ºC and an enzyme/substrate ratio of 0.1 (w/v), for 24 hours, temperature was raised up to 90ºC for 120 min to inactivate the enzymes. After cooling to room temperature, pH was adjusted to 7.0 with 1M NaOH, and centrifuged at 8000xg. Supernatant was collected and filtered through a 0.2mm membrane, using the filtrate as “crude A. bisporusextract”.ERAbE was prepared by UltraFiltration (UF) fractionation with a 50 KDaUFmembrane and concentration of the ultrafiltrateby reverse osmosis[20].
Cell culture
HepG2 cellswere routinely cultured in DMEM (Gibco) supplemented with 10% FBS and 1% penicillin and streptomycin in an incubator under an atmosphere of 5% CO2 at 37°C. The HepG2 cell model of OAinduced intracellular lipid accumulation was developed as previously describedby ChavesTapia et al., [21]. HepG2 cells were cultured with 1mM of OA for 48h, presence and absence of ERAbE (0.1mg/ml) or quercetin (50µM).
Oil Red O (ORO) cell staining
Lipid droplets were detected by fluorescent microscopy. Briefly, neutral lipids stored into thelipid droplets were visualized by fluorescence microscopy using Oil Red O staining (SigmaAldrich Co. LLC), a vital lipophilic dye used to label fat accumulation in the cytosol[22]. To analyse fat accumulation20,000 HepG2 cells were plated and grown on coverslips in 24 wells plate. HepG2 cells were cultured in presence of OA1mM diluted in DMSO (less than 0.01% of total volume) and treated for 48h with 0.1mg/ml of ERAbE or 50μM of quercetin (HWI ANALYTIK GmbH German) used as positive control. The cells were rinsed two times with PhosphateBuffered Saline (PBS) pH 7.4, fixed with 4% paraformaldehyde in PBS for 10min and permeabilised with 0.2% Triton X100 for 2min. Nuclei cells were stained with 4´,6-Diamidino-2-Phenylindole (DAPI) for 30min at room temperature, and neutral lipids were stained with Oil Red O (ORO) as previously described by [23]. Images were acquired with a fluorescence microscope (OLIMPUS BX41) equipped with the standard epifluorescence filter set up for DAPI and FITC. For determination of lipid droplets diameter images were captured under oil with a 100x plan apochromat objective. Analyses were performed on three independent experiments measuring at least 100 cells for each treatment using Imaging Software cell^F (Olympus Corporation, Tokyo, Japan).
Determination of intracellular fat content
Intracellular fat content was determined fluorimetrically based on Nile Red staining, a vital lipophilic dye used to label fat accumulation in the cytosol [24]. Ten thousand HepGe cells were grown in 96 plate wells, exposed to OA and treated with 0.1mg/ml ERAbE and 50µMquercetin for 48h. AdipoRed™ Reagent was added in each well and incubated at room temperature for 10min. Intensity fluorescence was quantified using a molecular image 485/572nm (Synergy HT, BioTeK) and normalizedto protein concentration.
Extraction of RNA and cDNA synthesis
Total RNA from HepG2 cells was isolated usingTRIsure Reagent (BIO38033, Bioline Reagents Ltd., London, UK) based on the guanidine isothiocyanate method [25]. Retrotranscriptase was performed using SensiFASTTM cDNA Synthesis Kit(Bioline®). RNA quantity was determined using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and the quality was assessed with an Agilent 2100 Bioanalyzer (Agilent technologies, Waldbronn, Germany). Reactions were performed in 48well plates in 10μl total volume containing 1μl of cDNA (100ng of cDNA), 1μl of the genespecific primer, 3μl of ultrapure H2O MilliQ and 5μl of the mixture GoTaq® qPCR Master Mix 2X(Promega®), for each sample and wellplate.
Quantitative Polymerase Chain Reaction (qPCR)
Primers for human genes, purchased from Qiagen (Qiagen, Hilden, Germany), were used. The sequence of the primers is shown in table1. Gene expression levels of SREBP1c, PPARγ, PPARα, ACAT, DGAT1, MTTP, APOB and APOEgeneswere analysed usingquantitative polymerase chain reaction.The conditions for amplification were as follows: Polymerase activation step of 95ºC 10 min, denaturation step 95ºC 10 seconds, alignment step of 65ºC 30 seconds and extension step of 72ºC 15 seconds. Quantitative PCR was performed in triplicate for each sample using Eco Realtime PCR System v3.0of the thermal cycler (Illumina® California, EEUU) software. Results are expressed as relative gene expression normalized to the expression levels of the reference gene Gapdh.
|
Gene |
Forward Sequence |
Reverse Sequence |
|
SREBP1c |
ACTTCTGGAGGCATCGCAAGCA |
AGGTTCCAGAGGAGGCTACAAG |
|
PPARγ |
AGCCTGCGAAAGCCTTTTGGTG |
GGCTTCACATTCAGCAAACCTGG |
|
PPARα |
TCGGCGAGGATAGTTCTGGAAG |
GACCACAGGATAAGTCACCGAG |
|
ACAT1 |
CCAGCCACTAAGCTTGGTTCCA |
GTAGGAGCTTGTCCTTCACCTC |
|
DGAT1 |
GCTTCAGCAACTACCGTGGCAT |
CCTTCAGGAACAGAGAAACCACC |
|
MTTP |
AGGCTGTCAGAAACTTCCTGGC |
GTCTGAGCAGAGGTGACAGCAT |
|
APOB |
AGAGGACAGAGCCTTGGTGGAT |
CTGGACAAGGTCATACTCTGCC |
|
APOE |
GAACCGCTTCTGGGATTACCTG |
GCCTTTACTTCCGTCATAGTGTC |
|
GAPDH |
GTCTCCTCTGACTTCAACAGCG |
ACCACCCTGTTGCTGTAGCCAA |
Table1:Forward and reverse sequence of primers used for qPCR.
Statistical analysis
Continuous variables are described as means±SD of minimum three independent experiments. The Student ttest was used for comparisons between groups. P values: p<0.05 (*), p<0.01 (**), and p<0.001 (***) were considered statistically significant.
RESULTS
To test OAinduced intracellular lipid accumulation in HepG2 cells, they were grown in a medium with and without 1mM OA. As shown in figure1A, untreated HepG2 shows a low content of intracellular lipiddroplets;however, as shown in figure1B after OA treatment, the number and size of lipid droplets were significantlyhigher.From these results we concluded that 1mM OAinduces lipid accumulation in HepG2 cells.Intracellular lipids contentwas analysed usingfluorescencebasedAdipoRedTMassay. Results areshown in figure1C.Data show that HepG2 cells grown with OA have a higher intracellular lipid contentcompared to untreated HepG2 cells, 4.6 fold (p<0.001). These results clearly show that this model can be used to study the effect on lipid accumulation of compounds of interest such as ERAbE.
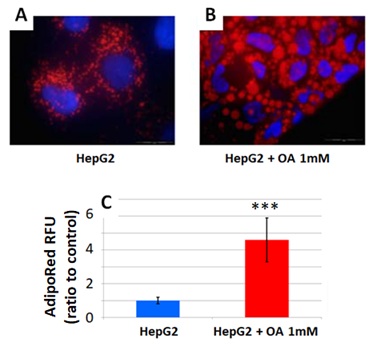 Figure1: Untreated HepG2 cells (A), and HepG2 cellscultured in the presence of 1mM OA (B), for 48 hours. Lipids droplets and nuclei were stained with ORO (red) and Dapi (blue), respectively. Images were acquired with a fluorescence microscope (OLIMPUS BX41). Intracellular triglycerides concentration of untreated (control) and OAtreated HepG2 cells, by the AdiposeRedTM assay. Stained intracellular triglycerides were quantified by fluorescence (assigning the value of 1 to the fluorescence of untreated hepatocytes) (C). Data are expressed as the mean±SD of three independent experiments. (* p<0.05; ** p<0.01; *** p<0.001).
Figure1: Untreated HepG2 cells (A), and HepG2 cellscultured in the presence of 1mM OA (B), for 48 hours. Lipids droplets and nuclei were stained with ORO (red) and Dapi (blue), respectively. Images were acquired with a fluorescence microscope (OLIMPUS BX41). Intracellular triglycerides concentration of untreated (control) and OAtreated HepG2 cells, by the AdiposeRedTM assay. Stained intracellular triglycerides were quantified by fluorescence (assigning the value of 1 to the fluorescence of untreated hepatocytes) (C). Data are expressed as the mean±SD of three independent experiments. (* p<0.05; ** p<0.01; *** p<0.001).
Effects ofERAbEon lipid accumulation
Before proceeding to the study of the effect of ERAbE on lipid accumulation, a toxicity study was carried out to exclude the possible effects of ERAbE. Nontoxicevidences were observed at 0.1mg/ml (data not shown). This was the chosen concentration of ERAbE in subsequent studies. The effect of ERAbE on lipid accumulation in HepG2 cells was tested with 1.0mM OA and in presence and absence of 0.1mg/mlERAbE, or 50µM quercetin used as a positive control[17]. As observed by OROstaining(Figure2A),treatment with 0.1mg/ml of ERAbE or 50µM of quercetin of HepG2 cells grown in presence of 1mM OA caused a significant reduction in lipid accumulation in treated cells compared to untreated.These results also show that a significant reduction of lipid droplets sizein HepG2 cells (Figure2B)withboth treatments(p<0.01), and that the effect of ERAbE at 0.1mg/ml is similar to that observed for quercetin (50µM) used as a positive control.Quantification of intracellular lipid (triacylglycerols) concentration by AdipoRedalso show lower lipid content in OAcultivated cells by treatment with ERAbE0.1mg/ml (70.4±2.2%),and with 50µMquercetin (69.1±1.9%) (Figure2C).Hence, both treatments are effective in the reduction of intracellular lipid accumulationsignificantly(p <0.01).
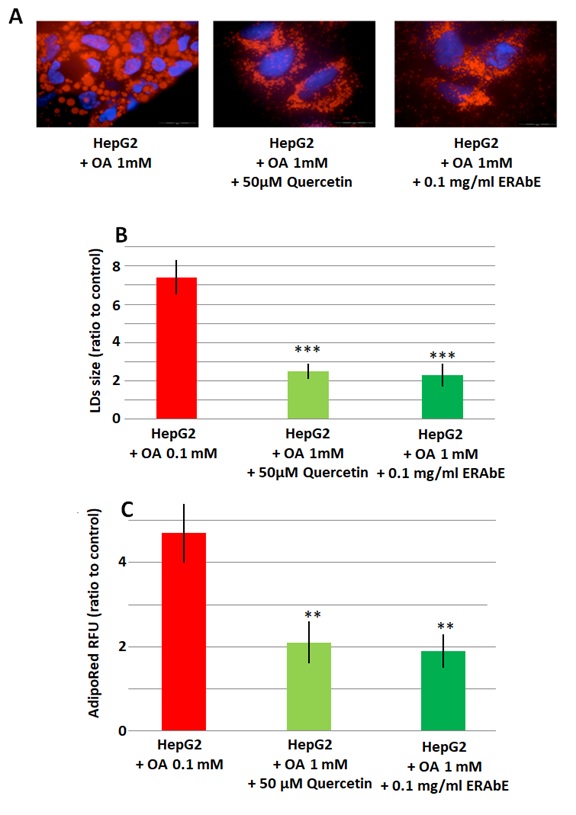 Figure2: HepG2 cells exposed to 1mM OA, untreated (control) and treated with ERAbE 0.1mg/ml and quercetin 50µM for 48h. (A) Lipid droplets and nuclei stained with ORO (red) and Dapi (blue), respectively. (B) Lipid droplets size measured by Imaging Software CellAF Software. Results are expressed as foldarea (µm2). (C) Intracellular triglyceride concentration determined by the AdipoRed Assay. The intracellular triglycerides were stained and the concentration of triglycerides was determined (quantified) by fluorescence (HepG2 fold=1). Data are expressed as the mean values±SD obtained from three independent experiments. (* p<0.05; ** p<0.01; *** p<0.001).
Figure2: HepG2 cells exposed to 1mM OA, untreated (control) and treated with ERAbE 0.1mg/ml and quercetin 50µM for 48h. (A) Lipid droplets and nuclei stained with ORO (red) and Dapi (blue), respectively. (B) Lipid droplets size measured by Imaging Software CellAF Software. Results are expressed as foldarea (µm2). (C) Intracellular triglyceride concentration determined by the AdipoRed Assay. The intracellular triglycerides were stained and the concentration of triglycerides was determined (quantified) by fluorescence (HepG2 fold=1). Data are expressed as the mean values±SD obtained from three independent experiments. (* p<0.05; ** p<0.01; *** p<0.001).
Modulation of lipogenesisrelated gene expression by ERAbE treatment
In order to investigate the mechanisms involved in the reduction of lipid accumulation in cells treated withERAbE, we have analysed gene expression of several proteins implicated in lipogenesis and lipolysispathways (Table1).
This analysis showed thatSREBP1c,PPARγand PPARαwere significantly increased in HepG2 cellsgrown in presence of OA (p<0.001)(Figure3)compared to untreated cells. These results also show thatthe treatment with ERAbE (0.1mg/ml) or quercetin (50µM)decrease expression of genes implicated in lipogenesis:SREBP1c(1.7±0.2and 1.3±0.1fold, respectively, p
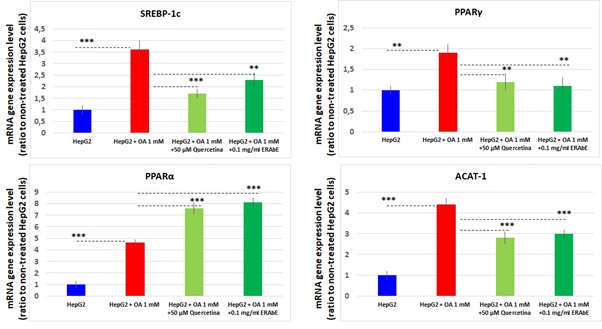 Figure3: mRNA gene expression levels of SREBP1c, PPARγ, PPARα and ACAT1, determined by RTqPCR. Results were normalized using GADPH, and HepG2 nontreated cells were used as reference. Data are expressed as the mean values±SD obtained from three independent experiments. (* p<0.05; ** p<0.01; *** p<0.001).
Figure3: mRNA gene expression levels of SREBP1c, PPARγ, PPARα and ACAT1, determined by RTqPCR. Results were normalized using GADPH, and HepG2 nontreated cells were used as reference. Data are expressed as the mean values±SD obtained from three independent experiments. (* p<0.05; ** p<0.01; *** p<0.001).
Our results also show that acyl-coenzymeA:cholesterol acyltransferase1 (ACAT1) gene expressionwassignificantly increasedin presence of OA1mM (p<0.001). After treatment with 0.1mg/ml ERAbEand with 50µM quercetin a significant reduction in mRNA expression(1.4±0.2 and 1.6±0.3 respectively, p
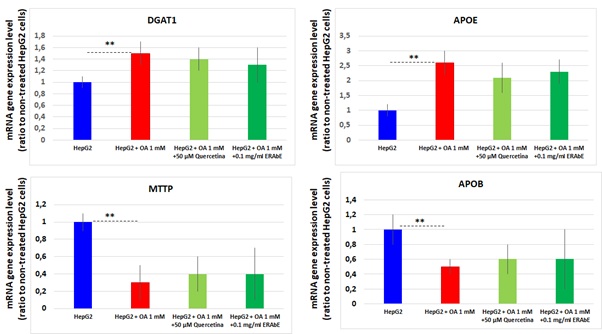 Figure4: mRNA gene expression levels of DGAT1, APOE, MTTP and APOB determined by RTqPCR. Results were normalized using GADPH, and HepG2 nontreated cells were used as reference. Data are expressed as the mean values±SD obtained from three independent experiments. (* p<0.05; ** p<0.01; *** p<0.001).
Figure4: mRNA gene expression levels of DGAT1, APOE, MTTP and APOB determined by RTqPCR. Results were normalized using GADPH, and HepG2 nontreated cells were used as reference. Data are expressed as the mean values±SD obtained from three independent experiments. (* p<0.05; ** p<0.01; *** p<0.001).
Other genes implicated on triacylglycerides and Very Low Density Lipoproteins (VLDL) metabolism was also modulated in OAtreated HepG2 cells.DGAT1 and APOEgenes were found significantly increased (p<0.01), while MTTP and APOBgenes were downregulated (p<0.05). The treatment of HepG2 cells cultured in presence of 1mM OA with 0.1mg/ml ERAbE or 50µM quercetindo not show statistically significant changes at mRNA level. Our results show that the treatment of OAcultured HepG2 with ERAbE or quercetin did not affect genes expression involved in triacylglycerol synthesis and VLDL excretion pathways.
DISCUSSION
Current therapies for NAFLD include lifestyle modifications, physical activity and medical intervention.In relation with the first one, nutritional intervention can be of great importance. Probiotics and nutraceuticals (i.e. resveratrol and quercetin[26], anthocyanins[27], vitamin E[28],became promising in helping therapeutic approaches, probably because of their antioxidant activity. On the basis of these data, it seems that rich foods in the natural antioxidant ERGmay be useful for the preventionand treatment of NAFLD. Taken into account thatmushroom extracts have been studied as potential agent for both prevention and treatment of hepatic steatosis[12] and that mushroom in general and A. bisporus in particular are excellentERG sources[19,29], ERAbE could be effective in NAFLD treatment via nutrition.
In this study we have shown that ERAbEmay be useful fortherapeutic interventions in lipid accumulationrelated liver pathologies like NAFLD reducing triglycerides concentration and lipid droplets size in an in vitro model, similar to that observed with quercetin [30].Beneficial effects from phenolic antioxidants in the prevention and treatment of liver steatosis have been widely reported[26,31]. These molecules presentedhepaticprotective effects because they could reduce liver fat accumulation, mainly by lowering lipogenesis and increasing fatty acid oxidation. Besides, these molecules are able toreduce oxidative stress and inflammation, the main factors responsible for liverdamage[32].
Lipid accumulation in the liver may be caused by enhanced de novo lipogenesis, activation of lipid uptake, and lowering of lipid catabolism. Fatty acids are known to be ligands for nuclear transcription factors, such as SREBP1c,PPARγ and PPARα[33]. PPARα activation is required to enhance hepatic lipid turnover to enable sufficient clearance of lipids from the liver, preventing lipid accumulation and peroxidation in murine NASH models[34].It has been shown that PPARα knockout (/) mice developed severe hepatic steatosis upon fasting as a result of failure to upregulate the fatty acid oxidation pathway [35]. Our result confirmedthat the therapeutic effect of ERAbE and quercetin on lipid metabolism in HepG2induced fatty liver cells could be partly due to PPARαupregulation(inducing lipolysis) and SREBP1c downregulation(reducing lipogenesis)[36,37].
ERAbEincreased PPARα gene expression levels in the model of steatosis which controls fatty acid degradation. It has been demonstrated that pathogenesis of NASH increased the pool of free fatty acids through de novolipid synthesis and nuclear receptors activation (SREBP1, ChREBP1, and PPARγ)[38].In our study we show that OA significantly increased SREPB1c gene expression which was disrupted by ERAbE and quercetin. In addition, PPARγgene expression was induced by OA and this effect was diminished after treatment with ERAbE and quercetin.We also observed that genes involved in triacylglycerols synthesis and VLDL secretion pathways were not affected by ERAbE or quercetin.
Our data demonstrated thatERAbEtreatment modified nuclear transcription factors leading toa significant decrease of intracellular lipid content and lipid droplets size.Therefore, we concluded that ERAbEmay interfere and prevent the development of metabolic disorders involved in NAFLD. ERAbEmay prevent the progression of liver damage related to NAFLD mainly bytwo independent mechanisms:i) inhibiting lipogenesis by reducing SREBP1c and ii) promoting lipolysisthrough PPARαinduction.However, further studies are required to clarify the molecular mechanism including their role on the oxidative stress and testing their effect on inflammation and fibrosis.
REFERENCES
- Bellentani S, Scaglioni F, Marino M, Bedogni G (2010) Epidemiology of nonalcoholic fatty liver disease. Dig Dis 28: 155-161.
- Diehl AM, Day C (2017) Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 377: 2063-2072.
- Postic C, Girard J (2008) Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J Clin Invest 118: 829-838.
- Sung KC, Kim SH (2011) Interrelationship between fatty liver and insulin resistance in the development of type 2 diabetes. J Clin Endocrinol Metab 96: 1093-1097.
- Contos MJ, Choudhury J, Mills AS, Sanyal AJ (2004) The histologic spectrum of nonalcoholic fatty liver disease. Clin Liver Dis 8: 481-500.
- Rosso N, ChavezTapia NC, Tiribelli C, Bellentani S (2014) Translational approaches: from fatty liver to nonalcoholic steatohepatitis. World J Gastroenterol 20: 9038-9049.
- Al Zarzour RH, Ahmad M, Asmawi MZ, Kaur G, Saeed MAA, et al. (2017) Phyllanthus niruri standardized extract alleviates the progression of nonalcoholic fatty liver disease and decreases atherosclerotic risk in Sprague-Dawley Rats. Nutrients 9: 766.
- Filippatos TD, Elisaf MS (2011) Role of ezetimibe in non-alcoholic fatty liver disease. World J Hepatol 3: 265-267.
- Nozaki Y, Fujita K, Yoneda M, Wada K, Shinohara Y, et al. (2009) Long-term combination therapy of ezetimibe and acarbose for nonalcoholic fatty liver disease. J Hepatol 51: 548-556.
- Milosevic N, Milanovic M, Abenavoli L, Milic N (2014)Phytotherapy and NAFLD--from goals and challenges to clinical practice. Rev Recent Clin Trials 9: 195-203.
- Dong H, Lu FE, Gao ZQ, Xu LJ, Wang KF, et al. (2005) Effects of emodin on treating murine nonalcoholic fatty liver induced by high caloric laboratory chaw. World Journal of Gastroenterology, 11: 1339-1344.
- Iñiguez M, Pérez-Matute P, Villanueva-Millán MJ, RecioFernández E, Roncero-Ramos I, et al. (2018) Agaricusbisporus supplementation reduces high-fat-diet-induced body weight gain and fatty liver development. J Physiol Biochem 74: 635-646.
- Liu Y, Zheng D, Su L, Wang Q, Li Y (2018) Protective effect of polysaccharide from Agaricusbisporus in Tibet area of China against tetrachlorideinduced acute liver injury in mice. Int J Biol Macromol 118: 1488-1493.
- Cheah IK, Tang R, Ye P, Yew TSZ, Lim KHC, et al. (2016) Liver ergothioneine accumulation in a guinea pig model of nonalcoholic fatty liver disease. A possible mechanism of defence? Free Radic Res 50: 14-25.
- Liu Y, Wang D, Zhang D,Lv Y, Wei Y, et al. (2011) Inhibitory effect of blueberry polyphenolic compounds on oleic acidinduced hepatic steatosis in vitro. J Agric Food Chem 59: 12254-12263.
- Hwang YJ, Wi HR, Kim HR, Park KW, Hwang KA (2014) Pinus densiflora Sieb. et Zucc. alleviates lipogenesis and oxidative stress during oleic acid-induced steatosis in HepG2 cells. Nutrients 6: 2956-2972.
- Rojas A, Gallego P, Gil-Gómez A, Muñoz-Hernández R, Rojas L, et al. (2018) Natural extracts abolished lipid accumulation in cells harbouting non-favourable PNPLA3 genotype. Ann Hepatol 17: 242-249.
- Liu Q, Bengmark S, Qu S (2010) The role of hepatic fat accumulation in pathogenesis of Nonalcoholic Fatty Liver Disease (NAFLD). Lipids Health Dis 9:42.
- Cremades O, Díaz-Herrero MM, Carbonero-Aguilar P, Gutierrez-Gil JF, Fontiveros E, et al. (2015) White button mushroom ergothioneine aqueous extracts obtained by the application of enzymes and membrane technology. Food Bioscience 10:42-47.
- Inca-Torres AR (2019) Valorization of subproducts of the industry of edible mushrooms. Application to obtain high value added products. PhD thesis, University of Seville, Sevilla, Spain.
- Chavez-Tapia NC, Rosso N, Tiribelli C (2012)Effect of intracellular lipid accumulation in a new model of nonalcoholic fatty liver disease. BMC Gastroenterol 12: 20.
- Hu KQ, Yu CH, Mineyama Y, McCracken JD, Hillebrand DJ, et al. (2003) Inhibited proliferation of cyclooxygenase2 expressing human hepatoma cells by NS-398 a selective COX-2 inhibitor. Int J Oncol 22: 757-763.
- Clement S, Juge-Aubry C, Sgroi A, Conzelmann S, Pazienza V, et al. (2008) Monocyte chemoattractant protein-1 secreted by adipose tissue induces direct lipid accumulation in hepatocytes. Hepatology 48: 799-807.
- McMilland MK, Grant ER, Zhong Z, Parker JB, Li L, et al. (2001) Nile Red binding to HepG2 cells: an improved assay for in vitro studies of hepatosteatosis. In Vitr Mol Toxicol 14: 177-190.
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156-159.
- Pisonero-Vaquero S, González-Gallego J, Sánchez-Campos S, García-Mediavilla MV (2015) Flavonoids and related compounds in non alcoholic fatty acid disease therapy.Curr Med Chem 22: 2991-3012.
- Zhang PW, Chen FX, Li D, Ling WH (2015) A consort-compliant randomized double blind placebo controlled pilot trial of purified anthocyaninin patients with non alcoholic fatty liver disease. Medicine (Baltimore) 94: 758.
- Ji HF (2015) Vitamin E therapy on aminotransferase level in NAFLD/NASH patients.Nutrition 31: 899.
- Kalaras MD, Richie JP, Calcagnotto A, Beelman RB (2017) Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem 233: 429-433.
- Li X, Wang R, Zhou N, Wang X, Liu Q, et al. (2013) Quercetin improves insulin resistance and hepatic lipid accumulation in vitro in a NAFLD cell model.Biomed Rep 1: 71-76.
- Van de Wier B, Koek GH, Bast A, Haenen GR (2017) The potential of flavonoids in the treatment of non alcoholic fatty liver disease. Crit Rev Food Sci Nutr 57: 834-855.
- Aguirre L, Portillo MP, Hijona E, Bujanda L (2014) Effect of resveratrol and other polyphenols in hepatic steatosis. World J Gastroenterol 20: 7366-7380.
- Jump DB, Tripathy S, Depner CM (2013)Fatty acid regulated transcription factors in the liver. Annu Rev Nutr 33: 249-269.
- Harano Y, Yasui K, Toyama T, Nakajima T, Mitsuyoshi H, et al. (2006) Fenofibrate a peroxisome proliferatoractivated receptor alpha agonist reduces hepatic steatosis and lipid peroxidation in fatty liver shionogi mice with hereditary fatty liver. Liver Int 26: 613-620.
- Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, et al. (2003) Central role of PPARalphadependent hepatic liver turnover in dietary steatohepatitis in mice. Hepatology 38: 123-132.
- Wang LL, Zhang ZC, Hassan W, Li Y, Liu J, et al. (2016) Amelioration of free fatty acid-induced fatty liver by quercetin3-O-β-Dglucuronide through modulation of peroxisome proliferatoractivated receptoralpha/sterol regulatory elementbinding protein1c signalling. Hepatol Res 46: 225-238.
- Vendrame S, Daugherty A, Kristo AS, KlimisZacas D (2014) Wild blueberry (Vacciniumangustifolium) enriched diet improves dyslipidaemia and modulates the expression of genes relates to lipid metabolism in obese Zucker rats. Br J Nutr 111: 194-200.
- Anderson N, Borlak J (2008) Molecular mechanism and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev 60: 311-357.
Citation: Carbonero Aguilar P, et al J food Sci Nutr 2019. 5:049.
Copyright: © 2019 Pilar Carbonero Aguilar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

