
Identification of Vector Borne Blood Protozoa in Cattle and Sheep in Bangladesh
*Corresponding Author(s):
Md Zakir HassanAnimal Health Research Division, Bangladesh Livestock Research Institute, Savar, Dhaka-1341, Bangladesh
Tel:+8801737840328,
Email:zakir.vet@blri.gov.bd; zhtitas@gmail.com
Abstract
Babesiosis, Anaplasmosis and Theileriais are the most common vector (Tick) borne blood protozoan diseases (TBDs) in Bangladesh. This study was conducted in cattle and sheep in a different area of Bangladesh. A total number of 1150 blood samples were randomly collected from Dhaka, Sirajganj and Nikhangsori for blood smear microscopy. However, co-infections, temperature, humidity, season, farming and prophylaxis were also under consideration. From the clinically positive sample PCR was done followed by gel electrophoresis. Prevalence of blood protozoa were 100% (55), 80% (n=320), 30% (n=120), 22% (n=44), 31% (n=22), 65% (n=16) in exotic sheep, intensive farming, milk-vita area, local cattle, hill tracts and native sheep respectively. The overall prevalence was 50.17% (n=577). Among the protozoa, Anaplasma spp. was 43%, Babesia spp. 19%, Anaplasma spp. with Babesia spp. 33%, Theileriaspp 4% and Anaplasma spp. with Babesia and Theileriaspp was 1%. The prevalence of blood protozoa in local breed ≥50%, up to 75% and above 75% cross or pure breed were 17.58% (n= 103), 31.91% (n=187) and 50.51% (n=296) respectively. Prevalence of blood protozoa during October to March was 16.041% (n= 94) and April to September was 83.959% (n=492). In PCR Anaplasma marginale showed positive band as 265 bp, Babesiabovis in 166 bp, and Theileriaannulata in 312 bp, Babesiaovis in 422bp and Babesiamotasi in 518bp respectively. Therefore, the tick is act as vector and high humidity and temperature is the main risk factor for vector borne diseases. In conclusion, blood protozoa are the silent emerging disease in livestock and need to improve the control strategy.
Keywords
Anaplasmaspp; Bangladesh; PCR; Prevalence; Vector
INTRODUCTION
Anaplasma spp is a gram negative rickettsial protozoan whereas Babesiaspp, and Theileriaspp are apicomplexan parasite which infects red blood cell (RBC), indeed transmission occurs to animal through vector bite, notably Ixodes and usually know as tick borne diseases (TBDs) (Karim et. al., 2012) and also worldwide distributed [1, 2]. Parasitism one of the major hinders in livestock farming in Bangladesh and hot humid climatic condition greatly favours the development and survival of ecto and endo parasite that makes the violence of parasitism and knows as endemic disease [3]. Cross-breed animals were more susceptible than indigenous cattle and summer season was predominant for blood protozoa followed by winter and rainy season in tropical and sub-tropical countries. Adult and female were more susceptible than young and male [4]. The clinical sign showed that high fever (105-1070F), anaemia, profuse diarrhea, ascites, sometime bloody diarrhea, coffee color urine at last stage of inBabesiosis[5]. About 80% of the world cattle population is affected by TBDs [6]. The TBDS in Bangladesh predominated in forest and hilly areas, high humidity and temperature aggravate the outbreaks and cross or pure animal breed is more vulnerable to infection [7]. Humid and hot climatic condition favors the growth, multiplication and survival of tick and blood protozoa in RBC that causes an outbreak of TBDs [8]. It causes anaemia, hides damage, reduces milk production and poor reproductive performance, increased mortality and global economic losses estimated at US$ 18.7 billion [9]. In blood smear microscopy,Babesia spp. resemble as short and long loop formation in RBC (Piroplasmosis), Anaplasmamarginale like as pointed round dot at periphery of RBC and in Anaplasmacentrale pointed round dot inside of RBC. Where as in Theileriaspp RBC was annular and, round, dot, rod shape was found [10]. Biting flies transmit the disease and multiplication is increased in sexual stages due to hot humid environment[11]. Usually recovered animal act as persistent carriers. Traditional impression smear staining is a routine test used to identify Babesia, Theileria and Anaplasma spp. Polymerase chain reaction (PCR) is now becoming a common tool for molecular detection [12]. Recovered animal act as carrier and notably, creating a potential source of infection [13]. The multiplex PCR with species specific primer gave positive bands at 166bp, 265bp and 312bp selective for B. bovis, A. marginaleand T. annulata in cattle respectively [14]. However,Anaplasma marginale, Anaplasmacentral, BabesiaOvis, Babesiamotasiand Theileriaannulataare the causal agent for TBDs in sheep [15]. Early diagnosis and specific treatment along with vector control are necessary to prevent death and production losses [16]. Therefore, considering the importance of TBDs the research work was done for the identification and molecular detection of vector borne blood protozoan infection notablyBabesiaspp, Anaplasmaspp and Theileriaspp in cattle and sheep in Bangladesh with seasonal variation.
MATERIALS AND METHODS
Study area
An epidemiological study was carried out in Parasitology Laboratory under Animal Health Research Division in Bangladesh Livestock Research Institute (BLRI), Savar, Dhaka from July 2016 to June 2017.
Sample collection
About 2.5 ml of peripheral blood samples were collected from different cattle and sheep farm from several of Savar, SirajganjSadar, ShajadpurUpazila and Nikhangsori, Chottrogram within Ethylene Diamine Tetra Acetate (EDTA) tube with ice-cool storage and shifted to the Parasitology laboratory in BLRI. A total number of 1150 blood samples were collected randomly on questionnaire basis, among them 55 from Australian sheep (BLRI), 400 from a high yielding dairy farm of Savar, 400 from high yielding cattle from milk vita Bathan area (Baghabari), 200 from native cattle (Sirajganj), 70 from native hilly cattle of Nikhangsori, 25 from native sheep.
Laboratory identification of blood protozoa through Giemsa’s stain Method
Samples were examined by Giemsa's stained blood smear (GMS) microscopy (FAO, 2016) and confirmatory diagnosis through Polymerase Chain reaction (PCR). The effect of topography, season, age and sex was remaining in consideration in this study. In GMS protocol thick and thin blood smear was done. After air dry absolute methanol fixation was done and stained with 10% Giemsa’s stain. After washing, air dry and emulsification, magnification under 100x objectives. The haemoprotozoa were microscopically identified based on the characteristic morphology illustrated by Soulsby[17].
Molecular Identification and confirmation of tick borne protozoa (Blood Protozoa)
DNA extraction of blood protozoa
Blood protozoan DNA was extracted using a commercially available kit (Invitrogen Purelink Genomic DNA mini kit, Cat. no. K1820-01) from blood sample through chloroform method. At a glance, 200 μl whole blood was mixed with 200 μl of lysis buffer containing 20μg/ml proteinase K and incubation was done at 55 0C for 10 minutes. Thereafter, washing and centrifugation was done at 13,000x g for 3 minutes. Finally, the spin column was discarded and collecting the eppendorf tube containing extracted DNA and stored in -20 0C in the refrigerator. The purity of genomic was visualized by using spectrophotometry (260°A/280°A) with 1.5% gel electrophoresis (Sigma Aldrich, USA). The compaction of the DNA genome was adjusted to 100ng/µl nuclease free water.
Multiplex Polymerase Chain Reaction (PCR)
PCR was carried out in a final reaction volume of 25μl in the thin walled PCR tubes to amplify genomic DNA of Babesia, Anaplasma and Theileria species. The commercially available master mix kit (Thermo Scientific) was used to amplify fragments of genomic DNA in a programmable thermocycler (Eppendorf, Germany). Furthermore, after an initial enzyme activation step at 95°C for 5 min, the reaction mixture was subjected to 35 cycles each containing a denaturation step at 95°C for 30 sec, an annealing step at 68°C for 30 sec, and an extension step at 72°C for 1.5 min. After a final elongation step at 72°C for 5 min, PCR products were resolved by agarose gel electrophoresis, stained with ethidium bromide, and then observed under UV light.
Oligonucleotide primers were used in the PCR amplification cycle (First BASE Laboratories sdnbhd, Malaysia).The PCR images were captured though computer software (Carl Zeiss, GmbH, Germany) and the positive samples were detected by specific band size of the PCR product(Table 1).
|
SL No. |
Primers Name |
Sequence (5'-3') |
Genes targeted/ |
References |
|
Amplicon size |
||||
|
1 |
B. Ovis F1 |
[CCTGGGTAATGGTTAATAGGAACGG] |
Multi-copy VESA- 1a/ 422bp |
Bilgic et al., |
|
2017 |
||||
|
2 |
B. Ovis R1 |
[GCAGGTTAAGGTCTCGTTCGTTAAC] |
Multi-copy VESA- 1a/ 422 bp |
Bilgic et al., |
|
2017 |
||||
|
3 |
B. Motasi F1 |
[CTCTGGTACAATATGCATTGC] |
Multi-copy VESA- 1a/ 518 bp |
Bilgic et al., |
|
2017 |
||||
|
4 |
B. Motasi R1 |
[CTGGTTCCCAGATATGGTAGC] |
Multi-copy VESA- 1a/ 518bp |
Bilgic et al., |
|
2017 |
||||
|
5 |
A. Ovis F1 |
[CAGCCAGGCACTCTGCACCAC] |
Multi-copy VESA- 1a/ |
Bilgic et al., |
|
265bp |
2017 |
|||
|
6 |
A. Ovis R1 |
[CAACAATTGATGTGAGTGCGC] |
Major surface protein-1b / 265bp |
Bilgic et al., |
|
2017 |
||||
|
7 |
A. Margin F1 |
[GCTCTAGCAGGTTATGCGTC] |
Major surface protein-1b / 265bp |
Bilgic et al., |
|
2013 |
||||
|
8 |
A. Margin R1 |
[CTGCTTGGGAGAATGCACCT] |
Major surface protein-1b / 265 bp |
Bilgic et al., |
|
2013 |
||||
|
9 |
T. Anulata F |
[ACTTTGGCCGTAATGTTAAAC] |
Cytochrom b /312 bp |
Bilgic et al., |
|
2013 |
||||
|
10 |
T. Anulata R |
[CTCTGGACCAACTGTTTGG] |
Cytochrom b /312 bp |
Bilgic et al., |
|
2013 |
||||
|
11 |
B. Bovis F |
[CAAGCATACAACCAGGTGG] |
Multi-copy VESA- 1a/ 166bp |
Bilgic et al., |
|
2013 |
||||
|
12 |
B. Bovis R |
[ACCCCAGGCACATCCAGCTA] |
Multi-copy VESA- 1a/ 166bp |
Bilgic et al., |
|
2013 |
Table 1: Positive samples were detected by specific band size of the PCR product.
RESULTS AND DISCUSSION
In blood smear microscopy prevalence of TBDs was 100% (n=55) in Australian sheep, 80% (n=320) in dairy farm, 30% (n=120) in Bathan area, 22% n= (44) in native cattle, 31% (n=22) in hilly cattle, and 65% (n=16) in native sheep, this findings strongly supported where he had found that prevalence of TBDs was significantly varied on area, season and breed(Table 2)[18].
|
Type of Sample area |
No. of total sample |
Positive Sample |
Negative sample |
% of Positive sample |
|
Australian sheep/BLRI (Pure) |
55 |
55 |
0 |
100% |
|
High Yielding cattle (Cross), Milk Vita Baghabari. |
400 |
120 |
280 |
30% |
|
Native cattle, Sirajganj |
200 |
44 |
156 |
22% |
|
Native Hilly cattle, Nikhongchori |
70 |
31 |
39 |
44% |
|
On recognized Dairy Farm,Jamghora ,Savar |
400 |
320 |
80 |
80% |
|
Native sheep,Savar |
25 |
16 |
9 |
21.29% |
|
Total |
1150 |
586 |
564 |
50.96% |
Table 2: Prevalence of Blood Protozoa on region basis.
The overall prevalence of TBDs was 50.17% (n=577) in cattle and sheep in which Anaplasma spp was 43%, Babesia spp 19%, Anaplasma spp. and Babesiaspp 33%, Theileriaspp 4% and Anaplasma spp. with Babesia and Theileriaspp 1% of blood protozoa (Table 3).
|
Total Number of Positive Sample |
Type of blood protozoa |
Positive no. of spp. of blood protozoa |
% of positive spp. of blood protozoa |
|
|
586 (Out of 1150) blood sample) |
Anaplasmaspp |
251 |
43% |
|
|
Babesiaspp |
113 |
19% |
||
|
Anaplasmaspp + Babesiaspp |
193 |
33% |
||
|
Theileria spp. |
23 |
4% |
||
|
Anaplasma spp. withBabesiaandTheileriaspp |
6 |
1% |
Table 3: Prevalence of Blood Protozoa on the basis of type.
This result was almost similar with where stated that in Turkey, the overall prevalence was 74.78%, Anaplasma spp and Babesia spp was 41.99%, and slightly higher from where over all prevalence was 38%, there was some variation due tropical and subtropical regions variation [19, 20]. In positive case the blood protozoa magnify slight purple color. In case of Babesia spp. short and long loop formation was found at the periphery of RBC (Piroplasmosis). In case of Anaplasma marginale pointed round dot at periphery of RBC and in Anaplasmacentrale pointed round dot inside of RBC. In case of Theileriaspp RBC was slight triangle in shape and ring form Theileriaspp was found (annular), sometimes oval, round, dot, rod shape was found, this findings were notably similar with(Figure 1)[21].
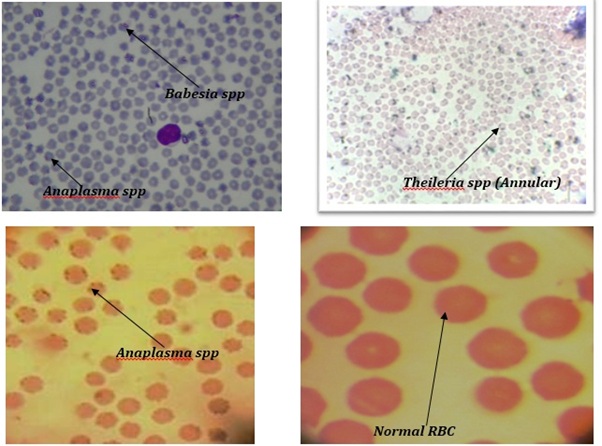 Figure 1: Blood smear microscopy of Babesiaspp, Anaplasmaspp and Theileriaspp within RBC in 100x objectives.
Figure 1: Blood smear microscopy of Babesiaspp, Anaplasmaspp and Theileriaspp within RBC in 100x objectives.
However, Anaplasma marginaleshown positive band as 265 bp, Babesiabovisin 166 bp, and Theileriaannulata in 312 bp, in cattle blood whereas Anaplasma marginalein 265 bpBabesiaovisin 422bp, Babesiamotasiin 518bp and Theileriaannulata in 312 bp in sheep blood respectively, this finding was clearly significant with (Figure 2)[22].
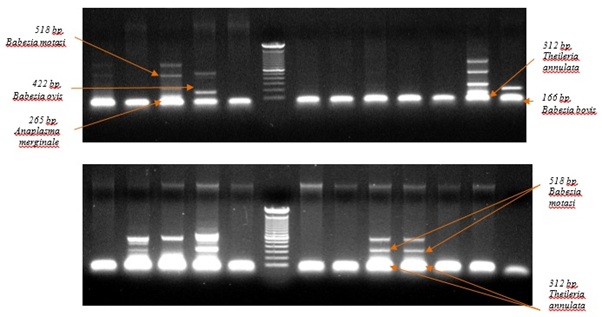 Figure 2: Molecular detection of Anaplasmaspp, Babesiaspp and Theileria spp. by multiplex PCR (Gel electrophoresis amplified DNA).
Figure 2: Molecular detection of Anaplasmaspp, Babesiaspp and Theileria spp. by multiplex PCR (Gel electrophoresis amplified DNA).
However in seasonal study in Bangladesh it was observed that April to September environmental temperature is arise (above 300°C, sometimes 400°C) and humidity is above 70% (sometimes above 90%) that triggers the multiplication of tick biologically and also multiplication of TBDs protozoa both in tick and animal blood that progresses havoc of TBDs in high yielding animal and local animal act as carrier, this clarification also clearly justified the same findings, there he stated that infection in sheep 52% due to hot humid environmental condition[23].
In addition, when environmental temperature is 300C or below and humidity is below 70% notably during October to March animal act as carrier but not showing clinical sign, and prevalence of blood protozoa during October to march was 16.041% (n= 94) and April to September was 492 83.959% (n=492), this result was verified the findings(Table 4)[24].
|
Name of Month |
Average temperature in BD |
Average Humidity in BD |
Prevalence of Blood protozoa |
|
|
October to March |
300C or below |
70% or below |
94 (16.041%) |
|
|
April to September |
30°C to 40° |
70% to 90% |
492 (83.959%) |
|
|
Total number of positive sample (Out of 1150) |
586 |
|||
Table 4: Prevalence of Blood protozoa on the basis of temperature and humidity.
In case of high yielding animal (above 60% cross breed) and 100% pure breed show high clinical sign and even death in high percentage and response to treatment is low. In local breed or 50% crossed breed, upto 75% crossed breed and above 75% or even pure prevalence of blood protozoa was 17.58% (n= 103), 31.91% (n=187) and 50.51% (n=296) respectively (Table 5). The findings compared with where signifying that the crossbred are more susceptible to TBDs than local animal and consequently, strongly supported by where they were stated that, TBDs caused high morbidity, mortality and economic losses in high yielding animal than local breed of ruminants [25-27].
|
Type of breed (on the basis of farmers history) |
Positive number of sample |
Prevalence of blood protozoa |
|
Local or Crossed up to 50% |
103 |
17.58% |
|
Crossed above 50% up to 75% |
187 |
31.91% |
|
Crossed above 75% to above or pure) |
296 |
50.51% |
|
Total number of positive sample 586(out of 1150 sample) |
||
Table 5: Prevalence of blood protozoa on the basis of breed.
CONCLUSION
Tick borne blood protozoan disease (Babesiosis,AnaplasmosisandTheileriosis) are now a days a crucial factor for livestock production in Bangladesh. Local animal act a as carrier but it indicating future havoc in livestock industry especially high yielding exotic animal (70 % to 100 % pure breed). Moreover, they are more susceptible to TBDs and it is very difficult to control because high temperature and humidity provoke the tick multiplication. To introduce high yielding animal in a farm strict biosecurity is essential for farming.
ACKNOWLEDGEMENT
The author would like express his gratefulness to all the research personnel in Dept. of Parasitology, Animal Health Research Division, Bangladesh Livestock Research Institute, Savar, Dhaka-1341, for conducting this problem oriented parasitic research surveillance.
DISCLAIMER
The authors had declared that, no conflict of interest for the publication of this manuscript
REFERENCES
- Vieira LL, Canever MF, Cardozo LL, Cardoso CP, Herkenhoff ME et al (2019) Prevalence of Anaplasma marginale, Babesiabovis, and Babesiabigemina in cattle in the Campos de Lages region, Santa Catarina state, Brazil, estimated by multiplex-PCR. Parasite Epidemiol Control.
- Kundave VR, Ram H, Banerjee PS, Garg R, Mahendran K et al (2018) Development of multiplex PCR assay for concurrent detection of tick borne haemoparasitic infections in bovines. Acta parasitological 63: 759-765.
- Alim MA, Das S, Roy K, Masuduzzaman M, Sikder S et al (2012) Prevalence of Haemoprotozoan Diseases in Cattle Population of Chittagong Division, Bangladesh. Pak Vet J 32: 221-224.
- Aouadi A, Leulmi H, Boucheikhchoukh M, Benakhla A, Raoult D et al (2017) Molecular evidence of tick-borne hemoprotozoan-parasites (Theileriaovis and Babesiaovis) and bacteria in ticks and blood from small ruminants in Northern Algeria. Comp ImmunolMicrobiol Infect Dis 50: 34–39.
- Alim Md. A, Das S, Roy K, Masuduzzaman Md., Sikder S et al (2011)Prevalence of Hemoprotozoan Diseases in Cattle Population of Chittagong Division, Bangladesh.Pak Vet 2: 221-224.
- Ghosh S, Azhahianambia P, Yadavb MP (2007) Upcoming and future strategies of tick control: a review. J Vector Borne Dis 44: 79–89.
- Bhat SA, Singh NK, Singh H, Rath SS (2017) Molecular prevalence of Babesiabigemina in Rhipicephalusmicroplus ticks infesting cross-bred cattle of Punjab, India. ParasitEpidemiol Control 2: 85-90.
- Lee SH, Mossaad E, Ibrahim AM, Ismail AA, Moumouni PF (2018) Detection and molecular characterization of tick-borne pathogens infecting sheep and goats in Blue Nile and West Kordofan states in Sudan. Ticks Tick Borne Dis 9: 598-604.
- Ananda KJ, Adeppa J (2016) Prevalence of Haemoprotozoan infections in bovines of Shimoga region of Karnataka state. J Parasit Dis 40: 890-892.
- Song R, Wang Q, Guo F, Liu X, Song S et al (2018) Detection of Babesia spp., Theileria spp. and Anaplasma ovis in border regions, northwestern China. Transboundary and emerging diseases 65: 1537-1544.
- Ghosh S, Nagar G (2014) Problem of ticks and tick-borne diseases in India with special emphasis on progress in tick control research: a review. J Vector Borne Dis 51: 259-270.
- Bock RE, Jackson L, de Vos A, Jorgensen W (2004) Babesiosis of cattle. Parasitology 129: 247-269.
- Zhou M, Cao S, Sevinc F, Sevinc M, Ceylan O (2017) Molecular detection and genetic characterization of Babesia, Theileria and Anaplasma amongst apparently healthy sheep and goats in the central region of Turkey. Ticks Tick Borne Dis 8: 246-52.
- Kocan KM, de la Fuente J, Blouin EF, Garcia-Garcia JC (2004) Anaplasmamarginale (Rickettsiales: An¬aplasmataceae): Recent advances in defining host-pathogen adaptations of a tick-borne Rickettsia. Parasitology 129: 285–300.
- Bilgiç HB, Karagenç T, Simuunza M, Shiels B, Tait A (2013) Development of a multiplex PCR assay for simultaneous detection of Theileriaannulata, Babesiabovis and Anaplasma marginale in cattle. ExpParasitol 133: 222-229.
- Bary MA, Ali MZ, Chowdhury S, Mannan A, Nur e Azam, Hossain MA et al (2018) Prevalence and molecular identification of haemoprotozoan diseases of cattle in Bangladesh. Adv. Anim. Vet. Sci 6: 176-182.
- Nair AS, Ravindran R, Lakshmanan B, Kumar SS, Tresamol PV et al (2011) Haemoprotozoa of cattle in northern Kerala, India. Trop Biomed 28: 68-75.
- Karim MA, Rima UK, Hossain MZ, Habib MA, Islam MS (2012) Adoption of Polymerase Chain Reaction Techniques for the Detection and Differentiation of Babesiosis, Anaplasmosis and Theileriosis in Clinically Infected and Slaughtered Cattle. Bangladesh J Vet Med 46: 31-43.
- Soulsby EJI (1982) Helminths, Arthropod and Protozoa of Domesticated Animals, 7th edition, Bailliere Tindal, London, pp 136-346, 365-491, 763-778.
- Jayalakshmi K, Sasikala M, Veeraselvam M, Venkatesan M, Yogeshpriya S (2019) Prevalence of haemoprotozoan diseases in cattle of Cauvery delta region of Tamil Nadu. J Parasit Dis 43: 308-12.
- Bilgic HB, Bak?rc? S, Kose O, Unlu AH, Hac?larl?oglu S et al (2017) Prevalence of tick-borne haemoparasites in small ruminants in Turkey and diagnostic sensitivity of single-PCR and RLB. Parasit Vectors 10: 211.
- Kundave VR, Ram H, Rafiqi SI, Garg R, Tiwari AK et al (2017) Comparative evaluation of microscopy and PCR assay for detection of Theileriaannulata infection in ruminants. J Anim Res 7: 699-703.
- Ros-García A, Barandika JF, García-Pérez AL, Juste RA, Hurtado A (2013) Assessment of exposure to piroplasms in sheep grazing in communal mountain pastures by using a multiplex DNA bead-based suspension array. Parasit Vectors 6: 277.
- Maharana BR, Tewari AK, Saravanan BC, Sudhakar NR (2016) Importanthemoprotozoan diseases of livestock: Challenges in current diagnostics and therapeutics: An update. Vet World 9: 487-495.
- Chowdhury S, Hossain MA, Barua SR, Islam S (2006) Occurrence of common blood parasites of cattle in sirajgonjsadar area of Bangladesh. Bangladesh J Vet Med 4: 143–145.
- Uilenberg G (2006). Babesia—a historical overview. Vet Parasitol 138: 3-10.
- Jonsson NN, Bock RE, Jorgensen WK (2008) Productivity and health effects of anaplasmosis and babesiosis on Bosindicus cattle and their crosses, and the effects of differing intensity of tick control in Australia. Vet Parasitol 155: 1-9.
Citation: Hassan MZ, Giasuddin M, Rahman MM, Ershaduzzaman M, Hasan M, et al.(2019) Identification of Vector Borne Blood Protozoa in Cattle and Sheep in Bangladesh, Bangladesh. J Virol Antiviral 2: 004.
Copyright: © 2019 Md Zakir Hassan, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

