
First Hydroacoustic Assessment of Fish Abundance and Distribution in the Shallow Sub-basin of Lake Titicaca
*Corresponding Author(s):
Erick LoayzaUnidad De Ecología Acuática, Instituto De Ecología (IE), Carrera De Biología-Facultad De Ciencias Puras Y Naturales, Universidad Mayor De San Andrés (UMSA), La Paz, Bolivia
Tel:+591 70691599,
Email:erickz.loayzatorrico@gmail.com
Abstract
For the last two decades, a rapid eutrophication process impacts Lake Titicaca, the largest tropical freshwater lake in South America and the main highest Great Lake. This is especially notorious in the Bolivian sector of its shallow Lago Menor sub-basin. Lago Menor is deteriorated by the combination of multiple contaminations (domestic, industrial and mining) from untreated wastewater discharged from the urban area of El Alto, indiscriminate overfishing, and climate change. These threats particularly affect the native Andean killifish genus Orestias, the ecology and dynamics of which require in-depth studies with non-invasive techniques. Here, we use hydroacoustic method to evaluate fish patterns of distribution and abundance in the Lago Menor. Hydroacoustic data were collected during the 2015 rainy season (November) with vertical beaming at 120 kHz along transects that sum a total length of 140 km. Our results showed that the proxy of fish biomass was linked to Lago Menor bathymetry. Furthermore, the vertical fish biomass proxy was steady from 3 to 20 m. This pilot study provides the first image of fish, mostly Orestias spp., distribution and opens future studies to deepen knowledge on their ecology and ethology, and regular monitoring of their population and stock for the fisheries assessment in Lake Titicaca.
Keywords
Conservation; High altitude tropical lake; Hydroacoustic; Native fish; Shallow waters
INTRODUCTION
During the last decades, substantial decreases in fish stocks have been evidenced at local and global scales, with freshwater ecosystems being the most endangered [1-3]. On the South-American Altiplano, one of the largest high plateau in the world, Lake Titicaca (Figure 1), transboundary between Bolivia and Peru, is one of the highest (3,810 m a.s.l.) Great Lakes. Lake Titicaca is also the largest freshwater lake (8,372 km2) in South America. It provides the major source of fish for about 3 million people in the region [4-6]. For the last two decades, Lake Titicaca has been facing a rapid eutrophication process, most intensively in the shallow Lago Menor sub-basin [6]. This deterioration results from mining, domestic and industrial contamination generated by the fast demographic expansion of El Alto city (more than 1.2 million inhabitants), that prior to 1985 was a suburb of La Paz, the Bolivian capital. Almost raw sewage waters from the urban area of El Alto drains into the Cohana bay in the Bolivian sector of Lago Menor (Figure 1). Indeed, the only water treatment plant in operation is undersized (initially designed to treat the domestic waters of 500,000 inhabitants), insufficient and deficient in performance [7]. The rapid increasing human impact was magnified by an intense climate warming due to the combined effects of tropical location (16°S) and altitude, resulting in twice the planet average warming towards 2100 [8-10]. This pressure particularly threatens most of the vulnerable native Andean killifish genus Orestias [11], that are endemic to Peruvian, Bolivian and Chilean isolated high-altitude lakes [12].
Lake Titicaca is divided into two sub-basins (Figure 1): Deep Lago Mayor (mean depth 150 m, maximum depth 285 m), and shallow Lago Menor (9 m and 40 m, respectively) where large areas encompass depth less than 5 m [13]. Overall, Lago Mayor is mainly oligotrophic, while Lago Menor is mesotrophic to eutrophic in the shallowest littoral areas. Due to its tropical location, temperature and light conditions remain almost constant, yearly. Altitude-related intense UV radiations generates a strong surface photo-inhibition of phytoplankton photosynthesis [14]. The lake typically experiences a dry season (April-November), and a rainy season (November-March) [15,16].
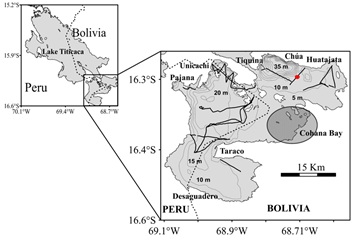 Figure 1: Left-hand map: Lake Titicaca with its two sub-basins. The dotted line shows the border between Peru and Bolivia. Right-hand map: Zoom on Lago Menor. The thin lines represent the bathymetry isolines every 5-m depth. The thick lines represent the hydroacoustic transects. The shaded ellipse highlights the area of influence of the Cohana Bay. The red dot corresponds to the stretch shown in the echogram in figure 2.
Figure 1: Left-hand map: Lake Titicaca with its two sub-basins. The dotted line shows the border between Peru and Bolivia. Right-hand map: Zoom on Lago Menor. The thin lines represent the bathymetry isolines every 5-m depth. The thick lines represent the hydroacoustic transects. The shaded ellipse highlights the area of influence of the Cohana Bay. The red dot corresponds to the stretch shown in the echogram in figure 2.
Lake Titicaca ichthyofauna is mainly composed by species of the native genera Trichomycterus and Orestias [5,17-19]. Orestias [11] comprises 23 species, with some authors’ divergence on species validity, based on genetic traits [5,12,16,20,21]. Among them, four Orestias species, O.agassizii, O.luteus, O.ispi and O.olivaceus, are well distributed throughout Lake Titicaca. O.ispi, a small pelagic species, is the only one living in schools in deep waters (Lago Mayor, Tiquina straight, and Chúa trench) [19,22]. O.olivaceus is a small demersal species and the most abundant in Lago Menor [23].
The few studies carried out on the feeding habits of Orestias species, described that, with the exception of O.ispi, Orestias diet is based on benthic resources, amphipods (Hyalella spp.) and mollusks. They are thus categorized as benthic species [19], they feed mainly over submerged macrophytes with microcarnivorous (O.luteus and O.mulleri), and omnivorous tendencies (O.agassizii) (Loayza et al., unpublished data). However, this information, as well as the distribution patterns of O.agassizii and O.luteus, only come from fishing-based studies performed during the 1980s in Lago Mayor [24,25], with very few studies [19] carried out in the Bolivian sector. It is therefore necessary to evaluate whether Orestias of Lago Menor are associated with submerged vegetation, as in Lago Mayor.
Since the 1940s, Lake Titicaca native ichthyofauna has been perturbed repeatedly due to the introductions of exotic piscivores e.g., rainbow trout (Oncorhynchis mykiss) from California, and the pejerrey silverside (Odontesthes bonariensis) [26] from Argentina. Competition and predation by trout and silverside may have caused the disappearance of two Orestias species (O.cuvieri and O.pentlandii), in addition to occasional epizooties of parasitic protozoan and bacteria locally affecting Orestias populations [27].
Despite their ecological and commercial importance and their vulnerability, Orestias spp. stock level in Lake Titicaca is poorly known [5]. Regulation and management programs for fisheries are not enforced in Lake Titicaca. In both countries, management measures (reproductive closures, minimum catch and mesh sizes) are not complied with. To develop an effective fisheries management plan, both a strict control of fishing activities and a better knowledge on catches, effort and fish stock distribution and abundance are required, especially in the Bolivian sector of Lake Titicaca. The use of gillnets for assessing fish population is likely to provide a biased and incomplete picture [28]. By contrast, hydroacoustics, despite of its limitations (e.g., difficulty in detecting fish near the surface, the bottom and in shallow waters), is a non-intrusive widely used method that provides high-resolution observations [29], especially in lakes [30]. Worldwide, hydroacoustic methods are increasingly used to study the abundance, distribution and behavior of fish and zooplankton communities, and offer a great variety of applications for studying fish in their habitats, especially in lakes [31-34]. However, hydroacoustic methods are still not widely applied in freshwater ecosystems in South America. Here, we performed a hydroacoustic survey to investigate the distribution and biomass patterns of fish, mostly native Orestias species, in Lago Menor, and discuss the possible causes of their spatial distribution patterns.
MATERIAL AND METHODS
Study site
A dense and extensive bed of submerged aquatic macrophytes, mostly Chara spp. (down to 10 m depth), dominates the Lago Menor sub-basin. Littoral emergent macrophytes, mostly endemic ‘Totora’ (Schoenoplectus californicus subsp. tatora), extend at < 4 m depth, which are important areas of feeding, reproduction and shelter for fish, and for traditional fisheries [22,35]. Lago Menor is divided into three bathymetric zones: The deepest zone in the northwestern sector (Chúa trench, 40 m); the mildly deep west zone (Peruvian sector, down to 20 m), southwest of the island line; and the shallow eastern zone (≤ 5 m), mostly within the Bolivian sector (see the bathymetric isolines in figure 1) [13]. Certain littoral areas of this shallow zone are affected by eutrophication. In particular, Cohana bay has been enriched for the last two decades by organic matter, nutrients, and other pollutants transported by the wastewaters from the 1.2 million inhabitants and industries of El Alto city [6].
The acoustic survey was carried out in the northern, central and southern regions of Lago Menor sub-basin during the 2015 rainy season (November; Figure 1). This study was performed in the framework of the annual fisheries binational monitoring program conducted by IMARPE (Peruvian Sea Institute), coupled with a limnological survey implemented as a collaboration between the French Research Institute for Development (IRD) and the Universidad Mayor de San Andrés (UMSA) in La Paz (Bolivia) [36]. During the rainy season, the surface water temperature increases to 16°C, unlike the dry season (11°C), and corresponds to one of the reproductive peaks of the native species (September - October; IMARPE, unpublished data). The Lake Titicaca acoustic surveys are generally conducted by IMARPE during the dry season (July - August). Herein, our acoustic survey was conducted for the first time during the rainy season, since baseline information was missing, and to complement our July 2015 (dry season) acoustic survey in Lago Menor.
We covered approximately 140 km of acoustics transects (Figure 1) at an average speed of 10 km h-1. For safety reasons the survey was performed only at daytime. Indeed, there are no luminous landmarks, while undetected reefs exist, and violent winds with strong waves can arise suddenly. Acoustic data were acquired using a scientific echo-sounder SIMRAD EK60 (Simrad - Kongsberg maritime, Horten, Norway) connected to a split-beam transducer operating at 120 kHz (ES120-7G). The transducer was frame-mounted from the port side of the ship at 0.5 m below the water surface. The equipment was calibrated as described by Foote et al. [37] and according to the constructor’s manual (SIMRAD). The pulse length was set at 0.512 ms with a maximum ping rate and 100 W power transmission. Data were recorded using the SIMRAD ER60 v2.00 software and stored digitally in SIMRAD Raw format. We estimated the sampling effort with the degree of coverage defined by Aglen [38] as the ratio between the total transect length (T in km) and the square root of the surveyed area (A in km2), that is D = T/√A. Admitted as a general guide, the degree of coverage should be at least 3.0 and preferably near to or above 6.0 [39]. With a Lago Menor surface A = 1,470 km2 [13] and a total transect length T = 140 km, our cover ratio attains D = 3.6. Although we did not reach a recommended cover ratio close to 6 [40], we slightly exceeded the 3.0 minimum level to provide relevant information about the horizontal and vertical distribution of fish in the Lago Menor sub-basin.
Acoustic data were processed using the software Matecho [41], developed in Matlab (MATLAB, release 2014a), and based on the movies3d software [42]. From visual scrutinizing and other works [43-45] we set a processing threshold at -68 dB when performing the echointegration (Figure 2). With this threshold, according to Love’s [46] equation in Parker-Stetter et al. [47], we should extract fish considering native fish size from 2.5 cm. Considering the transducer depth (0.5 m), the near field (about 1 m) where backscattering measurements are unreliable [47], and the presence of some noisy signals in the first meters (e.g. waves, remaining transducer face vibration), we excluded the layer close to the surface and sampled the water column from the 3 m depth down to 0.3 m above the bottom. The behavior of native fish known so far in Lake Titicaca indicates that they are benthic, associated with the vegetation covered bottoms [19]. So, the layer close to the surface is likely empty of this fish population. The nautical acoustic scattering coefficient (NASC or sA, in m2 nmi-2) [48] was used as an acoustic proxy to study fish biomass and distribution. We processed data by 100 m long Elementary Sampling Distance Units (ESDU). Vertically, the sA was integrated by 1 m depth layers. To test for statistical difference in fish biomass by depth strata, we applied an ANOVA coupled with a tukey’s HSD post-hoc test (α = 0.05) on the mean sA by depth layers of 5 m.
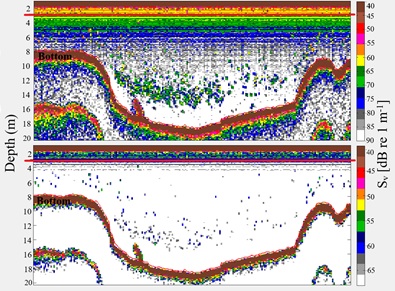 Figure 2: Typical daytime 120 kHz 20 log R echogram at -90 dB (up) and -68 dB (down) thresholds along a transect segment (near Huatajata) in Lago Menor, Lake Titicaca (see red dot in figure 1). The red line represents the 3 m layer.
Figure 2: Typical daytime 120 kHz 20 log R echogram at -90 dB (up) and -68 dB (down) thresholds along a transect segment (near Huatajata) in Lago Menor, Lake Titicaca (see red dot in figure 1). The red line represents the 3 m layer.
RESULTS AND DISCUSSION
Fish biomass distributions
The sA horizontal distribution (Figure 3) followed the bathymetric characteristics of Lago Menor. The area from Taraco peninsula and Unicachi (depth between 10 and 15 m), and the transect from Tiquina strait to Huatajata, near Chúa trench (> 20 m) showed a greater biomass in relation to the areas between the internal islands and the southern sector of Taraco (depth mainly ≤ 10 m). This distribution pattern shows that fish biomass was concentrated in specific areas, mainly in the north, Unicachi bay and between Huatajata and Tiquina strait connecting with Lago Mayor. Similarly, this pattern shows that most of fish biomass was concentrated in the Peruvian sector of Lago Menor.
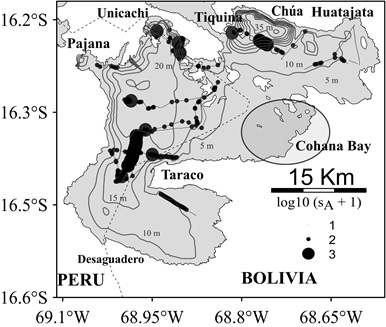 Figure 3: sA horizontal distribution during the survey in Lago Menor, Lake Titicaca. The diameter of the circles represents log10 (sA + 1). The thin lines represent the bathymetry isolines every 5 m depth.
Figure 3: sA horizontal distribution during the survey in Lago Menor, Lake Titicaca. The diameter of the circles represents log10 (sA + 1). The thin lines represent the bathymetry isolines every 5 m depth.
Vertically, the sA distribution was steady from 3 to 20 m, with a slight, but not significant, increase between 10 and 20 m, and decreased significantly at depths greater than 20 m, e.g., the euphotic zone depth (p < 0.001, f = 9.162; figure 4).
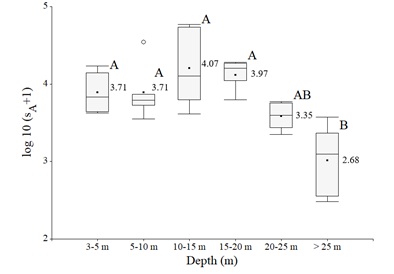 Figure 4: log10 (sA + 1) of fish acoustic measurements by depth layer. The black dots and values indicate the means, the vertical bars indicate the medians, the whiskers on the boxes extend out to the smallest and largest values within the inner fences, and the white dot denote individual observation that lies beyond the inner fences. Boxes with different letter are significantly different (P < 0.05).
Figure 4: log10 (sA + 1) of fish acoustic measurements by depth layer. The black dots and values indicate the means, the vertical bars indicate the medians, the whiskers on the boxes extend out to the smallest and largest values within the inner fences, and the white dot denote individual observation that lies beyond the inner fences. Boxes with different letter are significantly different (P < 0.05).
The vertical attenuation of the visible light (PAR, 400-700 nm) sets the lower limit for the submerged macrophytes growth [19]. Similarly, phytoplankton photosynthesis is constraint by the depth of the euphotic zone, defined as 1% of the surface irradiance [49]. Fifty-six percent of Lago Menor bottom are covered with submerged macrophytes, which maximum depth reaching 12 m (e.g., the mean PAR visible light extinction depth) [50]. Lauzanne [19] suggested that adult O.luteus and O.olivaceus were frequently found along the bottom of the Chara zone (3-10 m). Yet, most of Orestias species fed and sheltered in the submerged macrophytes zone [5,18,22], so the observed vertical distribution could reflect this behavior.
Likewise, horizontally fish biomass was mainly concentrated in areas with depth ranging from 10 to 20 m (Unicachi, northern Taraco, and Tiquina to Huatajata transect), in accordance to the vertical distribution patterns. This dense layer of fish biomass could be related to the intense solar radiation that penetrates deep (up to 12 m) [14], which makes the plankton submerge and causes that most of the aquatic biota, including fish, to find food in parts closer to the bottom. Orestias species mainly feed on amphipods and mollusks, the predominant macroinvertebrates in the whole lake, closely related to macrophytes [51], so they do not necessarily need light to forage, as they exploit the most profitable resources. Ninety-five percent of the benthic populations are found in the first 15 m of depth in Lago Menor [51]. Macrophytes areas also provide a shelter from the predation of exotic piscivorous fish, particularly the silverside, which is usually found dispersed in the south of Lago Menor, between Taraco and Desaguadero [52].
Methodological considerations
Our results interpretation depends on our assumption that the observed echoes mostly belong to Orestias. Hydroacoustic techniques alone cannot distinguish between fish species, and thus represent a major challenge [53]. However, previous knowledge on native fish in Lago Menor [5,19,23] suggest that most of the acoustically detected fish were Orestias, because they are known to dominate the fish community in Lago Menor [19], and to remain associated with the bottom, mainly not forming apparent aggregation. But O. olivaceus or Orestias gr gilsoni have been observed aggregated (pers. obs.). One species, O.ispi [19,23] is known to wander into the deep entrance sector of Tiquina strait in Lago Menor (local fishermen, pers. obs.), even though knowledge about such behavior is still limited.
Orestias demersal behavior challenges the acoustic assessments, as Orestias can hide among submerged macrophytes [22] so our acoustics assessment may have been underestimated. However, the choice of the threshold (-68 dB) to observe small fish was based on Love’s equation [46], which is successfully used in freshwater ecosystems [39,43-45,54].
Sound pulses have undoubtedly some limitations in detecting objects located near highly reflective surfaces such as the bottom [55], and the use of vertical beaming by itself also results in a rough underestimation of fish biomass or stocks particularly in the surface layers [56]. Because of the intense solar radiation in Lake Titicaca previously mentioned, the surface layer lacks fish during the day, so further hydroacoustic research into behavior of native fish will be required.
CONCLUSION
By applying for the first time the hydroacoustic method in Titicaca Lago Menor, we provide a preliminary image of fish distribution. We improve the understanding of the Orestias spatial distribution within the water column. This study encourages the implementation of hydroacoustic surveys for proper fisheries management in the shallow Bolivian and Peruvian sectors of Lake Titicaca, as well as studies on anthropogenic impacts on fish distribution. Likewise, hydroacoustic surveys can be a valuable tool for monitoring fish stock changes over years and disturbances in the fish behavior of Lago Menor of Titicaca caused by eutrophication and habitat loss. An acoustic survey is one of the tool for a complete study of the environment. In addition, seasonal studies must be carried out to analyze the influence of hydrological parameters on the behavior of fish, and therefore the impact on acoustic estimates, and thus determine which season is best for carrying out such surveys.
ACKNOWLEDGEMENT
We deeply thank IPD-PACU (Institución Pública Desconcentrada de Pesca y Acuicultura-Bolivia), MMAyA (Ministerio de Medio Ambiente y Agua-Bolivia), and ALT (Autoridad Autónoma Binacional del Lago Titicaca) for the opportunity to study the poorly known fish community distribution at the scale of Lago Menor. We are particularly grateful to LEMAR (Laboratoire des Sciences de l’Environnement Marin) for providing the acoustic equipment, and to IPD-PACU for providing the research boat and pilot.
Finally, we would like to give special acknowledgement to Prof. Dr. Tarik Méziane, Director of BOREA Laboratory (Biologie des Organismes et Écosystèmes Aquatiques, Muséum National d’Histoire Naturelle-Paris), for his support to the publication of this article.
SOURCES OF FUNDING
Our study logistics was funded by IPD-PACU, MMAyA, ALT, the research units Biologie des Organismes et Écosystèmes Aquatiques (BOREA) and Laboratoire des Sciences de l’Environnement Marin (LEMAR) of the Institut de Recherche pour le Développement (IRD), and the Instituto de Ecología (IE) of the Universidad Mayor de San Andrés (UMSA).
CONFLICT OF INTEREST
None to report.
REFERENCES
- Pauly D, Watson R, Alder J (2005) Global trends in world fisheries: Impacts on marine ecosystems and food security. Philos Trans R Soc B Biol Sci 360: 5-12.
- Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, et al. (2006) Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol Rev 81: 163-182.
- Funge-Smith S (2018) Review of the state of the world fishery resources: Inland fisheries. Food and Agriculture Organization of the United Nations, Rome.
- De Sostoa A, Caiola N, Rodríguez S, Prado P, Flores O, et al. (2010) Estudio de las comunidades de especies nativas de peces del Lago Titicaca: Caracterización ecológica y su uso como biondicadores del estado de conservación. Universidad de Barcelona/CSIC/PELT/IRTA, Lima-Perú.
- Ibañez C, Hugueny B, Garrigos YE, Zepita C, Gutierrez R (2014) Biodiversidad ictica en el Lago Titicaca. Quito, Ecuador.
- Molina C, Lazzaro X, Guédron S, Achá D (2017) Contaminación de la bahía de Cohana, Lago Titicaca (Bolivia): Desafíos y oportunidades para promover su recuperación. Ecol en boliv 52: 65-76.
- MMAyA/VRHR (2018) Plan director de la cuenca Katari y su estrategia de recuperación integral de la cuenca y del Lago Menor del Titicaca. Minisiterio de Medio Ambiente y Aguas.
- Bradley RS (2006) Climate change: Threats to water supplies in the tropical Andes. Science 312: 1755-1756.
- Valdivia C, Thibeault J, Gilles JL, García M, Seth A (2013) Climate trends and projections for the Andean Altiplano and strategies for adaptation. Adv Geosci 33: 69-77.
- Rangecroft S, Suggitt AJ, Anderson K, Harrison S (2016) Future climate warming and changes to mountain permafrost in the Bolivian Andes. Clim Change 137: 231-243.
- Cuvier GL, Valenciennes A (1846) Des Orestias. In: Cuvier GL, Valenciennes A (eds.) Histoire naturelle des poissons. Library, Paris.
- Lüssen A, Falk TM, Villwock W (2003) Phylogenetic patterns in populations of Chilean species of the genus Orestias (Teleostei: Cyprinodontidae): Results of mitochondrial DNA analysis. Mol Phylogenet Evol 29: 151-160.
- Dejoux C, Iltis A (1992) Lake Titicaca: a synthesis of limnological knowledge. Springer, Dordrecht, Netherlands.
- Lazzaro X, Gamarra C (2014) Funcionamiento limnológico y fotobiología del Lago Titicaca. In: Línea base de conocimientos sobre los recursos hidrológicos e hidrobiológicos en el sistema TDPS con enfoque en la cuenca del Lago Titicaca. Quito, Ecuador.
- Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403: 853.
- Vila I, Pardo R, Scott S (2007) Freshwater fishes of the Altiplano. Aquat Ecosyst Health Manag 10: 201-211.
- Parenti L (1984) A taxonomic revision of the Andean killifish genus Orestias (Cyprinodontiformes, Ciprinodontidae). Bull Am Mus Nat Hist 178: 214
- Loubens G (1989) Observations on the fishes of the Bolivian part of Lake Titicaca. Iv. Orestias, Salmo gairdneri and Management problems. Rev Hydrobiol Trop 22: 157-177.
- Lauzanne L (1992) Native species the Orestias. In: Dejoux C, Iltis A (eds.) Lake Titicaca: A synthesis of limnological knowledge. Springer, dordrecht, Netherlands.
- Garrigos YE, Hugueny B, Koerner K, Ibañez C, Bonillo B, et al. (2013) Non-invasive ancient DNA protocol for fluid-preserved specimens and phylogenetic systematics of the genus Orestias (Teleostei: Cyprinodontidae). Zootaxa 3640: 373-394.
- Takahashi T, Moreno E (2015) A rad-based phylogenetics for Orestias fishes from Lake Titicaca. Mol Phylogenet Evol 93: 307-317.
- Guerlesquin M (1992) Charophythes. In: Dejoux C, Iltis A (eds.) Lake Titicaca: A synthesis of limnological knowledge. Springer, Dordrecht, Netherlands.
- Lauzanne L (1982) Les Orestias (Pisces, Cyprinodontidae) du petit Lac Titicaca. Rev d’Hydrobiologie Trop 15: 39-70.
- Treviño H, Levy DA, Northcote TG (1984) Pesca experimental en aguas negras y limpias del litoral de la Bahia de Puno, Lago Titicaca, Boletin. puno, Perú. Pg no: 8: 34.
- Northcote TG (1989) Pollution in Lake Titicaca, Peru: Training, research, and management. IUCN, Puno, Peru.
- Loubens G, Osorio F (1992) Introduced species 2. Basilichthys bonariensis (the “pejerrey”). In: Dejoux C, Iltis A (eds.) Lake Titicaca: A synthesis of limnological knowledge. Springer, Dordrecht, Netherlands.
- Wurstbaugh WA, Tapia RA (1988) Mass mortality of fishes in Lake Titicaca (Peru-Bolivia) associated with the protozoan parasite ichthyophthirius multifiliis. Trans Am Fish Soc 117: 213-217.
- Deceliere-Vergès C, Guillard J (2008) Assessment of the pelagic fish populations using cen multi-mesh gillnets: consequences for the characterization of the fish communities. Knowl Manag Aquat Ecosyst 16.
- Simmonds J, MacLennan D (2005) Fisheries acoustics: Theory and practice, (2nd edn). Blackwell Science, Oxford, UK.
- Rudstam LG, Jech M, Parker-Stetter SL, Horne JK, Sullivan PJ, et al. (2012) Fisheries Acoustics. In: Zale AV, Parrish DL, Sutton TM (eds.) Fisheries techniques, (3rd edn). American Fisheries Society, Bethesda, MD, USA.
- Godlewska M, ?wierzowski A, Winfield I (2004) Hydroacoustics as a tool for studies of fish and their habitat. Ecohydrol Hydrobiol 4: 417-
- Guillard J, Darchambeau F, Mulungula PM, Descy JP (2012) Is the fishery of the introduced tanganyika sardine (Limnothrissa miodon) in Lake Kivu (east Africa) sustainable? J Gt Lakes Res 38: 524-533.
- Yule DL, Evrard LM, Cachera S, Colon M, Guillard J (2013) Comparing two fish sampling standards over time: Largely congruent results but with caveats. Freshw Biol 58: 2074-2088.
- Tessier A, Richard A, Masilya P, Mudakikwa ER, Muzana A, et al. (2020) Spatial and temporal variations of limnothrissa miodon stocks and their stability in Lake Kivu. J Gt Lakes Res 10: 380-1330.
- Monroy-López M (2014) Principales impactos antrópicos y sus efectos sobre la comunidad de peces del Lago Titicaca. Tesis doctoral, Universidad de Barcelona.
- Lazzaro X, Alcoreza P, Lanza WG, Flores A, Fernández P, et al. (2016) Expedición binacional de evaluación de recursos pesqueros y condiciones limnológicas del Lago Titicaca – Cr.1507-08 – jul-ago 2015-Informe técnico del equipo boliviano. IE-UMSA & BOREA-IRD, La Paz, Bolivia.
- Foote KG, Knudsen H, Vestnes D, Maclennan D, Simmonds E (1987) Calibration of acoustic instruments for fish density estimation: A practical guide. Int Counc Explor Sea Journal Mar Sci 144: 1-69.
- Aglen A (1983) Rrandom errors of acoustic fish abundance estimates in relation to the survey grid density applied. In: Nakken O, Venema SC (eds.). FAO Fisheries Report-Food and Agriculture Organization of the United Nations. FAO Fish. Rep, Bergen, Norway.
- Emmrich M, Winfield IJ, Gguillard J, Rustadbakken A, Vergès C, et al. (2012) Strong correspondence between gillnet catch per unit effort and hydroacoustically derived fish biomass in stratified lakes. Freshw Biol 57: 2436-2448.
- Aglen A (1989) Empirical results on precision-effort relationships for acoustic surveys. International council for the exploration of sea council meeting paper.
- Perrot Y, Brehmer P, Habasque J, Roudaut G, Behagle N, et al. (2018) Matecho: An open-source tool for processing fisheries acoustics data. Acoust Aust 46: 241-248.
- Trenkel VM, Berger L, Bourguignon S, Doray M, Fablet R, et al. (2009) Overview of recent progress in fisheries acoustics made by ifremer with examples from the Bay of Biscay. Aquat Living Resour 22: 433-445.
- Guillard J, Lebourges-Daussy A, Balk H, Colon M, Jó?wik A, et al. (2014) Comparing hydroacoustic fish stock estimates in the pelagic zone of temperate deep lakes using three sound frequencies (70, 120, 200 kHz). Inland Waters 4: 435-444.
- Mouget A, Goulon C, Axenrot T, Balk H, Lebourges-Dhaussy A, et al. (2019) Including 38 kHz in the standardization protocol for hydroacoustic fish surveys in temperate lakes. Remote Sens Ecol Conserv 5: 332-
- Tessier A, Cottet M, Kue K, Chanudet V, Descloux S, et al. (2020) Low input of offshore areas to fisheries in a large tropical reservoir in Lao PDR. Limnology 21: 73-86.
- Love RH (1977) Target strength of an individual fish at any aspect. J Acoust Soc Am 62: 1397-1403.
- Parker-Stetter SL, Rudstam LG, Sullivan PJ, Warner DM (2009) Standard operating procedures for fisheries acoustic surveys in the great lakes: Prepared for the Study Group on Fisheries Acoustics in the Great Lakes, Great Lakes Fishery Commission. Great Lakes Fishery Commission, USA.
- Maclennan D, Fernandes P, Dalen J (2002) A consistent approach to definitions and symbols in fisheries acoustics. ICES J Mar Sci 59: 365-369.
- Lazzaro X (1981) biomasses, peuplements phytoplanctoniques et production primaire du Lac Titicaca. Rm Hydrobiol trop 1: 319-380.
- Northcote TG (2000) Ecological interactions among an Orestiid (Pisces: Cyprinodontidae) species flock in the littoral zone of Lake Titicaca. Advances in Ecological Research 31: 399-420.
- Dejoux C (1992) Benthic populations. Distribution and annual variations. In: Dejoux C, Iltis A (eds.). Lake Titicaca: A synthesis of limnological knowledge. Springer, Dordrecht, Netherlands.
- IMARPE (2015) Anuario científico tecnológico IMARPE. Instituto del Mar del Perú, lima, Perú.
- Horne JK (2000) Acoustic approaches to remote species identification: A review. Fish Oceanogr 9: 356-371.
- Sobradillo B, Boyra G, Martinez U, Carrera P, Peña M, et al. (2019) Target strength and swimbladder morphology of Mueller’s pearlside (Maurolicus muelleri). Sci Rep 9: 17311.
- Mitson RB (1983) Acoustic detection and estimation of fish near the sea-bed and surface. FAO, Rome.
- Kubecka J, Wittingerova M (1998) Horizontal beaming as a crucial component of acoustic fish stock assessment in freshwater reservoirs. Fish Res 35: 99-106.
Citation: Loayza E, Bertrand A, Guillard J, La Cruz L, Lebourges-Dhaussy A, et al. (2020) First Hydroacoustic Assessment of Fish Abundance and Distribution in the Shallow Sub-basin of Lake Titicaca. J Aquac Fisheries 4: 034.
Copyright: © 2020 Erick Loayza, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

