
Evaluation of the Functional and Structural Properties of Collagen Extracted from Tilapia Co-Product
*Corresponding Author(s):
Flávia Aparecida Reitz CardosoPost Graduation Program Of In Technological Innovations Ppgit, Federal University Of Technology-Paraná, Campo Mourão, Brazil
Tel:+55 4435181402,
Email:reitz@utfpr.edu.br; flaviareitz@gmail.com
Abstract
Tilapia is of a great importance in the aquaculture sector, is one of the species more produced commercially. The collagen is a found fibrous protein in conjunctive fabrics of the body, that has the function to contribute with the resistance and elasticity of fabrics, most of the collagen is obtained by means of residues generated from cattle, pigs and fish, among others. The attainment of the extracted collagen of co-product of the tilapia of the Nile (Oreochromis niloticus) indicates an alternative in the use of the generation of residues of the production. The present study it was carried through by neutral daily pay-treatment with water, having varied time and temperature and had been evaluated characteristic as centesimal composition (moisture, ashes and proteins), solubility and Distinguishing Exploratory Calorimetry (DSC). The obtained collagen presented low 1.45 content of ashes texts 2.18%. In relation to the humidity text, the same it presents texts of 9.56 to 11.67% and one high protein text of 83.46 to 87.71%. The extracted collagen possess high solubility and good thermal stability. In this way, it was possible to conclude that the extraction from the tilapia co-product is a viable alternative for attainment of this product.
Keywords
Collagen; Extraction; Tilapia of the Nile
INTRODUCTION
The aquaculture is expanding in a sustainable manner and is currently the segment wherein more are implanted projects, mainly in the global fishing sector, represented as an alternative form of greater viability to supply the growing demand for fish, both marine origin, as fresh water. With the fall of the fishing extractive sector in recent decades, the rapid growth of aquaculture has been the only way to keep this high demand of world fish consumption [1]. Tilapia (Oreochromis niloticus) is one of the most cultivated species in the country for having great ability to adapt to production systems, be easily reproducible and easy to dispose growth.
The fish is an excellent source of protein, because their muscles have high nutritional value they contain higher portion of essential amino acids, particularly those limiting in vegetable proteins [2]. The fish proteins are divided into classes based on their solubility. water soluble proteins and albumins are called soluble proteins salts are known as globulins.
One of the biggest problems in the production chain of fishing is the large amount of waste generated after filleting [3]. In the case of tilapia, waste amount to 75% of the gross weight of the fish in organic waste and, of these, approximately 30% consists of skin and bones with high nutritional quality for obtaining different products, which can generate extra profits for producers and reduce the harmful effect on the environment.
According to Sader [4], Collagen is a fibrous protein found in multicellular animals, secreted by various types of cells and major structural protein found in the extracellular matrix and connective tissues. It is also found in most tissues: Bones, ligaments, tendons, cartilage and soft tissue. Collagen is characterized by high content of glycine, proline and hydroxyproline and denatured in the presence of acids diluted standards and converted into soluble protein such as gelatin, when dissolved in heated solutions [5].
The process of obtaining collagen is crucial to their properties because their quality depends on the physicochemical properties, they are strongly influenced by the severity of the manufacturing process [5]. Achieving higher yields in tilapia skin collagen extraction process is essential to enable its use as a potential source of production. Collagen is an excellent nutrient for most microorganisms, so care must be taken during its manufacture to avoid possible contamination [6].
Extraction of collagen may be carried out in such a way as to obtain the fiber and its by-product, the powder both in the raw state and having the same composition. With this type of extraction, the collagen may exhibit desirable characteristics for use in biofilms, as it has an elongated physical layout and large particles [7].
Collagen in its purified form, has several applications in the pharmaceutical and cosmetics industry. The quality and specific application of the extracted collagen are directly related to its functional properties and purity [8].
Additionally, many studies were conducted to evaluate the application of collagen as a functional ingredient in food. There is an increased interest in the food industry by collagen and gelatin due to their emulsifying properties, foaming agents, colloidal stabilizers, biodegradable films and forming microencapsule agents, with the trend to replace the synthetic material by natural. In addition to exploring various kinds of bioactive, antimicrobial agents, antioxidants and anti-hypertensive, studies have also concentrated on the effect of oral intake in animals and humans. Thus, an increase of research on the enzymatic hydrolysis of collagen and gelatin for the production of bioactive peptides [9].
MATERIALS AND METHODS
The skins of tilapia (Oreochromis niloticus), used as raw material for collagen extraction, were donated by a private fishing of Campo Mourão region. The skins were purchased fresh, washed in water and kept frozen until the start of pre-treatments. When starting the pre-treatment, the samples were thawed and washed again in running water to remove any residues. To the extraction was used the amount of 150g of raw material (skin). The study of collagen extraction process coproduct tilapia and evaluation of factors that influence the performance, we used the 22 factorial design with three center points for tests shown in table 1.
|
Test |
Time (Hours) |
Temperature (°C) |
|
1 |
16 |
25 |
|
2 |
16 |
35 |
|
3 |
18 |
25 |
|
4 |
18 |
35 |
|
5 |
17 |
30 |
|
6 |
17 |
30 |
|
7 |
17 |
30 |
Table 1: Values of the factors time and temperature of collagen extraction for each test.
For the extraction of collagen was used a methodology proposed by Yan et al. [10], with some modifications. The skins of tilapia were cut with the help of scissors into small pieces of 1cm2; Following this procedure, the washing of the hides with sodium hydroxide was made (NaOH) 0.1M 1:20w/v (1g skin NaOH for 20ml) during 24 hours with gentle agitation, by means of a magnetic stirrer. This step was performed to remove proteins. Then, descaling was performed skins with 0.5M EDTA (pH 7.5) in a 1:10w/v for 5 days with the EDTA solution being renewed daily. After descaling, the skins were washed with cold distilled water (5°C). Subsequently, they were soaked in 10% butanol solution in a ratio of 1:20w/v, left for 24 hours and it is renewed every eight hours. The degreased skins, washed with cold distilled water (5°C) and then immediately were immersed in a 0.05M sulfuric acid solution (1:20w/v) at room temperature (25°C) for 3 hours. After this stage, the skins were rinsed with distilled water until neutral pH. The skins were on continuously stirring in water in the thermostatic bath with a ratio of 1:30w/v, varying the time and temperature, according to table 1.
Percent composition of collagen
The chemical composition is needed to enable the classification of foods by verifying the identity and purity of organic and inorganic nature substances [11], depending on moisture content, lipid, protein and ash. This information contributes to the implementation of goals as the standardization of food productsbased on nutritional criteria in addition to providing benefits in dietario character [12].
Moisture
The humidity of the powdered collagen was defined as drying the sample in an oven at 105°C for a period of 6 hours, according to methods Physicochemical Adolfo Lutz Institute for Food Analysis [13]. The moisture analysis is based on weight loss experienced by the sample when it is heated conditions in which water is removed. For moisture analysis was performed using 3g 5g of sample.
Ash content
For the determination of ash was used the methodology of the Institute Adolfo Lutz [13], where the sample was carbonized in Bunsen burner and then heated in oven at 550°C for 4 hours (until obtaining a clear gray). For this analysis we were also used were 3g 5g of sample.
Proteins
The protein analysis was carried out using the Kjeldahl method as reported in Methods Physical-Chemical Food Analysis Institute Adolfo Lutz [13], formed by the sample digestion step in which organic nitrogen is converted into ammonia and organic components they are converted into CO2 and H2O. The second step, which is the distillation consists in capturing ammonia gas released into the receptor solution (boric acid) and the final stage, where the titration is carried out the quantitative determination of ammonia contained in the receiver solution.
Differential Scanning Calorimeter (DSC)
DSC analyzes were carried out on a machine Q20 (TA Instruments) in hermetically sealed aluminum pan with about 50mg of each sample. The experiment was conducted at a heating rate of 20°C/min at an initial temperature of 0°C and a final temperature of 300°C. The data were plotted using the OriginPro software.
RESULTS AND DISCUSSION
Composition Analysis Proximate
The results of the collagen powder chemical composition can be observed in table 2.
|
Test |
Time |
Temperature |
Moisture (%) |
Ash (%) |
Proteins (%) |
Lipids (%) |
|
1 |
15 |
25 |
11,67a±0.17 |
0.05±1,48c |
86,54a±0.31 |
0.49 |
|
2 |
20 |
35 |
0.44±9,56d |
1,60b±0.01 |
85,64a±0.17 |
3.19 |
|
3 |
15 |
25 |
11,27ab±0.11 |
2,18a±0.05 |
83,47a±0.33 |
3.20 |
|
4 |
20 |
35 |
10,44c±0.18 |
1,54bc±0.07 |
85,35a±0.06 |
2.68 |
|
5 |
17.5 |
30 |
10,24c±0.11 |
1,56bc±0.01 |
87,71a±0.48 |
0.50 |
|
6 |
17.5 |
30 |
10,70bc±0.19 |
0.04±1,46c |
87,25a±0.08 |
0.60 |
|
7 |
17.5 |
30 |
10,75bc±0.10 |
1,50bc±0.01 |
87,42a±0.34 |
0.20 |
Table 2: Results of centasimal composition of collagen extraction of the coproduct tilapia.
Note: *Means in the same column followed by different letters differ by Tukey test at a significance level of 5%.
Moisture is one of the determining factors for the microbiological processes, such as bacterial growth. The moisture content of raw materials is of fundamental importance for the conservation and storage. And the moisture content values found varied between 9.56 and 11.67% which is below that of Ahmed and 15.06% Prudêncio-Ferreira [14], cite the near and 8.31% by Olivo mentioned and Shimokomaki [15]. The humidity difference between samples may be explained by variation of temperature and time that they were exposed and samples 5, 6 and 7 were maintained under identical conditions and resulted in average significantly equal.
Analysis of ash as well as other analyzes was performed in triplicate to determine the amount of inorganic matter present in the samples. The results are shown in table 3. The ash values ranged between 1.45 and 2.18%. Only the test 3 was found above the value obtained by Ockerman and Hansen [12], which were below 2.0%. This variation is due to the process in which underwent the tests, the test being 3 presented the time value and lower temperature compared to other tests. The tests 4, 5 and 7 showed no significant difference between the means.
|
Test |
Td (°C) |
|
1 |
82.29 |
|
2 |
90.20 |
|
3 |
91.96 |
|
4 |
81.34 |
|
5 |
85.31 |
|
6 |
85.02 |
|
7 |
83.31 |
|
Collagen nova_prom |
107.92 |
|
Collagen pharmacy |
98.32 |
Table 3: Collagen denaturation temperatures of samples.
The ashes analysis provides background information on the nutritional value of the food in relation to its mineral content. The amount of ash present in food refers to the inorganic residue remaining from the incineration of the organic matter [16].
The values found in the analysis of powdered collagen proteins are shown in table 3. For the protein content, the results ranged from 83.46 to 87.71% and showed no significant difference between the means, as can be seen in table 3. As compared to the literature, the protein levels were close to 86.35% by Alves mentioned Prudêncio and Ferreira [14] and Santana et al. [17] and above were 76.27 to 81.41% and obtained by Olivo Shimokomaki [15].
For the lipid analysis values found are shown in table 3. The percentage of lipids collagen powder was 0.20 to 3.19%. When comparing the values with the literature we have left out the assays found by Cho et al. [18] and Muyonga et al. [19], 0.21 and 0.20% respectively. The only assay that was within those obtained by the authors was 7 with the test value of 0.20%. Songchotikunpan et al. [20], reported value of 1.1% for lipids. Low fat are characteristic of this species, which is considered lean.
Differential Scanning Calorimetry (DSC)
In the Differential Scanning Calorimetric analysis (DSC), samples of bovine collagen obtained from a compounding pharmacy, nova_prom collagen and collagen extracted from tilapia the coproduct were heated and simultaneously subjected to a controlled temperature program. DSC analysis is generally used to determine the thermal stability of collagen. The DSC curves obtained for each test sample of collagen and collagen standard samples are shown in figure 1.
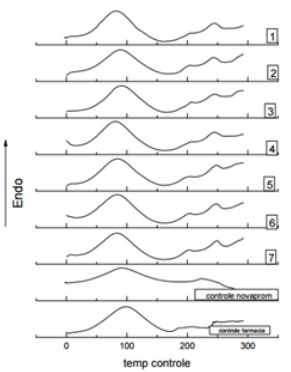 Figure 1: DSC curves.
Figure 1: DSC curves.
The Denaturation Temperatures (Td) for each test for 1 to 7 and collagen standard samples are shown in table 3.
It is observed that the curves for the control of pharmacy, nova_prom control and the curves of tests 1 to 7 show a transition around 100°C, which can be attributed to the denaturation of collagen [21], the longer the treatment time of the sample, the lower the Denaturation Temperature (Td). The data collected by DSC analysis indicate that the collagen has good thermal stability, which means that it might be used as polymer matrix for the formulation of composites [22].
CONCLUSION
The results in this study express the skin of tilapia has great potential for collagen extraction and the extraction carried out by the proposed methodology proved satisfactory. And it was verified that the present values in the analysis approach of literature, except for a few who were different due to the change of time and temperature at the time of extraction. However, this difference did not express a result that could alter the final characteristics of the product. The high content of proteins identified in collagen extraction obtained show the importance of the search for new sources of raw materials that can replace the extraction with the by-products of mammals, for example. It can be concluded that the use of co-product of tilapia shown a good alternative to the seafood industry, since this waste generated in production has a high added value. It is also a way to decrease the improper disposal of the same, preventing damage to the environment.
REFERENCES
- Sebrae (2015) Aquicultura No Brasil: Série Estudos Mercadológ Sebrae, Brazil.
- Vidotti RM, Gonçalves GS (2006) Produção e caracterização de silagem, farinha e óleo de tilápia e sua utilização na alimentação Instituto de Pesca: São José do Rio Preto, Brazil.
- Bueno CM, Alvim ID, Koberstein TCRD, Portella MC, Grosso C (2011) Production of tilapia skin gelatin and its use to obtain microparticles containing salmon oil. Braz J Food Technol 14: 65-73.
- Sader MS (2010) Tricalcium phosphate replaced by magnesium and magnesium composite-apatite carbonate-anionic collagen as a potential bone substitute. Doctoral Thesis (Postgraduate Program in Metallurgical and Materials Engineering). Alberto Coimbra Institute of Graduate Studies and Research in Engineering, Federal University of Rio de Janeiro.
- Alfaro AT (2008) Optimization of extraction conditions and characterization of tilapia skin gelatine (Oreochomis urolepishornorum). Doctoral Thesis (Graduate Program in Science and Technology Agroindustrial). Federal University of Pelotas.
- Cole CG, Roberts JJ (1997) Gelatine colour measurement. Meat Sci 45: 23-31.
- Basso TR, Urnau RM, Brandalize C, Simoes MR (2013) Extraction and characterization of collagen obtained from skins from tilapia processing. III Paraná Meeting of Engineering and Science. Toledo-Paraná.
- Rustad T (2003) Utilization of marine by-products. EJEAFChe 2: 458-463.
- Gómez GMC, Giménez B, López CME, Montero MP (2011) Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll 25: 1813-1827.
- Yan M, Quin S, Li J (2015) Study on the self-assembly property of type I collagen prepared from tilapia (Oreochromis niloticus) skin by different extraction methods. Food Sci Technol 50: 2088-2096.
- Silva D J, Queiroz AC (2002) Análise de alimentos: Métodos químicos e biológi (3rd edn). Viçosa: UFV, Brazil.
- Contreras-Guzmán ES (1994) Bioquímica de pescados e derivados. Jaboticabal: FUNEP,
- Instituto Adolfo Lutz (2008) Normas analíticas do Instituto Adolfo Lutz. São Paulo, Brazil.
- Alves SG T, Prudêncio-Ferreira SH (2002) Propriedades funcionais de material colagenoso de pés de frango. Arch Latinoam Nutr 52: 289-293.
- Olivo R, Shimokomaki M (2001) Carnes: No caminho da pesquisa. Cocal do Sul: Imprint.
- Chaves MC, Gouveia JPG, Almeida FAC, Leite CA, Silva FLH (2004) Caracterização físico-química do suco de acerola. Rev Biol Ciênc Terra 4: 23-31.
- Santana RC, Sato ACH, Cunha RS (2012) Emulsions stabilized by heat-treated collagen fibers. Food Hydrocoll 26: 73-81.
- Cho SY, Rhee C (2004) Mechanical properties and water vapor permeability of edible films made from fractions soy proteins with ultrafiltration. LWT 37: 833-839.
- Muyonga JH, Cole CGB, Duodu K (2004) Extraction and physicochemical characterization of Nile Perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll 18: 581-492.
- Songchotikunpan P, Tattiyakul J, Supaphol P (2008) Extraction and electrospinning of gelatin from fish skin. Int J Biol Macromolecules 42: 247-255.
- Monterrey-Quintero ES, Sobral PJA (2000) Preparo e caracterização de proteínas miofibrilares de tilápia-do-nilo para elaboração de biofilmes. Pesquisas Agropecuárias do Brasil 35: 179-189.
- Samouillan V, Delaunay F, Dandurand J, Merbahi N, Gardou JP, et al. (2011) The Use of Thermal Techniques for the Characterization and Selection of Natural Biomaterials. J Funct Biomater 2: 230-248.
Citation: Harati JB, Droval AA, Marques LLM, Fuchs RHB, Cardoso FAR (2020) Evaluation of the Functional and Structural Properties of Collagen Extracted from Tilapia Co-Product. J food Sci Nutr 6: 056.
Copyright: © 2020 Jamal Basam Harati, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

