
Growth and Absorption Response of Perna viridis (Lin, 1758) To Fish Farm Water Quality in Valladolid
*Corresponding Author(s):
Melendres ARDepartment Of Fisheries, Cebu Technological University, Carmen Campus, Cebu, Philippines
Tel:+63 9089607537,
Email:adrianojr.melendres@ctu.edu.ph
Abstract
The extractive species, Perna viridis has been integrated into the existing commercial finfish culture of Chanos chanos and Trachinotus blochii in Valladolid, Carcar Bay, Eastern Cebu, Philippines, following the Integrated Multitrophic Aquaculture (IMTA) concept. Two sampling sites (573 m apart) were selected; the experimental site, and control site. Biomass and growth rates of this species were measured monthly, along with physico-chemical parameters in the two sites, monitored for a period of up to one year. Results showed that P.viridis adopted in the fish farming site, where water temperature, salinity, pH, dissolved oxygen were found to be well within the optimal range. Growth of P.viridis however, aside from the seasonal changes in the weather pattern that influenced water movement, was mainly affected by predators and fouling organisms that were present during culture. These factors have reduced their potential biomass and growth rates. The introduction of green mussels in the fish farm appeared to have checked the adverse effects of fish culture activities whose daily inputs of commercial feeds, could led to the suspended solids. Transplanted green mussels grew into marketable after 7 months with average Specific Growth Rate (SGR) reached highest level at 1.03% in February 2019. The use of IMTA concept, following the culture of green mussels within the cages area is found to work well in the culture of milkfish/pompano, and should be promoted and expanded in the Philippines.
Keywords
Commercial fish culture; Chanos chanos; IMTA; Perna (=Mytilus) viridis; Trachinotus blochii
Introduction
A study by Liutkus, et al. [1], shows that mussels can absorb up to 86% of feces (organic) and 90% of dissolved feeds (inorganic form) from salmon farming. The growth and biochemical profile of mussels are greatly affected by the environment in which they exist such as the type of organic matter from fish farm (biodeposition) [2]. However, storms, tidal cycles, and current speed can resuspend bottom materials, increasing the concentration of seston in the upper portion, thus reducing the food available for bivalve filter-feeders at the bottom [3,4]. Further, current speed and flow also affect phytoplankton transport, mussel clearance rate, and settling of organic and inorganic material on the cultured bivalves, while extreme waves can cause mortality and limit food intake [5]. Complex interaction between all the aforementioned environmental parameters that influence growth and health of the animal must be considered. Furthermore, Bayne & Worrall [6], showed that food quantity, quality, physiological availability, and temperature were all affecting Perna viridis somatic growth and fecundity, thus emphasizing the importance of evaluating environmental characteristics and how they alter mussel condition, and the final quality of the harvested product.
Most of the fish farming activities using the IMTA approach have usually been done in temperate areas, but such concept can also be applied to tropical settings [7]. The increasing demand for green mussels from the current world markets will have positive and negative implications for the businesses providing these products and regarding its environmental sustainability due to culture operations. On the other hand, aquaculture activities must be ecologically and environmentally responsible to satisfy the increasingly discriminating consumers who demand the highest quality standard for seafoods. The IMTA concept is yet to be fully developed in Asian countries to realize the expectations of the market. In the Philippines, this concept is not yet widely acceptable to the traditional fish farm operators from the fact that it is still under the preliminary stages of development in the country and due to the lack of knowledge on its applicability. In fact, proper IMTA management must think of a wide assortment of culture procedures, species, and area, yet has a last objective to environmental sustainability and monetary steadiness through improved quantity and quality yield, lower expenses, and product diversification [8].
Feed loss has been diminished lately, because of the technological innovations for detecting the feed waste [9,10]. The estimation of feed wastage is routinely below 5% [11] because of the innovation. Be that as it may, actual percentage of feed loss is hard to determine for it can differ from fish farm operations and feeding schemes with an anticipation that feed loss in open water aquaculture was to be 20% [12]. The use of biofilters such as shellfish, could allow some biological control of outbreaks of pathogens and parasites, hence reducing the number of costly chemical treatments. Shellfish can ingest parasitic zooplanktons such as sea lice and protozoan parasites [13]. Consequently, shellfish rafts could be strategically placed to serve as a protection around fish cages to combat some diseases. Being filter-feeders, feeding bivalves can remove both phytoplankton and inorganic particles from the water column; hence, water turbidity is being reduced. Nutrients and physicochemical factors in seawater have essential roles in the existence and biological activity of green mussels. Fish-shellfish systems convert the fish waste into bivalves, which have a potential market in Cebu and region-wide. Green mussel culture production in the Philippines is contributing to alleviate socio-economic condition of the growers [14], but in the recent 2019 data, it showed a declined from 26, 302 MT in 2018 to 25, 421 MT in 2019 with CALABARZON, Western Visayas, and Eastern Visayas as the top producing regions (FSP Report 2017 - 2019). The significance of using and absorbing nutrient loads such as phosphate and nitrate by shellfish placed beside or near the commercial fish cages was an advantage to the present study. The main objective of this study therefore is to co-culture the green mussel Perna viridis with finfish (milkfish and pompano) cultured in cages following the concept of IMTA, in Valladolid, Carcar, Cebu Philippines. The following specific objectives were; to determine the growth of Perna viridis, to estimate the extraction of Perna viridis of inorganic nutrients (ammonia, nitrate, and phosphate) and organic particulates (total dissolved solids and total suspended solids) and to monitor changes in the physico-chemical parameters (such as, pH, DO, temperature and salinity) in the fish farm.
Materials And Methods
Study site description
The capacity of the green mussel, Perna viridis in removing both suspended particles and dissolved inorganic nutrients was investigated within the fish farm located in Sitio Tawog, Valladolid, Carcar, south of Cebu as described in Chapter 2 (Figure 1). Here, the green mussels were deployed in the vacant area between two rows of fish cages designated here as left and right cages (Figure 2).
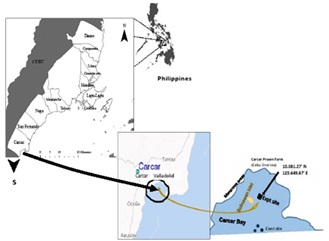 Figure 1: Fish farm site of Cebu Oversea Corp. in Valladolld, Carcar, Cebu, Philippines where the study was conducted.
Figure 1: Fish farm site of Cebu Oversea Corp. in Valladolld, Carcar, Cebu, Philippines where the study was conducted.
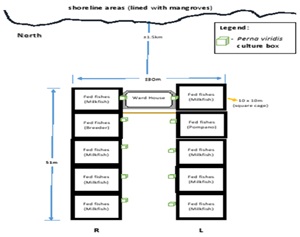 Figure 2: Culture design of the IMTA species (Perna viridis) placed side by side in left (L) and right (R) cages.
Figure 2: Culture design of the IMTA species (Perna viridis) placed side by side in left (L) and right (R) cages.
Collection and culture scheme of green mussel shells
Juvenile shells of Perna viridis were obtained from bivalve collectors in the western part of Negros Occidental, Philippines on December 2018. About 5,000 pcs of the young shellfish specimens were brought to the experimental IMTA site in Valladolid, Carcar and Cebu where they were transferred to a nylon net bag and acclimatized for 1 week below the sea surface (30 cm deep) beside a floating plastic frame near the fish farm’s ward house. The condition of the shells was checked daily prior to their deployment and dead shells were removed. After 1 week of acclimatization, at the start of culture, shell length of P.viridis individuals were measured again, after which they were transferred to nylon net bags following the design of Wong and Cheung [15]. The net bags containing 300 pcs of juvenile green mussels were tied with rope to the fiber frame of the fish cages (Figure 3). This suspension culture technique was a modification of that of Langan [16], to suit the experimental site, wherein each net bag was lined with multiple vertical branching ropes (0.6 m long abaca fiber) tied inside the net bag. Following the method of Azpeitia, et al. [17], individual mussel shells were carefully detached from the ropes and cleaned from encrusting organisms. All ropes and net bags were cleaned as well from fouling organisms twice a month as part of a standard maintenance protocol.
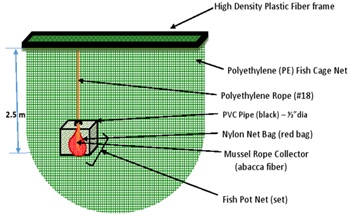 Figure 3: Illustration of the fish pot used for the culture of green mussel Perna viridis (red bag) inside a plastic box (0.6 m L x 0.6 m W x 0.4 m H) with mesh net deployed at 2.5 m depth.
Figure 3: Illustration of the fish pot used for the culture of green mussel Perna viridis (red bag) inside a plastic box (0.6 m L x 0.6 m W x 0.4 m H) with mesh net deployed at 2.5 m depth.
Growth monitoring
A total of forty-five pieces or 15% of the cultured green mussels were randomly sampled from two culture nets (20 and 25 pcs each from two selected nets) for monthly growth measurements, namely: 1) total shell length, 2) shell width, and 3) whole wet weight (shell plus meat). The shell length and width were measured using a plastic ruler (fixed in a plastic cardboard) and a plastic Vernier caliper while shell weight was measured using a weighing balance. Another batch of 45 pcs of juvenile P.viridis from another culture unit was also measured. The growth of green mussels deployed besides the fish cages (i.e. left and right of the cages) were physically compared in terms of their biomass, shell length and width, after which they were returned back to the culture nets. All measurements were done in the morning (9 to 10 AM). Percent mortality and survival rates were monitored weekly in the entire duration of the study. Dead and lost shells were replaced with new ones from the reserved stock maintained near the ward house.
Growth performance of the green mussels was calculated using the formula of Reid, et al. [18]: SGR (%biomass ÷ d) = 100 x LN (Wf – Wi) / d
Where; Wf and Wi are the final and initial wet weights of the shells sampled in each station (g); d = the duration of the experiment in days; LN = normal logarithm.
Water quality monitoring, including physico-chemical parameters
Water samples for nutrient analysis (ammonia, nitrate and phosphate) and for TDS and TSS were taken at 2.5 m deep using a water sampler, from both the experimental and control sites, for the whole duration of the study. These were done in two phases - the first phase or a total of 4 months (August to November 2018) was before the deployment of extractive species (pre-IMTA) to serve as baseline data; and the second phase or a total of 7 months (January to July 2019) during the IMTA implementation. The samples, in 1.5 L volumes for nutrients and 1.5 L for TDS and TSS, were taken between 1:00 to 2:00 PM. The samples from the first phase (pre-IMTA) were collected into polyethylene and glass bottles, placed in a chest cooler and brought to the Water Quality First Analytical Services and Technical Cooperative (FAST) Laboratories in Highway, Mandaue City, Cebu, within less than 24 h from collection. Water samples from the second phase, instead of submitting to FAST Laboratories (which charged higher analysis fees), were submitted to the USC Water Laboratory, in Talamban Campus, Cebu City. In both laboratories, water samples were analyzed using colorimetric and gravimetric methods.
Water samples for the physico-chemical parameter measurements were also taken from 2.5 m depth in both the experimental and control sites at twice a month. Water temperature was immediately measured in situ at twice a month, such as; water temperature and dissolved oxygen using an YSI 550A DO meter, salinity using a digital refractometer (Trans Instruments), and pH using a digital pH meter (American Marine Inc. brand), respectively. Four to five replicates of water samples were randomly obtained at 2.5 m deep within the fish cage perimeter.
Fish feed profile and composition analysis
Same proximate analysis content profile of fish feed as presented in Chapter 4 (Table 1). Meat sample from January and another batch in July 2019 of the green mussel weighing 200 g each were submitted for proximate analysis of protein, glycogen and ash content to the Laboratory of the Department of Science and Technology (DOST) - 7, Lahug, Cebu City.
|
Parameter |
|
Perna viridis |
|
|
Initial |
< 0.35 |
|
Total Fat (%) |
Final |
< 0.35 |
|
|
Increment |
|
|
|
Initial |
15.6 |
|
Crude Protein (%) |
Final |
16.7 |
|
|
Increment |
1.1 |
|
|
Initial |
2.70 |
|
Ash (%) |
Final |
4.37 |
|
|
Increment |
1.67 |
Table 1: Growth and proximate composition of Perna viridis (n = 30±SD) from the experimental culture fish cage for 7 months.
Initial = January 2019; Final = June 2019.
Statistical Analysis
All water quality parameters were analyzed using F comparison between the experimental and control sites with an independent two-tailed test application to check the differences between the nutrients in the two sites. All data are presented herein as means ± Standard Deviation (SD). Physico-chemical parameters namely DO, temperature, salinity and pH were analyzed for minimum and maximum values using Excel 2013.
Results And Discussion
Growth performance of Perna viridis under IMTA condition
P.viridis showed monthly average Specific Growth Rate (SGR) from January to July 2019 of 0.84 ±0.48 % day-1, 1.03 ±0.84, -0.23 ±0.81, 0.67 ±0.27, 0.65 ±0.23, 0.08 ±0.29, and 0.09 ±0.40 % day-1, respectively (Figure 4). The negative SGR value in March 2019 was due to predation of the still young mussels but SGRs recovered in the succeeding months of April to July 2019. Monthly average biomass (n = 90), on the other hand, increased from an initial weight of 225.40 +2.92 g in January to reach a maximum weight of 447.80 ±4.20 g in July or after 7 months (Figure 5). Although P.viridis had consistent positive growth rates, mortality was observed starting April which could be attributed to an abundance of predatory crabs and blister worms and the proliferation of sponges inside the culture nets which could have competed for food for this filter-feeding mollusc. These factors (i.e. predation and competition) resulted to an overall P.viridis mortality of 21.9% for 7 months, with highest single month mortality rate of 6.7% obtained in July and affected the biomass production for that month as shown in figure 5.
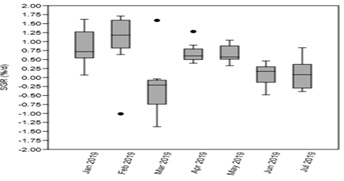 Figure 4: Specific growth rate (% d-1) of the cultured Perna viridis (n=45) in the experimental site with upper and lower outliers (with dots) for 7 months monitored from January to July 2019.
Figure 4: Specific growth rate (% d-1) of the cultured Perna viridis (n=45) in the experimental site with upper and lower outliers (with dots) for 7 months monitored from January to July 2019.
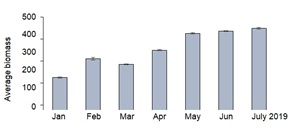 Figure 5: Monthly average biomass production of Perna viridis (n=90) cultured in close proximity to fish cages at a depth of 2.5m in the experimental site monitored for 7 months from January to July 2019. Vertical lines are standard deviation.
Figure 5: Monthly average biomass production of Perna viridis (n=90) cultured in close proximity to fish cages at a depth of 2.5m in the experimental site monitored for 7 months from January to July 2019. Vertical lines are standard deviation.
Table 2 shows the specific growth rate of green mussel spats in terms of Shell Length (SL) and Shell Width (SW) from its initial size measured in December 2018 or during pre-IMTA phase and during IMTA implementation from January to July 2019 parallel the fish culture. Thirty-five (35) pcs of green mussel shells were randomly picked from 2 selected fish pots representing the same colony from the wild cohorts. A sudden increase in shell size was observed in March and May followed by a slight decrease in shell length June. Highest shell size in terms of width (SW) and length (SL) after July 2019 or 7 months obtained 20.90 ±2.12 mm and 50.60 ±3.25 mm, from an initial SL of 28.06 ±2.68 mm and SW of 12.39 ±1.69 mm in December 2018, respectively. Power, et al. [19], obtained maximum shell size for P.viridis from 80 to 100 mm after 1 year, while both Soon, et al. [20] and Layugan, et al. [21] obtained a maximum size of over 40 mm after 6 months. Results in this study for 6 months alone (January to June 2019) showed a comparable growth and have reached SL of 49.70 ±4.16 mm in a random selection of shells from the net bags. Based on personal interviews with a local grower and supplier of green mussels in Pasil Market, Cebu City, green mussels can have better growth when deployed or positioned near the river mouths, where there is abundance of organic particulates as their food. However, more research is needed to support this claim and whether it is also applicable to Carcar Bay area. Up to what extent will this maximum growth of bivalves (in terms of commercial size) can be achieved in the IMTA site remains to be determined, which is beyond the scope of the present study.
|
Pre-IMTA (initial) |
During IMTA Implementation |
|||||||
|
Dec '18 |
Jan '19 |
Feb '19 |
Mar '19 |
Apr '19 |
May '19 |
Jun '19 |
Jul '19 |
|
|
SL (mm) |
28.06 ±2.68 |
32.14 ±3.05 |
34.33 ±3.79 |
40.57 ±4.80 |
45.14 ±4.20 |
50.74 ±2.52 |
49.70 ±4.16 |
50.60 ±3.25 |
|
SW (mm) |
12.39 ±1.69 |
13.41 ±1.56 |
14.30 ±2.12 |
18.00 ±2.35 |
18.00 ±1.57 |
18.43 ±1.58 |
18.66 ±2.84 |
20.90 ±2.12 |
Table 2: Average growth of Perna viridis as extractive species. Shown are values for Shell Length (SL) and Width (SW) (n=35) measured during pre-IMTA (Dec. 2018) and during IMTA operation (January to July 2019).
The transplanted green mussel grew into marketable size after 7 months based on weight and shell size (length and width) with lesser mortality after 4 months onward. The site where the mussels originated has a significant impact on bivalve mortality [22], because it will create more efforts for the transplanted species to adjust in the new culture environment. Site suitability according to Sallih [23] was very important for the culture of bivalves. Consequently, the growth of the bivalves in the IMTA set up can be affected by the conditions of surrounding environment within the bay, with significant contribution from the river run-offs containing anthropogenic wastes. Seawater quality depends on several internal (autochthonous) and external (allochthonous) factors, such as particulate matters that are brought down into the bay during rainfall, freshwater inflow carried by tidal movement and biological activities. These factors have been found to contribute to water quality in the coastal environment of the southeast coast of India during summer [24].
More detailed analysis of planktons in the site should be able to provide detailed information about the composition and quantity of food organisms present in the area. A site with high diatom concentration is considered well for green mussel farming, while an area with high turbidity, low salinity and low dissolved oxygen should be avoided [25]. The culture of bivalves close to the fish farm has many benefits because it will buffer estuaries and coastal ocean waters against excessive phytoplankton blooms, counteracting the symptoms of eutrophication; they may also remove inorganic sediments from suspension, counteracting coastal water turbidity according to National Research Council of the United States [26]. However, in Cavite, Philippines, a decline in mussel production was due to water pollution [21], which means that the optimal range of water quality for mussels to survive has to be observed. The extractive species being added to the monoculture of finfish (e.g. Chanos chanos and Trachinotus blochii), has been proven to use the waste products in the form of dissolved nutrients (nitrate, ammonia and phosphate) and suspended solids. The efficiency of such waste extraction would depend, to some degree, on the positioning of the extractive species vis-à-vis to fed species. Being filter-feeders it was found in this study that they were better positioned in the water column (at 2.5 m deep) where the fed fish are more concentrated, to allow for the removal of suspended organic particles.
To attain sustainability, fish farming operators must know the kind of fish species to be cultured, feeds being supplied, feed ingredients and temperature of the seawater [27-31]. It is worth noting that such information could be helpful to sea farming industries in facilitating the proper adoption technique specific to tropical countries like the Philippines. It is the responsibility of the aquaculture industry to enhance the culture techniques and to discover the equitable balance between economic return and sustainability of the cultured species. There is no doubt, however, that these efforts are being pursued to meet the ecological carrying capacity of the environment where the species is cultured. Sustainability is not an endpoint, but rather a trajectory of constant improvement [32]. This initial culture development can work as a basis for future ventures to integrate several species of different trophic levels in a holistic approach. Yet, the proximity of IMTA market and consumers is also a factor to be thought of, among others.
Physico-chemical factors
Physico-chemical parameters showed monthly variations at the water column (i.e., at 2.5 m deep) where the mussels were cultured in the two culture sites (Table 3, Figure 6). The monthly water temperature over a 12-month monitoring period, i.e. from August 2018 to July 2019, in the experimental and control sites had average values of 29.14 ±0.16 and 28.86 ±0.42°C, respectively. The monthly average temperatures were 29.14°C and 28.86°C in experimental and control site, respectively. However, at 2.5 m, the water temperatures could be as low as 26.62°C in the control site which is situated at the deeper portion of Carcar Bay. Generally, all these values are within the optimal temperature requirements of the green mussel Pernia viridis. Water temperature is one of the most important water quality parameters in an aquatic environment because it determines certain biological activities [33].
pH values, on the other hand, ranged from 7.60 ±0.03 in March to 8.21 ±0.27 in July 2019 in the experimental site and from 7.55 ±0.19 in August 2018 to 8.36 ±0.13 in July 2019 in the control site. Most of these values fall below the normal value of 8.2 for marine waters. However, during hot weather conditions on selective days of June and July 2019, when the temperature rose from 29 and 30°C, could be the leading factor for the maximum increase of pH to 8.21, as reflected in figure 6. The uniform decrement of pH every monsoon period could be linked to the confluence of fresh water and decomposition of organic matters carried by the floods [34]. The slightly higher values of above normal in the control site could be due to organic pollutants from human settlements [35] being delivered from the river outlet located 575 m away from the experimental site of the fish farm. On the other hand, low pH could be attributed to the organic effluents from land [36]. Considering the excessive introduction of CO2 into the oceans, the pH of seawater is expected to shift towards the acidic side although current model calculated pH show values to still hover around 8.2 with variation between 8.08 and 8.33 [37].
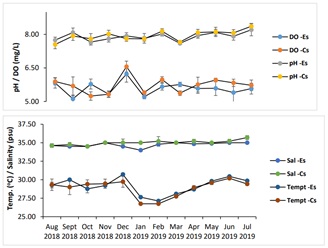 Figure 6: Monthly average values of the physico-chemical parameters (DO, pH Salinity, Temperature (oC)) between Experimental (Es) and Control site (Cs) at 2.5 m depths for 12 months. Vertical lines are standard deviation (n=8-10).
Figure 6: Monthly average values of the physico-chemical parameters (DO, pH Salinity, Temperature (oC)) between Experimental (Es) and Control site (Cs) at 2.5 m depths for 12 months. Vertical lines are standard deviation (n=8-10).
In terms of salinity, an average value of 35.01 ±0.23 psu was obtained in the control site, wherein a maximum value of 35.70 ±0.27 psu was observed in July and a minimum of 34.50 ±0.00 psu was observed in October 2018. This high salinity range was recorded during the hotter period of the year in the control site. In the experimental site, an average salinity of 34.72±0.07 psu was obtained, with minimum and maximum values of 34.00±0.00 and 35.00 ±0.00 psu were obtained, respectively. Salinity in both experimental and control site ranged 34 and 35 psu which are well within the normal salinity range for marine waters and the optimal growth requirements for P.viridis [38,39]. A slight salinity drop in both sites in December 2018 and January 2019 could be due to the successive heavy rains before the measurements were being conducted. These salinity values hovered around 35 psu in the both sites, but with few instances where salinity could go up to 36 psu for certain months, even during and after heavy rains. Fish farm sites in Carcar Bay are in quite deep water as compared to other sites in the province of Cebu where there are also fish farming activities, thus leaving a little chance for a rapid salinity drops, except from the river outlet of the bay, which is facing less than 1 km from the experimental site.
|
Location |
|
DO (mg/L) |
pH |
Salinity (‰) |
Temp (oC) |
|
|
|
12 |
12 |
12 |
12 |
|
Experimental |
Ave |
5.59±0.23 |
7.90±0.15 |
34.72±0.07 |
29.14±0.16 |
|
|
Min |
5.12±0.14 |
7.60±0.03 |
34.00±0.00 |
27.13±0.04 |
|
|
Max |
6.23±0.36 |
8.21±0.27 |
35.00±0.00 |
30.71±0.18 |
|
|
|
12 |
12 |
12 |
12 |
|
Control |
Ave |
5.73±0.19 |
7.94±0.15 |
35.01±0.23 |
28.86±0.42 |
|
|
Min |
5.24±0.19 |
7.55±0.19 |
34.50±0.00 |
26.74±0.09 |
|
|
Max |
6.56±0.24 |
8.36±0.13 |
35.70±0.27 |
30.19±0.16 |
Table 3: Physico-chemical parameters (random replicates, n= 8 to 10±SD per month) before and during deployment of green mussels at 2.5m depth in the experiment site and in the control site (no green mussels deployed).
DENR Administrative Order (DAO) 2016-08-WQG under the classification of the marine waters in the Philippines for its commercial use placed water quality standard to have a pH range of 6.5 - 8.5 and dissolved oxygen of 5.0 mg/L. An Ideal range of several physico-chemical factors for the culture of green mussel Perna (as Mytilus) edulis as found in neighboring Malaysia, including dissolved oxygen at more than 8 mg/L, salinity of between 27 and 32, water temperature of 26-32°C and pH of 7.9 to 8.2 [40]. The aquaculture sites of Carcar Bay have values falling within these ranges.
In terms of Dissolved Oxygen (DO) concentration, readings varied between the two sampling sites wherein highest value of 6.23 ±0.36 mg/L was obtained in December 2018 and lowest value of 5.12±0.14 mg/L obtained in August 2018 in the experimental site. These months were before the deployment of the green mussels (pre-IMTA). On the other hand, highest value of 6.56±0.24 and lowest value of 5.24±0.19 mg/L were obtained in October and December 2018, respectively. The occurrence of successive rains and two typhoons in Central Visayas during the study period could have altered the dissolved oxygen concentrations due to stronger waves compared to other times of the year in 2018. Strong waves also occurred on certain days in May 2019. Consequently, the growth of bivalves in the experimental site could have been affected by the prevailing environmental conditions on the bay together with inputs of wastes from the nearby river. Dissolved Oxygen (DO) levels in both sites which went from 5.05 - 6.77 mg/L conformed to the threshold set by DENR DAO 2016-08 to be at least 5 mg/L. Adequate DO is essential to fish farm operation [41]. The highest DO readings occurred in December 2018, while the low DO reading of 5.12 mg/L occurred in September 2018. If dissolved oxygen values were also to be used as an indicator of flushing activity, its higher average value in the control site would show that this was the case compared to the experimental site where its average value was lower. This can also be seen in the average pH value over an 11-month period where the experimental site had lower average pH than the control site. Moreover, DO remained high in the control site at deeper waters, indicating no issues on this parameter that would deprive the cultured animal species - both the fed species (fish) and the extractive ones - with oxygen that usually happens in more stagnant or less dynamic water bodies [41].
Therefore, the environmental conditions were fundamental to understanding the overall bivalve growth and physiology. As observed, heavy siltation from the surrounding environment fouled the culture fish pots containing the mussels in less than two weeks, especially during undisturbed times of the month.
Nutrient profile
Nutrient concentrations showed monthly fluctuations during the IMTA implementation from January to July 2019 with the deployment of green mussels in the experimental site (Figure 7). High monthly average concentrations of 0.057 mg/L for ammonia, 0.264 mg/L for nitrate and 0.034 mg/L for phosphate were observed in the experimental site as compared to the control site where lower concentrations of 0.043, 0.157 and 0.022 mg/L were observed for ammonia, nitrate and phosphate, respectively (Table 4). High ammonia and phosphate compounds could be related to increased water temperature during summer period and the prevalence of anoxic conditions in deep waters would give rise to organic compound decomposition that produce ammonia and phosphate compounds [42,43]. Generally, 0.02 mg/L value of ammonia in water is harmless [44]. Mussels cultured on one side of the fish cages (“Left side”) (Figue 1b) showed better condition and growth profile at the time of sampling and final harvest of July 2019 than its “right side” counterpart, supporting the hypothesis of a more stable parameter for Perna viridis growth. In our opinion, in order to see more marked differences between sites, the distance from the river mouth of the bay should be considered. Based on our interviews to local farmers, Perna viridis grow better when the bivalve is deployed or positioned near the river due to an abundance of organic particles serving as food. Whether maximum growths for mussels are achievable in the study site is a function of many biophysical parameters, not pursued in this study. However, knowledge of seawater nutrient changes prior to and during the study can be useful to frame up expectations of nutrient extraction and to assist in the interpretation of the augmented growth of this bivalve [45] and a tracer data as a means to infer nutrient reduction is also needed. Absorption efficiency is a common goal of IMTA involving different species such as combining the sea cucumber (P.californicus) with Pacific oyster (C.gigas) [46], sea cucumber (C.frondosa) and blue mussel (M.edulis) with salmon (S.salar) [47,48] and C.gigas with sea bass (D.labrax) [49].
Although inorganic nutrients in the form of ammonia, nitrate and phosphate showed little variations in average values between the experimental and control sites (Table 4), fluctuations were observed on a monthly basis before and during IMTA implementation in both experimental and control sites (Figure 7). Phosphate had the highest concentration in August 2018 (0.78 mg/L), which was before the implementation of IMTA in the experimental site. It was then taken over by nitrate during IMTA implementation in March (0.547 mg/L) and April 2019 (0.376 mg/L) as the dominant nutrient with highest concentration in the experimental and control sites. The increase in nitrate from March and April 2019, matched the peak production periods of the cultured milkfishes in both experimental and control cages entailing the increased amount of commercial feeds provided to the fish. Ammonia, which is among the waste products of the cultured aquatic animals, remained low before IMTA (0.027 mg/L in August - November 2018). As indicated, it rose up higher than phosphate during the IMTA implementation (0.058 mg/L from January to July 2019) in the experimental site (Figure 7), even when the extractive species was deployed. Generally, 0.02 mg/L value of ammonia in water is harmless [44]. At 2.5 m water column, all three nutrient compounds had high average concentrations at the experimental than in the control site (Table 4). The study sites can be contrasted more in terms of their hydrodynamics function which could explain the difference in their water quality parameters. For instance, the INCA circular fish cages (as control site) being positioned in a deeper portion (about 35 m deep), has a more dynamic water exchange and efficient flushing capacity for wastes coming from the fed fish. As it was being indicated by the lower concentration of nutrients, except for ammonia near the surface water. In contrast, the experimental site was set in a relatively shallower depths of only 13 to 15 m during low tide. In this site, the nitrate and phosphate have relatively higher average concentrations (0.226 and 0.149 mg/L, respectively) over an 11- month study period than the average concentrations of the same nutrients (0.215 and 0.085 mg/L, respectively) in the control site in the same period. An F comparison test between the two sites showed a significant difference for ammonia and phosphate but not in nitrate concentrations. A study on the biogeochemical responses on the removal of the mariculture structures in Tapong Bay, Taiwan, will explain of the added inputs of nutrients into the lagoon from external sources aside from the mariculture structures (i.e., discharges from land-based wastes) by the river into the sea [50]. This situation could be possible to happen in Carcar Bay, where the fish farm is located close to a river.
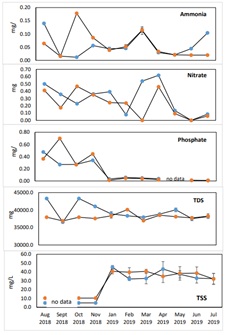 Figure 7: Monthly average water quality (n=3±SD) of the Experimental site (blue dot) and Control site (orange dot) at 2.5 m depth from January to July 2019. Vertical lines are standard deviations (n=3).
Figure 7: Monthly average water quality (n=3±SD) of the Experimental site (blue dot) and Control site (orange dot) at 2.5 m depth from January to July 2019. Vertical lines are standard deviations (n=3).
|
Location |
Depth (m) |
|
NH3 (mg/L) |
NO3 (mg/L) |
PO4 (mg/L) |
TSS (mg/L) |
TDS (g/L) |
|
|
|
Mos |
7 |
7 |
6* |
7 |
7 |
|
|
|
Ave |
0.057 |
0.264 |
0.034 |
36.571 |
38.540 |
|
Experimental |
2.5 m |
Min |
0.021 |
0.001 |
0.010 |
32.000 |
37.551 |
|
|
Max |
0.113 |
0.618 |
0.057 |
45.333 |
40.061 |
|
|
|
|
Var |
0.001 |
0.061 |
0.004 |
31.656 |
676.683 |
|
|
|
SE |
0.014 |
0.094 |
0.008 |
2.127 |
310.916 |
|
|
|
Mos |
7 |
7 |
6* |
7 |
7 |
|
|
|
Ave |
0.043 |
0.157 |
0.022 |
37.857 |
38.298 |
|
Control |
2.5 m |
Min |
0.020 |
0.001 |
0.008 |
32.000 |
36.994 |
|
|
Max |
0.114 |
0.460 |
0.045 |
40.667 |
40.116 |
|
|
|
|
Var |
0.001 |
0.028 |
0.000 |
10.402 |
900.831 |
|
|
|
SE |
0.013 |
0.063 |
0.006 |
1.219 |
358.734 |
|
|
|
df |
6 |
6 |
5 |
6 |
6 |
|
|
|
F value |
1.139 |
2.195 |
1.607 |
3.043 |
0.751 |
|
|
|
F critical |
4.284 |
4.284 |
4.284 |
4.284 |
0.233 |
Table 4: Nutrients (with min. and max. range) at 2.5 m for 7 months (from Jan to Jul 2019) in experimental and control site.
*No analysis for Phosphate (PO4) was made for May 2019.
No large monthly fluctuation was observed in Total Dissolved Solids (TDS) concentrations in the experimental site, except in May 2019 where TDS reached an average value of 40 g/L. The rest of the monthly TDS values were below 40.06 g/L in the experimental site and during the deployment of the IMTA species. In contrast, high TDS concentration occurred in February 2019 in the control site with 40.12 g/L whereas the minimum average values of TDS for both experimental and control site were 37.55 g/L in June and 37.00 g/L in March 2019, respectively. The slightly lower TDS values in the control site is probably due to its location in the deeper portion of the bay where water current and waves easily move the water mass containing suspended and dissolved solids to the more open sea lowering the organic loads present in both sites. The average monthly concentrations for TSS, on the other hand, recorded higher values in January and April 2019 in the experimental site with 45.33 and 43.33 mg/L, and lower in February and July with 32.00 and 32.33 mg/L. In the control site, TSS values were relatively high in the months of January and March 2019 with 40.67±2.89 and 40.67±2.31 mg/L while the lows were in the months of April and July with 35.00 and 32.00±6.25 mg/L. Based on the F comparison for these two organic nutrients, the TDS was slightly reduced (Fstat = 0.751) than the TSS (Fstat = 3.043) in the experimental site which could be attributed to the deployment of the IMTA species in this site. The proximate composition of the experimental IMTA species Perna viridis was shown in table 1. The live weight increment of the green mussel for 7 month culture period obtained 5.38. Their crude protein, and ash content increment were; 1.1 and 1.67. However, the total fat content of the cultures were almost non-detectable (<0.35). A non-significant tendency for an increased percentage of crude protein with an increased dietary protein and body weight level was observed.
The biomass of fish in the control site, which were placed in INCA cages, was 3 to 4 times higher in stocking density (based on company information) and therefore requires more feeds than those in the experimental site, the released wastes coming from the cages are expected to be enormous. However, the average concentrations of these nutrients were relatively low in the control site which, as explained above, could be attributed to the location of this site being in the deeper portion of Carcar Bay. Inspite of the overall relatively high concentrations of ammonia, phosphate, and nitrate (Fstat = 1.139, 1.607, and 2.195, respectively) in the experimental site than in the control site, it would appear from this study that Perna viridis as a biofilter was working for these nutrients, especially if the monthly nutrient differences and feeds supplication between the experimental and control sites were being compared.
Conclusion
The average specific growth rate (SGR) of Perna viridis reached highest level at 1.03% in February 2019, but declined to -0.23% in March 2019 for the entire 7-month culture period. The green mussel grew to a marketable weight of 447 g after this period, despite the presence of predators (mainly crabs) and fouling organisms (e.g. sponges and blister worms), causing mortality to some shells and rapid drops in growth rates in March 2019. At the end of the 7-month period of green mussel deployment, the cumulative mortality reached 21.9%. Transplanted green mussel grew into marketable after this period attaining its biggest size (length and width) and weight.
Physico-chemical parameters namely water temperature, salinity, pH and Dissolved Oxygen (DO), were well within the optimal requirements for growth of Perna viridis. Only the TDS decreased during the entire culture period based on F-test comparison. At least P.viridis as among the IMTA species used in this study, appear to be working well as a biofilter to all other tested nutrients (i.e., phosphate, nitrate and TSS) based on the monthly data obtained in the experimental site where the species was deployed. The compatibility of this bivalve species (together with the oyster species to be discussed in the next chapter) with the fish culture, relied mainly on depth-specific culture used in this study, such that the fish pot worked well if suspended at a depth where the fish cages are located. Finally, for the IMTA to work well, maintenance of water quality is of paramount importance and should be constantly monitored both spatially and temporally within the farm sites.
Recommendations
The goal of commercial aquaculture production is to produce great number of the produce of a given size within the shortest possible time. In this case, a variety of considerations such as, economic, environmental effects, and social benefits should be considered for effective mass culture and production of IMTA species. Based on the findings of this study, the following are recommended:
- The use of green mussels which normally are not naturally obtained in Cebu, and has to be brought from the neighboring provinces (like Negros and Bohol areas) for this study, took so much time, effort and cost to transport, therefore the use of locally occurring filter feeders should be This will not only address stress of the organisms that is usually associated with long transportation but acclimatization may no longer be necessary.
- Corollary to the above, research on the population structure of the selected filter- feeding species (e.g. wild mussels) must be conducted to assess their potential genetic consequences of interbreeding between farmed and wild stocks. These studies should be done before the mass production of the wild stock to their new environment as part of the IMTA
- The incorporation of modern technologies (e.g. wireless sensors) for real-time water quality monitoring in the aquaculture system is becoming a necessity and is highly recommended for a timely response in case of water quality disruptions in the aquaculture
Acknowledgment
Special thanks are due to Carcar Cebu Prawn Farm Research Laboratory and its technical personnel, Fish Farm Manager - Peter Villamor and Supervisors - Eric Pulvera and Leonides T. Candado, for providing the necessary secondary data, accommodation, use of facilities and technical assistance for the accomplishment of the cultures. I also thanked my Doctorate Adviser, Dr. Danilo B. Largo for sharing me his expert ideas and for the improvement of this article.
References
- Liutkus M, Robinson SMC, Macdonald BA, Reid GK (2012) Quantifying the effects of diet and mussel size on the biophysical properties of the blue mussel, Mytilus , feces egested under simulated IMTA conditions. J Shell Res 31: 69-77.
- Sara G, Zenone A, Tomasello A (2009) Growth of Mytilus galloprovincialis (Mollusca, Bivalvia) close to fish farms: A case of integrated multi-trophic aquaculture within the Tyrrhenian Sea. Hydrobiologia 636: 129-136.
- Cranford P, Reid G, Robinson S (2013) Open water integrated multi-trophic aquaculture: Constraints on the effectiveness of mussels as an organic extractive component. Aqua Envi Int 4: 163-173.
- Karayücel S, Çelik MY, Karayücel I, Öztürk R, Eyüboglu B (2013) Effects of stocking density on survival, growth and biochemical composition of cultured mussels (Mytilus galloprovincialis, Lamarck 1819) from an offshore submerged longline system. Aqua Res 46: 1369-1383.
- Buck BH (2007) Experimental trials on the feasibility of offshore seed production of the mussel Mytilus edulis in the German Bight: Installation, technical requirements and environmental conditions. Helgoland Mar Res 61: 87-101.
- Bayne BL, Worrall CM (1980) Growth and production of mussel Perna viridis from two populations. Mar Ecol Prog Ser 3: 317-328.
- Chopin T (2013) Aquaculture, Integrated Multi-Trophic (IMTA). SpringerReference. Pg no: 184-205.
- Frankic AM, Hershner C (2003) Sustainable aquaculture: Developing the promise of aquaculture. Aqua Int’l 11: 517-530.
- Ang KP, Petrell RJ (1997) Control of feed dispensation in seacages using underwater video monitoring: Effects on growth and food conversion. Aqua Eng 16: 44-62.
- Parsonage KD, Petrell RJ (2003) Accuracy of a machine-vision pellet detection system. Aqua Eng 29: 109-123.
- Cromey CJ, Nickell TD, Black KD (2002) DEPOMOD—modelling the deposition and biological effects of waste solids from marine cage farms. Aquaculture 214: 211-239.
- Beveridge MCM (1987) Cage aquaculture. Fishing News Books, Farnham, Surrey. Pg no: 352.
- Srisuphanunt M, Wiwanitkit V, Saksirisampant W, Karanis P (2009) Detection of Cryptosporidium oocysts in green mussels (Perna viridis) from shell-fish markets of Thailand. Parasite 16: 235-239.
- Department of Agriculture (2009) Mussel Profile. Agriculture and Fisheries Market Information System.
- Wong WH, Cheung SG (2001) Feeding rates and scope for growth of green mussels, Perna viridis (L.) and their relationship with food availability in Kat O, Hong Kong. Aquaculture 193: 123-137.
- Langan R (2009) Opportunities and challenges for offshore farming. In Burnell G, Allen G (eds.). New technologies in aquaculture: Improving production efficiency, quality and environmental management. Woodhead Publishing Limited, Cambridge, UK.
- Azpeitia K, Urrutia MB, Mendiola D (2018) Analysis of culture production and growth rate patterns of mussel (Mytilus galloprovincialis ) cultured in the open ocean of the SE Bay of Biscay for the commercial product development. Aqua Int’l Spring. Pg no: 1-18.
- Reid GK, Lefebvre S, Filgueira R, Robinson SMC, Broch OJ, et al. (2018) Reviews in aquaculture. Wiley Pub Asia Pty Ltd 13: 1-29.
- Power AJ, Walker RL, Payne K, Hurley D (2004) First occurrence of the nonindigenous green mussel, Perna viridis in Coastal Georgia, United States. J Shellfish Res 23: 741-744.
- Soon TK, Denil DJ, Ransangan J (2016) High mortality and poor growth of green mussels, Perna viridis, in high chlorophyll-a environment. Ocean Sci J 51: 43- 57.
- Layugan EA, Tabasin JPB, Alejos MS, Pidoy LE (2018) Growth performance of green mussel Perna viridis transplanted in Buguey Lagoon, Philippines. Acta Sci Agri 2: 43-47.
- Fuentes J, Gregorio V, Giraldez R, Molares J (2000) Within-raft variability of the growth rate of mussels, Mytilus galloprovincialis, cultivated in the Ria de Arousa (NW Spain). Aquaculture 89: 39-52.
- Sallih K (2005) Mussel Farming in the State of Sarawak, Malaysia: A Feasibility Study. The United Nations University Fisheries Training Program. Pg no: 1-44.
- Satpathy KK, Mohanty AK, Sahu G, Sarguru S, Sarkar SK, et al. (2010) Spatio-temporal variation in physicochemical properties of coastal waters off Kalpakkam, Southeast coast of India, during summer, pre-monsoon and post-monsoon period. Environ Monitor Assess 180: 41-62.
- Sing OF, Ransangan J (2019) Effect of Physicochemical Parameters and Phytoplankton Composition on Growth Performance of Green Mussel (Perna viridis) in Ambong Bay and Marudu Bay, Sabah, Malaysia. J Fish Environ 43: 0-68.
- National Research Council (2010) 7 Ecosystem services of bivalves: Implications for restoration. The Nat Acad Press, Ecosystem Concepts for Sustainable Bivalve Mariculture. Washington, DC, USA.
- Blancheton JP, Piedrahita R, Eding EH, Roque d’orbcastel E, Lemarié G, et al. (2007) Intensification of landbased aquaculture production in single pass and reuse systems. In: Bergheim A (eds.). Aquaculture Engineering and Environment. Research Signpost. Pg no: 21- 47.
- Blancheton JP, Bosc P, Hussenot JMÉ, d' Orbcastel ER, Romain D (2009) The 'new' European fish culture systems: Recirculating systems, offshore cages, integrated systems. Cahiers Agriculture 18: 227-234.
- Schneider O, Sereti V, Eding EH, Verreth JAJ (2005) Analysis of nutrient flows in integrated intensive aquaculture systems. Aqua Eng 32: 79-401.
- Schneider O, Blancheton JP, Varadi L, Eding EH, Verreth JAJ (2006) Cost price and production strategies in European recirculation systems. Linking tradition and technology highest quality for the consumer, Firenze, Italy, WAS.
- Schneider O, Schram E, Poelman M, Rothuis A, van Duijn A, et al. (2010) Practices in managing finfish aquaculture using RAS technologies, the Dutch example. Paper presented at the OECD Workshop on Advancing the Aquaculture Agenda, OECD, Paris, France.
- Hargreaves J (2011) Molluscan shellfish aquaculture and best management practices. John Wiley & Sons. Pg no: 51-80.
- Gupta HK (2005) Oceanology. Private Ltd, Universities Press, India. Pg no: 232.
- Upadhyay S (1988) Physico-chemical characteristics of the Mahanadi Estuarine ecosystem, east coast of India. Indian Journ Mar Sci 17: 2-23.
- Tan KS, Ransangan J (2015) Factors influencing the toxicity, detoxification and biotransformation of paralytic shellfish toxins. Rev Environ Contam Toxicol 235: 1-25.
- Sany SBT, Hashim R, Rezayi M, Salleh A, Safari O (2014) A review of strategies to monitor water and sediment quality for a sustainability assessment of marine environment. Environ Sci Pollut Res 21: 813-833.
- Marion GM, Millero FJ, Camoes MF, Spitzer P, Feistel R, et al. (2011) pH of seawater. Mar Chem 126: 89-96.
- Rajagopal S, Venugopalan V, van der Velde G, Hener HA (2006) Greening of the coasts: A review of the Perna viridis success story. Aqua Ecol 40: 273-297.
- Tan KS, Ransangan J (2016) Feasibility of green mussel, Perna viridis farming in Marudu Bay, Malaysia. Aqua Rep 4: 130-135.
- Sivalingam PM (1977) Aquaculture of the green mussel, Mytilus viridis Linnaeus, in Malaysia. Aquaculture 11: 297-312.
- Price C, Black KD, Hargrave BT, Morris JA (2015) Marine cage culture and the environment: Effects on water quality and primary production. Aqua Environ Interact 6: 151-174.
- Markou DA, Sylaios GK, Tsihrintzis VA, Gikas GD (2007) Water quality of Vistonis Lagoon, Northern Greece: Seasonal variation and impact of bottom sediments. Desalination 210: 83-97.
- Spears BM, Carvalho L, Perkins RG, Paterson DM (2008) Effects of light on sediment nutrient flux and water column nutrient stoichiometry in a shallow lake. Water Res 42: 977-986.
- Philminaq (2008) Water quality criteria and standards for freshwater and marine aquaculture. Mitigating Impact of Aquaculture in the Philippines, Quezon City, Diliman, Quezon City.
- Petersen JK, Holmer M, Termansen M, Hasler B (2019) Nutrient Extraction Through Bivalves. Goods and Services of Marine Bivalves. Springer, Cham.
- Paltzat DL, Pearce CM, Barnes PA, McKinley RS (2008) Growth and production of California sea cucumbers (Parastichopus californicus ) co-cultured with suspended Pacific oysters (Crassostrea sp. T.). Aquaculture 275: 124-137.
- Reid GK, Liutkus M, Bennett A, Robinson SMC, MacDonald BA, et al. (2010) Absorption efficiency of blue mussels (Mytilus edulis and trossulus) feeding on Atlantic salmon (Salmo salar) feed and fecal particulates: Implications for integrated multi-trophic aquaculture. Aquaculture 299: 165-169.
- Nelson EJ, Macdonald BA, Robinson SMC (2012) The absorption efficiency of the suspension-feeding sea cucumber, Cucumaria frondosa, and its potential as an extractive integrated multi-trophic aquaculture (IMTA) species. Aquaculture 370371: 19-25.
- Lefebvre S, Barille L, Clerc M (2000) Pacific oyster (Crassostrea gigas) feeding responses to a fish-farm effluent. Aquaculture 187: 185-198.
- Hung JJ, Hung CS, Su HM (2008) Biogeochemical responses to the removal of maricultural structures from an eutrophic lagoon (Tapong Bay) in Taiwan. Mar Environ Res 65: 1-17.
Citation: Melendres AR (2021) Growth and Absorption Response of Perna viridis (Lin, 1758) To Fish Farm Water Quality in Valladolid. J Aquac Fisheries 5: 042.
Copyright: © 2021 Melendres AR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

