
Gold Nanopellets a Unique Platform for Electrochemical Ultra-Low Detection of Ochratoxin A
*Corresponding Author(s):
Tinku BasuAmity Institute Of Nanotechnology, Amity University Uttarpradesh, Noida, Uttar Pradesh, India
Tel:+91 1204392510,
Email:tbasu@amity.edu
Abstract
Anisotropic nano structures offer exceptional optoelectronic properties when contrasted with isotropic elements and discover application in therapeutics and molecular imaging separated from opto electronic gadgets. Attributable to remarkable optical properties, it is not exploited in electrochemistry up to the fullest extent. The present paper portrays the manufacture of an electrochemical transducer where Gold Nano Pellet (AuNPl) is permitted to develop directly on Indium Tin Oxide (ITO) covered glass electrode utilizing seed-mediated and template free approach which in turn is connected to create Ochratoxin A (OTA) immunosensor (BSA/aOTA/Cys/AuNPl/ITO). The structural and morphology of the proposed electrode are researched utilizing UV-Visible spectroscopy, Cyclic Voltammetry, EDAX, FESEM and AFM. The manufacturing procedure and sensing characteristics of OTA immunosensor are examined utilizing Cyclic Voltammetry (CV). Under optimum experimental conditions, the straight range created by the immunoelectrode is observed to be as 1-20pg mL-1. The LOD and sensitivity of the immunoelectrode are recorded as 0.6pg mL-1 and 2.89 × 105Apg-1 mL cm-2, individually. Ultralow sensing and high sensitivity can be ascribed to high conductivity of AuNPl/ITO electrode and low loading of monoclonal anti Ochratoxin A (aOTA). Least interference is seen in spiked coffee tests due to monoclonal aOTA. The created transducing platform demands a potential application in electrochemical ultra low detection of toxins.
Keywords
INTRODUCTION
Extensive insight over knowledge of a nanomaterial is a fundamental factor in building of later and present day gadgets with required functions. Because of this reason, much attention has been committed for outlining of materials in nano administration with complex shapes. Anisotropic nanomaterials are bearing ward showing startling highlights contrasted with isotropic particles to an outstanding degree with applications in numerous devices. Having diverse surface territory and crystallographic facets, anisotropic nanostructures offer highly tunable optical, optoelectronic, electrochemical and magnetic properties as for viewpoint proportion and shape. In the bunch of anisotropic nano structures, gold nanoparticles assume a pivotal role due to its unique shape dependent electron or hole confinement, surface plasmon, tunable electron transfer kinetics, biocompatibility and find enormous application in catalysis, molecular recognition, diagnostics, imaging and therapeutics.
The variation in longitudinal wave in nanobelts, nanocombs [1], nanotubes or nanowires [2], of gold like other noble metals showcase outstanding electro catalytic activities, magical detecting capacity to trace biomolecule with enhanced sensitivity [3,4], imaging of Bacillus subtilis spores (a simulant of Bacillus anthracis ) utilizing Two-Photon Radiance (TPR) microscopy [5], and recognizing ability of heavy metals for example, Cu2+ and Hg2+ ions [2], and so forth. The sensing ability of anisotropic nano structured gold is fundamentally limited to optical gadgets programmed on fluorescence, SERS and surface plasmon resonance [2]. Investigation on anisotropic nano sized gold as an electrochemical detecting framework is yet to be investigated. Few illustrations such as bimetallic Au-Pt nanowires for continuous no enzymatic impedimetric sensing of glucose [2,6], and one dimensional Gold Nanostructure (AuNs) relied upon thiol functionalized Graphene Oxide (GO-SH) for identification of free cholesterol up to a level of 0.2nM within 5seconds and so on [7], can be referred to.
Mutagenic and cancer-causing mycotoxins, for example, aflatoxins, sterigmatocysin, ochratoxins or fuminisin contaminate the food chain and impose a serious medical problem for both the animal and plant species [6]. Among every one of the mycotoxins, nine number of Ochratoxins have been recognized till date with Ochratoxin A (OTA) being one of the most pervasive and perilous mycotoxins and has received widespread attention in regards to the food pollution and safety [8,9]. It is recognized as a significant nephrotoxic, teratogenic, cancer-causing and immunosuppressive operator which significantly influences the animal, generally rodent, mice and other mammalian species. Studies have demonstrated that OTA at cell level outcomes in expanded oxidative stress and abnormal state of the poison in people causing “Balkan endemic nephropathy”. Numerous nations over the globe have set regulatory levels for the usage of OTA depending on the food commodities consumed [8,10]. Among all the countries, Europe is known to be a prolific consumer of the OTA-contaminated foods and the European Union has fixed the amount of OTA to be permitted in the food items which is 5ppb for crude grains, 2ppb for wines and 0.5ppb for child nourishments (Commission Regulation No. 123/2005) [8]. Usually, OTA is found in minute amount starting from nanograms to micrograms per gram of the food product. Subsequently, modern instrumental investigation, for example, High-Performance Liquid Chromatography (HPLC) or Gas Chromatography (GC) coupled to ultraviolet-visible, fluorescence or mass spectrometry [11], and molecular recognition element such as Enzyme-Linked Immunosorbent Assay (ELISA) are investigated to detect OTA precisely and particularly in the food products [12]. Attributable to high cost, need of sophisticated research laboratory and trained manpower, complex sample preparation steps, and lacking of exactness in the ultra low detection range, these instruments are not really used to detect OTA in the natural way of life unless it is compulsory which brings about constant intake of OTA contaminated food causing serious and interminable effects in animal and human health. This could raise the need of developing reliable, smart, portable, rapid and cost effective sensing tool for checking of OTA in food items.
In the past years, biosensors have emerged as an exceptionally assertive alternative to the conventional analysis protocols because of its high sensitivity, selectivity, technical simplicity, and portability [13,14]. Studies reveal that electrochemical (amperometric and impedimetric) biosensors are the most popular one due to low cost, ease of operation, direct measurement, high sensitivity, fast response time and ease of scale up as compared to piezoelectric or optical biosensors [15]. The last decade has seen the development of many immunosensors for the detection of OTA based on the strong interaction between the antibody-antigen [16]. Vidal et al., have demonstrated a labeled electrochemical immunosensor for OTA detection within wide range of (104 to 1000ng mL-1) on Screen Printed Electrode (SPE). They have employed an OTA-peroxidase enzyme conjugate and magnetic bead for signal amplification. Radi et al., have reported a label-free electrochemical impedimetric immunosensor for detection of OTA with a detection limit of 0.5ng mL-1 [17]. The Electrostatic Impedance Spectroscopy (EIS) has resulted in a linear relationship between the charge transfer resistance and the concentration of OTA within a range of 1-20ng mL-1. Electrochemical enzyme linked Immunosorbent assays based on screen printed electrodes have been developed by Alarcon et al., for the rapid detection of OTA in wheat with a detection limit of 0.4µg/kg and this method uses the samples directly with the assay without any clean-up [18]. Zamfir et al., have developed a label-free immunosensor based on Magnetic Nanoparticles (MNPs) for high selectivity of OTA [19]. Ngundi et al., have proposed an array biosensor based on competitive immunoassay format for the detection of OTA in cereals and beverages and achieved a detection limit of 3.8 to 100ng g-1 for cereals and a LOD between 7 to 38ng g-1 for coffee and wine [20]. Development of OTA biosensor using OTA specific aptamer has been extensively investigated [21,22]. The investigation on OTA biosensor highlights lights couple of vital confinement, for example, limit recognition run, low affectability, poor time span of usability, detection limit. The inspiration for the present examination is to build up a mark free direct immunosensor for ultra Low Recognition Limit (LOD) of OTA.
In the present investigation, anti OTA immobilized on thiol functionalized gold nano pellet, as grown directly on Indium Tin Oxide coated glass sheet (ITO) is employed to detect OTA up to a level of 0.6pg mL-1. The nano Gold Pellets (AuNPl) are grown on ITO surface using Au seeds, ascorbic acid and CTAB [23-26]. The fabricated AuNPl/ITO electrode is functionalized with cystamine to impart amine moieties on the surface. Further this functionalized electrode is allowed to bioconjugated with activated monoclonal antibodies of OTA (aOTA). The fabricated immunoelectrode is designated as BSA/aOTA/Cys/AuNPl/ITO is used to recognize OTA in spiked coffee samples.
MATERIALS AND METHODS
Chemicals and reagents
Instrumentation
Solution preparation
Preparation of ITO substrates
Fabrication of AuNPl/ITO electrode
Fabrication of the OTA immunoelectrode (BSA/aOTA/Cys/AuNPl/ITO)
Detection of OTA
Stability studies
RESULTS AND DISCUSSION
Morphological analysis
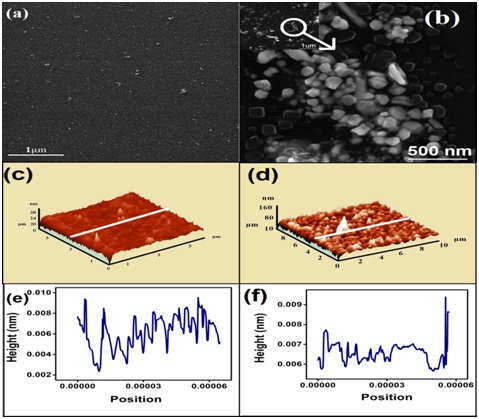 Figure 1: FESEM images of surface morphology of (a) Au seeds. (b) AuNPl in high magnification at 500nm. Inset of (b) shows FESEM image of AuNPl at low magnification of 1µm. AFM images of surface topography. (c) Au seeds for 5µm × 5µm area. (d) AuNP1 for 10µm × 10µm area of developed electrode. Line profile of AFM height image. (e) Au seeds/ITO and (f) AuNP1/ITO.
Figure 1: FESEM images of surface morphology of (a) Au seeds. (b) AuNPl in high magnification at 500nm. Inset of (b) shows FESEM image of AuNPl at low magnification of 1µm. AFM images of surface topography. (c) Au seeds for 5µm × 5µm area. (d) AuNP1 for 10µm × 10µm area of developed electrode. Line profile of AFM height image. (e) Au seeds/ITO and (f) AuNP1/ITO.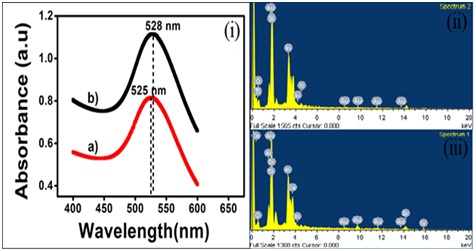 Figure 2: (i) UV-Vis Spectra of a) Au seed and b) Gold nanopellets within 400nm to 650nm and EDX analysis of. (ii) Au seeds/ITO and (iii) AuNPl/ITO.
Figure 2: (i) UV-Vis Spectra of a) Au seed and b) Gold nanopellets within 400nm to 650nm and EDX analysis of. (ii) Au seeds/ITO and (iii) AuNPl/ITO.Cyclic voltammetry:
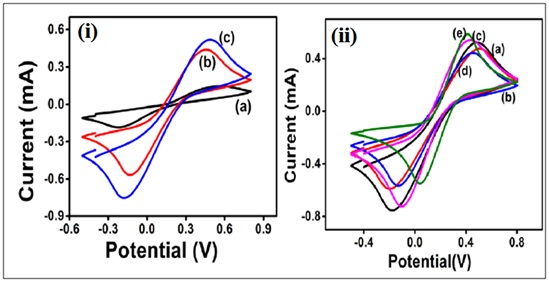 Figure 3: (i) CV of a) Bare ITO electrode b) Au seed/ITO and c) AuNPl/ITO at a scan rate of 100mV/S within a potential -0.4V to 0.8V in PBS buffer containing Fe(CN)63−/4−. (ii) CV of immunoelectrode at each modification step showing a) AuNPl/ITO b) Cys/AuNPl/ITO c) aOTA/Cys/AuNPl/ITO and d) BSA/aOTA/Cys/AuNPl/ITO (e) OTA/ BSA/aOTA/Cys/AuNPl/ITO at a scan rate of 100m V/S within a potential -0.4V to 0.8V in PBS buffer containing Fe(CN)63−/4−.
Figure 3: (i) CV of a) Bare ITO electrode b) Au seed/ITO and c) AuNPl/ITO at a scan rate of 100mV/S within a potential -0.4V to 0.8V in PBS buffer containing Fe(CN)63−/4−. (ii) CV of immunoelectrode at each modification step showing a) AuNPl/ITO b) Cys/AuNPl/ITO c) aOTA/Cys/AuNPl/ITO and d) BSA/aOTA/Cys/AuNPl/ITO (e) OTA/ BSA/aOTA/Cys/AuNPl/ITO at a scan rate of 100m V/S within a potential -0.4V to 0.8V in PBS buffer containing Fe(CN)63−/4−.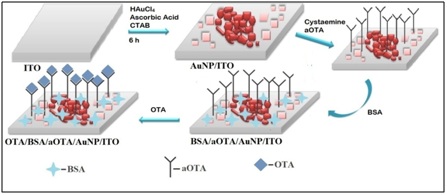 Figure 4: Schematic illustration of the stepwise of fabrication of BSA/aOTA/Cys/AuNPl/ITO immunoelectrode. (a) Bare ITO (b) AuNP1/ITO (c) aOTA/Cys/AuNP1/ITO (d) BSA/aOTA/Cys/AuNP1/ITO (e) OTA/BSA/aOTA/Cys/AuNP1/ITO.
Figure 4: Schematic illustration of the stepwise of fabrication of BSA/aOTA/Cys/AuNPl/ITO immunoelectrode. (a) Bare ITO (b) AuNP1/ITO (c) aOTA/Cys/AuNP1/ITO (d) BSA/aOTA/Cys/AuNP1/ITO (e) OTA/BSA/aOTA/Cys/AuNP1/ITO.Operational parameters of BSA/aOTA/Cys/AuNPl/ITO immunosensor
Response studies of the immunosensor
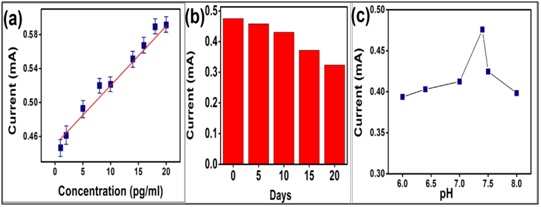 Figure 5: (a) Calibration curve obtained between the magnitudes of current (mA) versus concentration of aOTA (pg mL-1). (b) Shelf life study of the immunoelectrode for a period of 20 days. (c) Effect of pH on Ipa of BSA/aOTA/Cys/AuNP1/ITO electrode.
Figure 5: (a) Calibration curve obtained between the magnitudes of current (mA) versus concentration of aOTA (pg mL-1). (b) Shelf life study of the immunoelectrode for a period of 20 days. (c) Effect of pH on Ipa of BSA/aOTA/Cys/AuNP1/ITO electrode. The repeatability of the BSA/aOTA/Cys/AuNPl/ITO immunosensor is confirmed by measuring the same sample by five times and then the Relative Standard Deviation (RSD) is calculated. A 4.2% of RSD for the repeated measurement of the same sample demonstrates a good repeatability of the electrochemical immunosensor.
Minimal interference is observed due to the presence of monoclonal antibody while testing in OTA spiked coffee extract. Table 1 distinguishes the results with Relative Error (% RE). The relative error was found to be in the range of 14.5%. The error % is attributed to extremely low concentration of OTA (in the pico gram range). Table 2 shows the characteristics of our developed immunosensor and some latest sensors developed for the detection of OTA. A comparative study with the reported literature clearly indicates that the presently developed electrode exhibits an abnormally low detection limit and entire detection range lies within the pico gram range.
| Sample No. | Actual Spiked Concentration (pg/mL) | Experimental Concentration (pg/mL) |
% Relative Error (% RE) |
| 1 | 20 | 17.1 | 14.5 |
| 2 | 16 | 15.3 | 4.8 |
| 3 | 5 | 5.6 | 12 |
Table 1: Detection of OTA in spiked coffee extract.
| S. No | Matrix | Detection Range | LOD | Sensitivity | Reference |
| 1 | AptOTA-MBs/SPE | 0.78-8.74ng mL-1 | 0.07ng mL-1 | Bonel et al. [20] | |
| 2 | OTA-AP graphene/SPE | 0.05-2.5µgL-1 | 0.40µg/kg-1 | Alarcon et al. [18] | |
| 3 | DApt/MB | - | 0.11ng mL-1 | Barthelmebs et al. [28] | |
| 4 | 4-CP/aOTA | 1-20ng mL-1 | 0.5ng mL-1 | Radi et al. [17] | |
| 5 | aOTA/MNPSs/Au | 0.01-5ng mL-1 | 0.01ng mL-1 | Zamfir et al. [19] | |
| 6 | MIP/MWCNT/GCE | 0.050-1.0μM | 0.004μM | Pacheco et al. [29] | |
| 7 | ThiolatedaptOTA/Au | 10-8-102ng mL-1 | Cheng et al. [22] | ||
| 8 | aOTA/BSA/Au | 2.5-100ng mL-1 | Badea et al. [30] | ||
| 9 | aOTA-peroxidase/MB/SPE | 10-4-1,000ngL-1 | Vidal et al. [31] | ||
| 10 | Anti-OTA/Protein-A/PSi | 0.001-100ng/ML | 4.4pg mL-1 | 0.01-5ng/mL | Myndrul et al. [32] |
| 11 | Glass/ZnO-NRs/Protein-A/BSA/Anti-OTA | 10-4-20ng mL-1 | 10-2ng mL-1 | 0.1-1ng/mL | Viter et al. [33] |
| 12 | aOTA/AuNPl/ ITO | 1-20pg mL-1 | 0.6pg mL-1 | 2.89 × 10-5Apg mL-1 | Present |
Table 2: A comparative report from the literature on the various electrochemical sensors of OTA.
Note: AptOTA-OTA Aptamer; SPE-Screen Printed Electrode; DApt-DNA aptamer, MB-Magnetic Bead; BSA-Bovine Serum Albumin; CP-4-Carboxyphenyl; Psi-Porous Silicon.
Shelf life of BSA/aOTA/Cys/AuNPl/ITO immunoelectrode
CONCLUSION
In this work, a label free immunosensor is developed based on AuNPl for the ultra-low detection of OTA. The immunosensor exhibits a detection range of 1-20pg mL-1, LOD of 0.6pg mL-1, sensitivity of 2.89×10-5Apg-1 mLcm-2 and good association constant of 1.3mM is obtained.
The developed immunosensor is found to be highly propitious for ultra-low detection of OTA. AuNPl on ITO posses irregular geometry, indefinite shapes and sizes. The developed electrode is tunable and can be further modified to obtain a single morphology. Increasing the growth time to 18h-22h results in formation of gold nanorods. Preparation of nano rod also comprises various other shapes of nanopellet. The ultra low detection of OTA could be attributed by the anisotropic AuNPl, loading of very low concentration of a OTA and binding via Fc group with electrode surface to expose Fab part to expose towards OTA. The proposed electrode definitely promise high potential for application in ultra low detection of analyzes.
ACKNOWLEDGMENT
Authors gratefully acknowledge Inter University Accelerator Center, New Delhi for their support in characterizing the electrodes and materials which are developed in the present investigation.
REFERENCES
- Seo D, Park JC, Song H (2006) Polyhedra gold nanocrystals with O h symmetry: From octahedra to cubes. J Am Chem Soc 128: 14863-14870.
- Li N, Zhao P, Astruc D (2014) Anisotropic gold nanoparticles?: Synthesis, properties, applications, and toxicity. Angew Chem Int Ed Engl 53: 1756-1789.
- Au L, Chen Y, Zhou F, Camargo PHC, Lim B, et al. (2008) Synthesis and optical properties of cubic gold nanoframes. Nano Res 1: 441-449.
- Cobley CM, Xia Y (2010) Engineering the properties of metal nanostructures via galvanic replacement reactions. Materials Science and Engineering: R: Reports 70: 44-62.
- He W, Henne WA, Wei Q, ZhaoY, Doorneweerd DD, et al. (2008) Two-photon luminescence imaging of bacillus spores using peptide-functionalized gold nanorods. Nano Res 1: 450.
- Mayorga-Martinez CC, Guix M, Madrid RE, Merkoçi A (2012) Bimetallic nanowires as electrocatalysts for nonenzymatic real-time impedancimetric detection of glucose. Chem Commun 48: 1686-1688.
- Nandini S, Nalini S, Reddy MBM, Suresh GS, Melo JS, et al. (2016) Synthesis of one-dimensional gold nanostructures and the electrochemical application of the nanohybrid containing functionalized graphene oxide for cholesterol biosensing. Bioelectrochemistry 110: 79-90.
- Pohland AE, Nesheim S, Friedman L (1992) Ochratoxin A: A review. Pure & Appl Chem 64: 1029-1046.
- Petzinger E, Weidenbach A (2002) Mycotoxins in the food chain: The role of ochratoxins. Livestock Production Science 76: 245-250.
- Fernández-Cruz ML, Mansilla ML, Tadeo JL (2010) Mycotoxins in fruits and their processed products: Analysis, occurrence and health implications. Journal of Advanced Research 1: 113-122.
- Turner NW, Subrahmanyam S, Piletsky SA (2009) Analytical methods for determination of mycotoxins: A review. Anal Chim Acta 632: 168-180.
- Yu FY, Vdovenko MM, Wang JJ, Sakharov IY (2011) Comparison of enzyme-linked immunosorbent assays with chemiluminescent and colorimetric detection for the determination of ochratoxin A in food. J Agric Food Chem 59: 809-813.
- Chauhan R, Singh J, Sachdev T, Basu T, Malhotra BD (2016) Recent advances in mycotoxins detection. Biosens Bioelectron 81: 532-545.
- Chauhan R, Singh J, Solanki PR, Manaka T, Iwamoto M, et al. (2016) Label-free piezoelectric immunosensor decorated with gold nanoparticles: Kinetic analysis and biosensing application. Sensors and Actuators B: Chemical 222: 804-814.
- Kaushik A, Arya SK, Vasudev A, Bhansali S (2013) Recent advances in detection of ochratoxin-A. Open Journal of Applied Biosensor 2: 1-11.
- Vidal JC, Bonel L, Ezquerra A, Hernández S, Bertolín JR, et al. (2013) Electrochemical affinity biosensors for detection of mycotoxins: A review. Biosens Bioelectron 49: 146-158.
- Radi AE, Muñoz-Berbel X, Lates V, Marty JL (2009) Label-free impedimetric immunosensor for sensitive detection of ochratoxin A. Biosens Bioelectron 24: 1888-1892.
- Alarcón SH, Palleschi G, Compagnone D, Pascale M, Visconti A, et al. (2006) Monoclonal antibody based electrochemical immunosensor for the determination of ochratoxin A in wheat. Talanta 69: 1031-1037.
- Zamfir L, Geana I, Bourigua S, Rotariu L, Bala C, et al. (2011) Highly sensitive label-free immunosensor for ochratoxin a based on functionalized magnetic nanoparticles and EIS/SPR detection. Sensors & Actuators: B Chemical 159: 178-184.
- Ngundi MM, Shriver-Lake LC, Moore MH, Lassman ME, Ligler FS, et al. (2005) Array biosensor for detection of ochratoxin a in cereals and beverages. Anal Chem 77: 148-154.
- Bonel L, Vidal JC, Duato P, Castillo JR (2011) An electrochemical competitive biosensor for ochratoxin a based on a DNA biotinylated aptamer. Biosens Bioelectron 26: 3254-3259.
- Cheng L, Qu H, Teng J, Yao L, Xue F, et al. (2017) Extraordinary tunable dynamic range of electrochemical aptasensor for accurate detection of ochratoxin a in food samples. Food Science and Human Wellness 6: 70-76.
- Miranda A, Malheiro E, Eaton P, Carvalho PA, de Castro B, et al. (2011) Synthesis of gold nanocubes in aqueous solution with remarkable shape-selectivity. Journal of Porphyrins and Phthalocyanines 15: 441-448.
- Xia Y, Xiong Y, Lim B, Skrabalak SE (2009) Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew Chem Int Ed Engl 48: 60-103.
- Sajanlal PR, Sreeprasad TS, Samal AK, Pradeep T (2011) Anisotropic nanomaterials: Structure, growth, assembly, and functions. Nano Rev 2.
- Priecel P, Salami HA, Padilla RH, Zhong Z, Lopez-Sanchez JA (2016) Anisotropic gold nanoparticles: Preparation and applications in catalysis. Chinese Journal of Catalysis 37: 1619-1650.
- Debgupta J, Pillai VK (2013) Thiolated graphene--a new platform for anchoring CdSe quantum dots for hybrid heterostructures. Nanoscale 5: 3615-3619.
- Barthelmebs L, Hayat A, Limiadi AW, Marty JL, Noguer T (2011) Electrochemical DNA aptamer-based biosensor for OTA detection, using super paramagnetic nanoparticles. Sensors and Actuators B: Chemical 156: 932- 937.
- Pacheco JG, Castro M, Machado S, Barroso MF, Nouws HPA, et al. (2015) Molecularly imprinted electrochemical sensor for ochratoxin a detection in food samples. Sensors and Actuators B: Chemical 215: 107-112.
- Badea M, Floroian L, Restani P, Cobzac SC, Moga M (2016) Ochratoxin a detection on antibody- immobilized on BSA-functionalized gold electrodes. PLoS One 11: 0160021.
- Vidal JC, Bonel L, Ezquerra A, Duato P, Castillo JR (2012) An electrochemical immunosensor for ochratoxin a determination in wines based on a monoclonal antibody and paramagnetic microbeads. Anal Bioanal Chem 403: 1585-1593.
- Myndrul V, Viter R, Savchuk M, Shpyrka N, Erts D, et al. (2018) Porous silicon based photoluminescence immunosensor for rapid and highly-sensitive detection of Ochratoxin A. Biosens Bioelectron 102: 661-667.
- Viter R, Savchuk M, Iatsunskyi I, Pietralik Z, Starodub N, et al. (2018) Analytical, thermodynamical and kinetic characteristics of photoluminescence immunosensor for the determination of Ochratoxin A. Biosens Bioelectron 99: 237-243.
Citation: Nisar S, Jairath A, Basu T (2018) Gold Nanopellets a Unique Platform for Electrochemical Ultra-Low Detection of Ochratoxin A. J Food Sci Nut 4: 032.
Copyright: © 2018 Sumaya Nisar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

