
Immunomodulatory, Antimicrobial and Insecticidal Properties of Anisotes Trisulcus (Forssk.) Leaf Extract Containing Silver Nanoparticles
*Corresponding Author(s):
Essam H IbrahimResearch Center For Advanced Materials Science Rcams, King Khalid University, Biology Department, Faculty Of Science, Blood Products Quality Control And Research Department, National Organization For Research And Control Of Biologicals, Abha, Cairo, Egypt, Saudi Arabia
Tel:+966 0562668689,
Email:essamebrahim@hotmail.com
Abstract
The ecofriendly production of Silver Nanoparticles (AgNPs) is targeted nowadays. This work aimed to prepare Anisotes trisulcus (Forssk.) Nees leaf acetone extract and extract prepared AgNPs and testing their different bioactivity potentials. A. trisulcus leaves were collected from Abha (KSA) and acetone extract from these leaves was prepared. AgNPs were prepared using the extract. Synthesized AgNPs were examined using Scanning Electron Microscope (SEM) and UV/Vis spectra. Fourier-Transform Infrared Spectroscopy (FT-IR) was used to screen biomolecules present in the extract before and after synthesis of AgNPs. Biological activities found in the extract and extract containing AgNPs, including immunomodulatory (normal rat splenic cells proliferative/anti-proliferative effects), insecticidal, antibacterial, antifungal, anticancer (against Helen and HepG2 cell lines), safety on Red Blood Cells (RBCs) and vital organ were tested. Results showed that the extract has many functional groups. The size of AgNPs ranged from 40-60 NM. Both extract and extract containing AgNPs have antibacterial, antifungal, anticancer, rat splenic cell’s proliferation inhibition, insecticidal activities and safe on RBCs and liver. In conclusion, the extract has many functional groups capable of synthesizing and stabilizing AgNPs formation. The extract and extract containing AgNPs have antibacterial, antifungal, anti-proliferation, anticancer and insecticidal activities and safe for vital organs and RBCs.
Keywords
AgNPs; Anisotes trisulcus (Forssk.) Nees; Antimicrobial; Insecticidal; Splenic cells
INTRODUCTION
The family Acanthaceae, in which the plant Anisotes trisulcus (Forssk.) Nees is included, is rich with medicinal plants [1]. The plant Anisotes trisulcus (Forssk.) is found in a place between Abha and Najran called Wadi of Jabal Abu Hassan in Saudi Arabia [2]. The plant is not only found in Saudi Arabia, but also found in all the Arabian Peninsula. The plant has a local names of Moze, Madh and Moddaid [3]. The medicinal values of Anisotes trisulcus rely on its biological activities to hepatic disorders, gallstone, hepatitis and jaundice. The plant is used to suppress appetite and manage tobacco infliction. In addition, Anisotes trisulcus has bronchodilator, cytotoxic, antidiabetic, anti-bacterial, hypotensive, hepato-protective and local anesthetic activities [4-6].
The genus Anisotes was shown to be rich in compounds such as alkaloids [7]. Phytochemical studies done on A. trisulcus proved that the plant has many alkaloids like peganine, 5-hydroxy vasentine, anisotine, 7-hydroxy vasicine, vasicinone, 7-hydroxy vasicinone, trisulcusine, and 5-methoxypeganine [8,9].
The employments of Silver Nanoparticles (AgNPs) in the area of pharmaceutical ventures are wide [10], as these AgNPs have a strong inhibitory power against numerous microorganisms [11,12] and a good carrier for anti-cancer drugs [13]. Recently, AgNPs got an incredible interest. The production of AgNPs can be done through numerous methods, including chemical [14,15], photochemical [16,17], electrochemical reduction [18,19] and vaporization using heat [20,21]. These strategies use numerous dangerous chemicals to reduce the metal ions into nanoparticles. So, there is a need for alternative methods to replace these toxic materials seeking for better human health and the environment. To conquer the several side reactions represented in the use of poisonous chemical compounds to synthesize the AgNPs, plants or plant extracts have been utilized instead of chemical compounds (biosynthesis process). Plants and plant extracts have numerous several natural constituents/phyto-biomolecules that have defensive and reducing characteristics which are critical for the reduction of silver ions relying upon the natural biomolecules and reductive protein complexes. As of late, extracellular AgNPs were orchestrated utilizing diverse plant removes as a potential lessening specialist [22,23].
The current study tries to investigate the different biological potentials found in ethanol extract of Anisotes trisulcus (Forssk.) Nees leaves and extract prepared silver nanoparticles.
MATERIALS AND METHODS
Preparation of plant extracts
Anisotes trisulcus leaves were gathered from Abha, Aseer, and Kingdom of Saudi Arabia (KSA) in the month of February 2018. Identification of the plant was kindly done by a taxonomist at the Biology Department, King Abdul-Aziz University, Saudi Arabia. Ethanolic extract from A. trisulcus leaves was prepared according to Ghramh et al., [24]. Stock solutions (1%) in ethanol, acetone (to be used in cell culture) or in dimethyl sulfoxide (to be used in testing of antimicrobial activity) were prepared.
Synthesis of AgNPs using A. trisulcus extract
To Erlenmeyer flask, 197 mL 1 mM AgNO3, 1 mL Triton X-100 (both from Sigma-Aldrich) and 2 mL acetone dissolved plant extract solution were mixed together to prepare silver nanoparticles (AgNPs) according to Ghramh et al., [25]. The flask was kept at 23-26°C until the color of the mixture changed (about 30 h).
Characterization of A. trisulcus-produced AgNPs
After the change of the color of AgNO3/extract mixture changed, AgNPs formation was detected spectrophotometerically (UV-3600 Shimadzu spectrophotometer) at the wavelengths from 350 to 700 nm at 1 nm resolution according to Ghramh et al., [25]. To measure the sizes of the produced AgNPs, a scanning electron microscope (SEM, JEM-1011, JEOL, and Tokyo, Japan) was used at an accelerating voltage of 90 KV. The presence of functional groups in the extract was monitored using Fourier Transform Infrared (FT-IR) spectroscopy (Perkin- Elmer Spectrum 2000, USA) within the range 500-4500 cm−1 at a rate of 16 times and the clarity of 4 cm−1.
Antibacterial activities
The antibacterial potential of Anisotes trisulcus leaf extract and extract containing AgNPs was tested using agar well diffusion method according to Kilany [26], using Gram negative bacteria (Escherichia coli, Proteus mirabilis and Shigella flexneri) and Gram positive bacteria (Staphylococcus aureus). Positive control (Penicillin/Streptomycin at 20 units/20 µg respectively) solution and negative control (nutrient broth), 30 µL each, were included. Different plates were incubated for 24 h at 37ºC and zones (mm diameter) of inhibition around each well were measured in triplicate [27].
In vitro immunomodulatory potential test
The test was done according to Ibrahim et al., [28]. Splenic single-cell suspension was prepared from healthy adult male Sprague Dawley rat weighing about 280 g. The rat was kindly supplied by animal house found at King Khalid University. The cells were suspended at a density of 0.05X106/mL in complete RPMI-1640 medium. The cell culture was performed in 96-microwell tissue culture plate (Coaster) by adding 100 μL of cell suspension (5000 cells/well) and 100 μL Anisotes trisulcus leaves extract or extract prepared AgNPs at 200, 100 and 50 μg/mL separately. Control culture was prepared by adding 5000 splenic cells/well in 200 μL culture medium. All plates were incubated in a CO2-incubator (Memmert, Gmbh) at 37°C for 72 h. The viability of the cells in all plates were assessed using Vybrant® MTT Cell Proliferation Assay Kit (Thermo Fisher Scientific) according to manufacturer’s instructions. Following Oves et al., [29], the percent increase or decrease in cell number was evaluated.
Lytic effects of Anisotes trisulcus leaves extract and extract prepared AgNPs on Red Blood Cells (RBCs)
The hemolytic activity which may be found in A. trisulcus leaves acetone extract and its biosynthesized AgNPs was tested separately at final concentrations 200 μg/mL according to the method described by Ghramh et al., [24]. Positive control (1.5% Triton X-100 in PBS) and negative control (PBS), in triplicates were included.
Acute cytotoxicity study of Anisotes trisulcus leaves extract and extract prepared AgNPs
To test hepatic toxicity which may be found A. trisulcus leaves extract or extract prepared AgNPs, 5 adult healthy rats (200-250 g) were injected with a single dose regimen of 100 µg/mL A. trisulcus leaves extract or extract prepared AgNPs [29]. The rats were left for 24 h and then sacrificed and sera were obtained from their blood. Liver function was tested by assaying the level of serum Aspartate Aminotransferase (AST) colorimetrically according to the Reitman and Frankel [30], method using Randox Kit (UK).
Collection of Culex pipiens larvae
Larvae of Culex pipiens were gathered from various reproducing locales and identified in the Entomology Unit, Department of Biology, Faculty of Science, King Khalid University, Abha, KSA. The larvae were kept in several plastic plates containing tap water. They were raised in the research facility to mid fourth instars feeding on dog biscuits and yeast powder in a 3:1 ratio [31]. The fourth instar stage was gathered following six days post-hatching, with the larvae achieving a length of right around 0.5 inch.
Larval bioassay
The larval susceptibility test was done according the WHO [32], method. Cx pipiens 4th instar larvae (20/test), in five replicates, were treated with 100 mL different concentrations of A. trisulcus leaf extract and extract containg AgNPs for 24 h. Control group (20 larvae) was treated with tap water only. Using an environmental chamber (27°C), all groups were kept with a 16:8-h light/dark cycle. The larvae were given their food as above during the experiments. Larval mortalities in all groups were recorded after 24 h of initial treatment. The dead larvae were recognized when they did not move after being probed by a needle in siphon or cervical area.
Anticancer activity test
HepG2 and HeLa cell lines (Sigma-Aldrich) were cultured separately in DMEM (Sigma) supplemented with 10% foetal calf serum (Gibco BRL), penicillin/streptomycin (100 U/ml/100 mg/ml, Gibco BRL) and 2 mM L-glutamine (Gibco BRL) at a cell density of 5000 cells/well in 96-well tissue culture plate (Falcon). Plates were incubated at 37°C under 5% CO2 and 90% humidity for 24 h. The media were removed from each plate and replaced with 200 µL/well fresh media containing 100 µg/mL A. trisulcus extract alone or with nanoparticles separately. Cells with media only served as control culture. The cultures were continued for more 24 h at the same conditions described above. The cell viability assay was done as mentioned above. Results were expressed as the percentage of the control at the completion of each incubation period [33].
STATISTICAL ANALYSIS
The study was conducted in a Completely Randomized Design (CRD) in a factorial experiment. All data were analyzed statistically using Analysis of Variance (ANOVA) and means were compared by LSD at P ≤ 0.05 using Graphpad Prism (version 7). LC50, IC50 and IC95 were calculated according to probit analysis program [34]. Computerized log-probit analysis was used to analyze values, regression equations, the 95% confidence intervals and degrees of freedom of the χ2 goodness of fit tests. Using Abbot's formula [35], the mortality percent was corrected for control mortality.
RESULTS AND DISCUSSION
Characterization of AgNPs
The change in color extract mixed silver nitrate was monitored by naked eye. The solution color changed from light yellow to brown and finally to dark brown (Figure 1A and 1B). The change in color into dark brown was time-dependent and this characteristic brown color may be referred to the excitation of Surface Plasmon Response (SPR) with the silver nanoparticles as explained by Ranganathan et al., [36]. Color changes of the solutions may be attributed to some biochemical compounds like flavonoids, alkaloids, saponins and steroids. The active biomolecules present in the plant extract may act as reducing substances that reduced silver ions (Ag+) to a silver atom (Ag0) through the nitrate reductase enzyme. These biomolecules or enzymes that released into the solution may have the power to reduce the silver ions to silver nanoparticles through capping agents such as protein-like molecules [37].
The synthesis of silver nanoparticles was accomplished via one pot reaction involving the reduction of silver salt using the acetone extract of A. trisulcus. UV-v is absorption spectrum of the solution showed the surface Plasmon resonance derived from the silver nanoparticles at around 465-488 nm, specific area of silver nanoparticles (Figure 1C and 1D). The area under the curve may indicate the size and distribution of sizes of synthesized AgNPs [38].
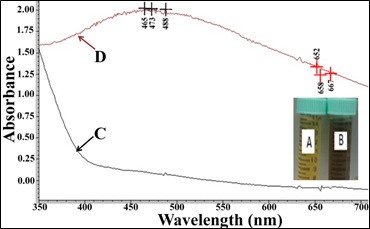
Figure 1: Silver Nanoparticles (AgNPs) synthesis by Anisotes trisulcus leaf extract; A: Extract alone; B: Extract after adding of silver nitrate; C: Light absorbance the extract alone; D: Light absorbance the extract with AgNPs.
Scanning electron micrographs facilitated the characterization of both size and shape of the AgNPs (Figure 2). SEM analysis of the synthesized AgNPs clearly showed clustered and irregular shapes, mostly aggregated and having an average size of 40-60 nm with inter particle distance, which are magnified at ×10,000 times. The shape of AgNPs is a reflection of the color of extract/silver nitrate mixture after the formation of AgNPs [38].
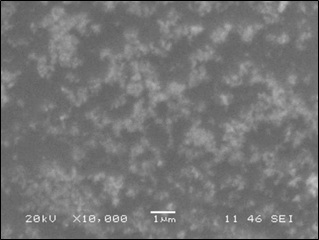
Figure 2: The SEM image showing the crystalline silver nanoparticles as uniform and aggregates.
Fourier Transform Infrared Spectroscopy (FTIR)
FT-IR spectroscopy is useful in probing the chemical composition of the surface of the silver nanoparticles synthesized by Anisotes trisulcus extract and the local molecular environment of the capping agents on the nanoparticles.
Figure 3 represents the FT-IR spectra were studied in the range of 400-4000 cm−1 (Figure 3). The peaks observed at 3125 cm−1 may be due to the stretching and bending vibration of the -OH, the functional group of alcohols and carboxylic acids. The strong peaks at 2350 and 2150 cm−1 correspond to (O=C=O) and (NH) C=O arising due to carbonyl stretch in proteins corresponding to bending of primary and secondary amines, in addition ester, ketone and carboxylic acid groups. The sharp peak at 1600 cm−1 corresponds to C=C aromatic stretch and/or CO2 -stretch of carboxylic acid salts and ketones. The weak bands at 1200 and 1100 cm−1 assigned to C=O vibrations of aliphatic ether and alcohols [39]. From this FT-IR examination, we can conclude that the extract contains alcohols, carboxylic acids, proteins, ketones and aliphatic ether.
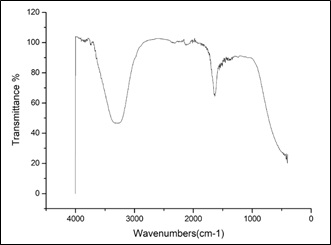
Figure 3: FT-IR spectra of leaf extract of Anisotes trisulcus after addition of AgNO3.
Antibacterial activity
Antibacterial activity (in vitro) results by A. trisulcus leaves extract and extract prepared AgNPs against Gram-positive (S. aureus) and Gram-negative bacteria (E. coli, P. mirabilis, and Shigella flexneri) are summarized in table 1. Both plant leaves extract and extract prepared AgNPs inhibited the bacterial pathogens. Average ZOI produced by plant extract + AgNPs were higher (minimum 12 mm in Shigella flexneri and maximum 19.33 mm in P. mirabilis) than that of produced by plant extract alone (minimum 6.67 mm in E. coli and maximum 7.66 mm in both P. mirabilis and Shigella flexneri). While the average ZOI produced by positive control was observed 21.67 mm (maximum) in S. aureus and 18.67 mm (minimum) in Shigella flexneri. In negative control no ZOI was found. The ZOI formed by A. trisulcus leaves extract mediated Ag NPs were statistically at par with positive control (Penicillin/Streptomycin at 20 units/20 µg respectively) against all selected bacteria. Our results are in accordance with Das et al., [40] and Pallela et al., [41]. The antibacterial activity A. trisulcus leaves extract + Ag NPs may be due to their small size and uniform distribution. The ultra-small size of nanoparticles is responsible for deep penetration and AgNPs diffuse through the cell membrane of microbial pathogens and disrupt the cell function causing cell death. Further, antibacterial activity is also boosted by Reactive Oxygen Species (ROS) generation and surface potential [41].
|
Treatments |
Zone of Inhibition (mm) |
|||
|
Bacterial Strains |
||||
|
Escherichia coli |
Proteus mirabilis |
Staphylococcus aureus |
Shigella flexneri |
|
|
Extract |
6.67 ± 0.58c |
7.67 ± 0.58b |
7.00 ± 1.00c |
7.67 ± 0.58c |
|
Extract + AgNPs |
18.33 ± 1.53b |
19.33 ± 1.15a |
14.67 ± 1.15b |
12.00 ± 2.00b |
|
Control (Positive) |
21.33 ± 0.58a |
19.67 ± 0.58a |
21.67 ± 1.53a |
18.67 ± 1.15a |
|
Control (Negative) |
0.00 ± 0.00d |
0.00 ± 0.00c |
0.00 ± 0.00d |
0.00 ± 0.00d |
Table 1: Antibacterial potentials of Anisotes trisulcus leaves extract and extract prepared Silver Nanoparticles (AgNPs).
Note: Zones of inhibitions are expressed as the average of three replicates ± SD. Means with same superscript letters are not significantly different.
Immunomodulatory effects
The cytotoxic or stimulatory properties that may be found in the A. trisulcus were studied at different concentrations. The results showed that there were stimulatory effects of the extract alone at high concentration (200 µg/mL) and then changed to the inhibitory effect with the decrease in concentration (100-25 µg/mL, figure 4). The stimulatory effect of A. trisulcus at higher concentration may be due to the stimulatory biomolecules found in the extract which may be dominated by inhibitory molecules when the extract diluted. The extract of A. trisulcus might contain growth inhibition mediators like polysaccharides that are known to be toxic to cells, which were obvious when the extract was diluted [42]. The inhibitory effects A. trisulcus leaves extract with AgNPS was dose-dependent where it decreased with the decrease in the extract concentration. The growth inhibition of splenic cells may be due the phagocytosis of the AgNPs found in the extract of A. trisulcus. These nanoparticle materials may lead to the activation of macrophages, which may initiate a cascade of events leading to some inflammatory responses that may produce and secrete some proinflammatory cytokines and chemokines [43,44].
The inhibitory effects of extract decreased with the decrease in the extract concentration. Also, the stimulatory effects decreased with the decrease in the nanoparticles containing extract.
The cytotoxic effects of A. trisulcus have been demonstrated by others [45]. In addition, A. trisulcus was found to have antitumor activity [46], indicating its toxic behaviors. The stimulatory effects of the extract in the presence of AgNPs may be due antagonistic effects of the nanoparticles with the active toxic compounds found in the extract.
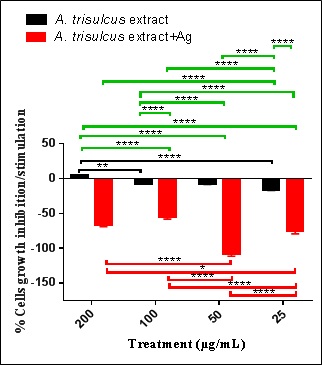
Figure 4: Percent normal splenic cells’ growth stimulation/inhibition after treatment with A. trisulcus leaf extract and extract containing AgNPs.
Note: Where: Black brackets refer to significance among the different concentrations of the extract; red brackets refer to significance among the different concentrations of the extract containing AgNPs; green brackets refer to significance among the two types of treatment. NB: *= 0.019; **= p=0.0071; **** = p<0.0001
Lytic effects of the extract and the extract containing AgNPs
The percentage of RBCs lysis was calculated by comparing the absorbance of sample to the positive and negative controls (Table 2). The positive control (1.5% Triton X-100) showed about 100% lysis, while the negative control (PBS) showed no lysis effects on the RBCs. The biosynthesized AgNPs by the plant leaf extract showed 100% RBCs lysis while, the plant leaf acetone extract alone showed 20.45% RBCs lysis (Figure 5).
|
No. |
Treatment |
Absorbance at Wavelength of 576 nm |
RBC Hemolysis (%) |
|
1 |
Plant leaf acetone extract |
0.667 |
20.45 |
|
2 |
Plant biosynthesized silver nanoparticles (AgNPs) |
> 3.00 |
100 |
|
3 |
Control (Negative) |
0.067 |
0 |
|
4 |
Control (Positive) |
> 3.00 |
100 |
Table 2: Absorbance of Anisotes trisulcus leaf acetone extract alone and its biosynthesized Silver Nanoparticles (AgNPs) with their Red Blood Cells (RBCs) lysis percentage.

Figure 5. The effect of (1) Anisotes trisulcus leaf acetone extract alone and (2) Its biosynthesized Silver Nanoparticles (AgNPs) on cow RBCs. Where, (3) Is negative control and (4) Is positive control.
The lysis effect of plant extract containing AgNPs may be due to direct effect of nanoparticles on the membranes of RBCs. Others referred the hemolytic effects as due to the induced release of oxidative stress products following exposure [47]. Our results of RBCs lysis by the plant leaf acetone extract alone were slightly higher than that of Zohra and Fawzia [48], who measured hemolytic activity of methanol extracts of nine plants from arid and sub-arid area on human erythrocytes. They observed the hemolytic activity in all extracts was not more than 15% and low lysis % age was observed at low concentrations while high hemolytic activity on high concentration. As the hemolytic activity is concentration-dependent therefore it is possibility that our results are slightly high. So, the plant extract should be used at low concentration with a great attention as high concentration will take will increase RBCs lysis.
Acute cytotoxicity effects
The hepatic toxicity effect which may be found in the prepared extracts was tested in adult healthy rats. The levels of serum Aspartate Aminotransferase (AST) showed a non-significant increase (1.16 fold increase) in extract alone and the same (1.09 fold increase) in extract with AgNPs. These results indicate the plant is safe and can be used without any systemic toxic effects. Many authors demonstrated the cytotoxic effects of AgNPs on liver either in vivo [49] or in vitro [50]. In our study, the extract containing AgNPs showed no toxic effects on liver in vivo. This may be due to the bioactive compounds found in the extract that abrogated the toxic effects of the nanoparticles on the liver.
Larvicidal potential
Susceptibility levels of Cx pipiens larvae following treatments with different concentrations of A. trisulcus leaf extract only and the extract containing AgNPs against 4th larval instars of Cx pipiens are shown in table 3. There was a significant (p>0.001) increase in mortality rate among 4th larval instars of Cx pipiens over the increase in the concentrations of A. trisulcus leaf extract only (1000-5000 ppm) and the same situation was observed when using A. trisulcus leaf extract containing AgNPs. There was no significant difference between the corresponding concentrations of A. trisulcus leaf extract only and the extract containing AgNPs. Taking LC50 values (concentration which to kill 50% of larvae) into consideration, the records showed that the A. trisulcus leaf extract with silver nanoparticles (214.047 ppm) proved to be more effective than A. trisulcus leaf extract only (2226.595 ppm) by about 10.4 folds (Figure 6). The results found from this study showed that the effect of the extract of A. trisulcus and the nanoparticles against the larvae of Cx pipiens are in the accordance with what was repeated by other workers while studying the efficacy of certain plant extracts with AgNPs against some Culex spp. [51-53].
|
Concentration (ppm) |
Observed Response % |
|
|
A. trisulcus Extract |
A. trisulcus Extract with AgNPs |
|
|
1000 |
28.571 ± 2.71 |
25.51 ± 2.23 |
|
2000 |
38.776 ± 3.78 |
38.776 ± 2.91 |
|
3000 |
53.061 ± 4.88 |
59.184 ± 3.99 |
|
4000 |
69.388 ± 5.93 |
76.531 ± 4.21 |
|
5000 |
87.755 ± 5.82 |
88.776 ± 3.98 |
Table 3: Susceptibility level of Culex pipiens larvae to A. trisulcus extract and extract containing AgNPs following continuous exposure for 48 h.
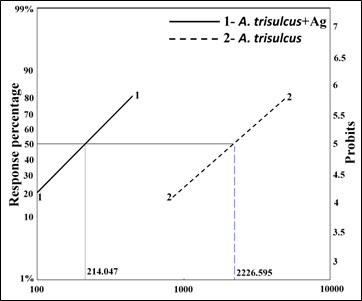
Figure 6. The relationship between concentrations of A. trisulcus leaf extract and mortality percentage of 4th instar larvae of Cx pipiens. Line 1: A. trisulcus +AgNPs; Line 2: A. trisulcus.
Effects of A. trisulcus extract on Hela HepG2 cancer cell lines
A. trisulcus extract showed an insignificant inhibitory effect on Hela cells (-1.86 ± 0.02) and moderate inhibitory effects on HepG2 cells (-56.85 ± 0.22; figure 7). The inhibitory effect on HepG2 was significantly higher (p>0.001) than that on Hela cell line. Also, A. trisulcus extract containing nanoparticles showed inhibitory effects on both Hela (-50.02 ± 0.10) and HepG2 (-61.82 ± 0.35) cells. These inhibitory effects of extract alone may be due the presence of active inhibitory molecules in the extract preparation. The inhibitory effect of the extract containing the nanoparticles was more on HepG2 than that shown on Hela cells and the inhibition that happened in both cell lines may be due to the uptake of AgNPs that lead to cell cytotoxicity [54]. Many of the phyto-compounds identified in the leaf of Anisotes strisulcus like phenolic compounds, tannin and flavonoids possess the anti-cancer properties [55]. This may explain the inhibitory behavior of the extract, alone or with AgNPs, against the cancer cell lines used in this study.
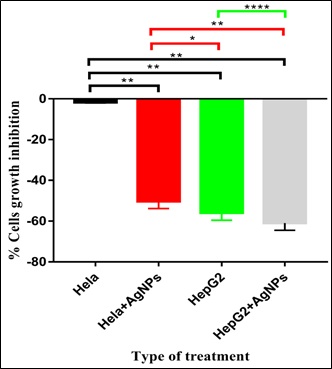
Figure 7: Effects of A. trisulcus leaf extract and extract containing AgNPs on Hela and GepG2 cancer cell lines growth.
Note: *= p=0.0289; **= p= 0.0032; **** = p<0.0001
CONCLUSION
From the results we can conclude that the leaves extract of A. trisulcus could green synthesize AgNPs with a size of 40-60 nm with. The extract contained functional groups that inhibited the growth of splenic, Hela and HePG2 cell proliferation. The extract and extract containing AgNPs are safe on RBCs and vital organ. The extract alone and with AgNPs showed insecticidal activity against Culex pipiens larvae. The extract alone or in combination with AgNPs can be used as immunomodulatory and insecticidal agents.
DATA AVAILABILITY
The data used to support the findings of this study are included within the article.
CONFLICT OF INTEREST
All authors state that they haven’t any financial/commercial conflict of interest regarding this work.
FUNDING STATEMENT
This work was funded by the Deanship of Scientific Research at King Khalid University for funding this work through the Research Groups Program (R.G.P.1) -11/40.
ACKNOWLEDGEMENT
The authors extend their appreciation to the Research Center for Advanced Materials Science (RCAMS), King Khalid University, P.O. Box 9004, Abha 61413, Saudi Arabia, for great help and assistance.
REFERENCES
- El-Shanawany MA, Sayed HM, Ibrahim SRM, Fayed MAA (2014) New nitrogenous compounds from Anisotes trisulcus. Z Naturforsch C J Biosci 69: 209-218.
- Collenette S (1985) An Illustrated Guide to the Flowers of Saudi Arabia. Scorpion Publishing Ltd., London.
- Al-Rehaily AJ (2000) Pharmacognostic Studies on the Leaf of Anisotes trisulcus (Forssk.) Nees Pakistan J Biol Sci 3: 1427-1430.
- Ali NAA, Jülich WD, Kusnick C, Lindequist U (2001) Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol 74: 173-179.
- Al-Rehaily AJ, Al-Said MS, Eltahir KEH (2011) Peganine isolated from Anisotes trisulcus as a smoking deterrent and anorexigenic agent. African J Pharm Pharmacol 5: 1342-1348.
- El-Shanawany MA, Sayed HM, Ibrahim SRM, Fayed MAA (2014) Chemical constituents, anti-inflammatory, and antioxidant activities of Anisotes trisulcus. Bull Fac Pharmacy Cairo Univ 52: 9-14.
- RR Arndt, SH Eggers, A Jordaan (1967) The alkaloids of anisotes sessiliflorus C.B.Cl. (acanthaceae)-five new 4-quinazolone alkaloids. Tetrahedron 32: 3521-3532.
- Al-Rehaily AJ, EL-Sayed KA, Al-Said MS, Ahmed B (2002) Trisulcusine: A novel spiro quinazoline alkaloid from Anisotes trisulcus. Indian J Chem Sect B Org Med Chem 41: 2385-2389.
- El-Shanawany MA, Sayed HM, Ibrahim SRM, Fayed MAA (2011) 5-Hydroxy vasentine, a new pyrroloquinazoline alkaloids from Anisotes trisulcus (Forssk.) Nees. J Nat Prod Plant Resour 1: 80-85.
- Ramanathan T, Satyavani K, Gurudeeban S (2011) Plant mediated synthesis of biomedical silver nanoparticles by using leaf extract of citrullus colocynthis. Res J Nanosci Nanotechnol 1: 95-1011.
- Majeed A, Ullah W, Anwar AW, Shuaib A, Ilyas U, et al. (2016) Cost-effective biosynthesis of silver nanoparticles using different organs of plants and their antimicrobial applications: A review. Mater Technol 33: 1-8.
- Mohanta YK, Panda SK, Jayabalan R, Sharma N, Bastia AK, et al. (2017) Antimicrobial, antioxidant and cytotoxic activity of silver nanoparticles synthesized by leaf extract of Erythrina suberosa (Roxb.). Front Mol Biosci 4: 14.
- Nayak D, Minz AP, Ashe S, Rauta PR, Kumari M, et al. (2016) Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J Colloid Interface Sci 470: 142-152.
- Wiley B, Sun Y, Mayers B, Xia Y (2005) Shape-controlled synthesis of metal nanostructures: The case of silver. Chem A Eur J 11: 454-463.
- Bindhu MR, Umadevi M (2015) Antibacterial and catalytic activities of green synthesized silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc 135: 373-378.
- Mallick K, Witcomb MJ, Scurrell MS (2005) Self-assembly of silver nanoparticles in a polymer solvent: formation of a nanochain through nanoscale soldering. Mater Chem Phys 90: 221-224.
- Zhang XF, Liu ZG, Shen W, Gurunathan S (2016) Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17: 1534.
- Liu YC, Lin LH (2004) New pathway for the synthesis of ultrafine silver nanoparticles from bulk silver substrates in aqueous solutions by sonoelectrochemical methods. Electrochem commun 6: 1163-1168.
- Sandmann G, Dietz H, Plieth W (2000) Preparation of silver nanoparticles on ITO surfaces by a double-pulse method. J Electroanal Chem 491: 78-86.
- Bae CH, Nam SH, Park SM (2002) Formation of silver nanoparticles by laser ablation of a silver target in NaCl solution. Appl Surf Sci 197-198: 628-634.
- Smetana AB, Klabunde KJ, Sorensen CM (2005) Synthesis of spherical silver nanoparticles by digestive ripening, stabilization with various agents, and their 3-D and 2-D superlattice formation. J Colloid Interface Sci 284: 521-526.
- Jain S, Mehata MS (2017) Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and their Enhanced Antibacterial Property. Sci Rep 7: 15867.
- Rheder DT, Guilger M, Bilesky-José N, Germano-Costa T, Pasquoto-Stigliani T, et al. (2018) Synthesis of biogenic silver nanoparticles using Althaea officinalis as reducing agent: evaluation of toxicity and ecotoxicity. Sci Rep 8: 12397.
- Ghramh HA, Kkan KA, Ibrahim EH, Setzer WN (2019) Synthesis of gold nanoparticles (AuNPs) using ricinus communis leaf ethanol extract, their characterization, and biological applications. Nanomaterials 9: 765.
- Ghramh HA, Al-Ghamdi KM, Mahyoub JA, Ibrahim EH (2019) Chrysanthemum extract and extract prepared silver nanoparticles as biocides to control Aedes aegypti (L.), the vector of dengue fever. J Asia Pac Entomol 21: 205-210.
- Kilany M (2017) Isolation, screening and molecular identification of novel bacterial strain removing methylene blue from water solutions. Appl Water Sci 7: 4091-4098.
- Cockeril F, Wikler M, Bush K, Dudley M, Eliopoulos G, et al. (2012) Performance standards for antimicrobial disk susceptibility tests. CLSI, Pennsylvania, USA.
- Ibrahim EH, Kilany M, Ghramh HA, Khan KA, Islam S (2018) Cellular proliferation/cytotoxicity and antimicrobial potentials of green synthesized silver nanoparticles (AgNPs) using Juniperus procera. Saudi J Biol Sci 26: 1689-1694.
- Oves M, Khan MS, Zaidi A, Ahmed AS, Ahmed F, et al. (2013) Antibacterial and cytotoxic efficacy of extracellular silver nanoparticles biofabricated from chromium reducing novel OS4 strain of Stenotrophomonas maltophilia. PLoS One 8: 59140.
- Reitman S, Frankel S (1957) A Colorimetric Method for the Determination of Serum Glutamic Oxalacetic and Glutamic Pyruvic Transaminases. Am J Clin Pathol 28: 56-63.
- Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K (2008) Larvicidal activity of some euphorbiaceae plant extracts against aedes aegypti and culex quinquefasciatus (Diptera: Culicidae). Parasitol Res 102: 867-873.
- WHO (2005) Prevention and control of dengue and dengue hemorrhagic fever. WHO Regional Publication, Serial No.29. 134 pages.
- Chai YS, Hu J, Lei F, Wang YG, Yuan ZY, et al. (2013) Effect of berberine on cell cycle arrest and cell survival during cerebral ischemia and reperfusion and correlations with p53/cyclin D1 and PI3K/Akt. Eur J Pharmacol 708: 44-55.
- Finney D (2009) Probit analyses. Cambridge University Press, Cambridge, United Kingdom.
- Abbott WS (1987) A method of computing the effectiveness of an insecticide. 1925. J Am Mosq Control Assoc 3: 302-303.
- Ranganathan R, Madanmohan S, Kesavan A, Baskar G, Krishnamoorthy YR, et al. (2012) Nanomedicine: towards development of patient-friendly drug-delivery systems for oncological applications. Int J Nanomedicine 7: 1043-1060.
- Manivasagan P, Venkatesan J, Senthilkumar K, Sivakumar K, Kim SK (2013) Biosynthesis, antimicrobial and cytotoxic effect of silver nanoparticles using a novel Nocardiopsis sp. MBRC-1. Biomed Res Int 2013: 287638.
- Wiley BJ, Im SH, Li ZY, McLellan J, Siekkinen A, et al. (2006) Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J Phys Chem B 110: 15666-15675.
- Umoren SA, Obot IB, Gasem ZM (2014) Green synthesis and characterization of silver nanoparticles using red apple (malus domestica) fruit extract at room temperature. J Mater Environ Sci vol 5: 907-914.
- Das R, Gang S, Nath SS (2011) Preparation and antibacterial activity of silver nanoparticles. J Biomater Nanobiotechnol 2: 472-475.
- Pallela PNVK, Ummey S, Ruddaraju LK, Pammi SVN, Yoon SG (2018) Ultra Small, mono dispersed green synthesized silver nanoparticles using aqueous extract of Sida cordifolia plant and investigation of antibacterial activity. Microb Pathog 124: 63-69.
- Nam KS, Shon YH (2009) Chemopreventive effects of polysaccharides extract from Asterina pectinifera on HT-29 human colon adenocarcinoma cells. BMB Rep 42: 277-280.
- Yue H, Wei W, Yue Z, Wang B, Luo N, et al. (2012) The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials 33: 4013-4021.
- Zhang B, Wei P, Zhou Z, Wei T (2016) Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights. Adv Drug Deliv Rev 105: 145-162.
- Ivanova A, Mikhova B, Najdenski H, Tsvetkova I, Kostova I (2005) Antimicrobial and cytotoxic activity of Ruta graveolens. Fitoterapia 76: 344-347.
- Preethi KC, Kuttan G, Kuttan R (2006) Anti-tumour activity of ruta graveolens extract. Asian Pacific J Cancer Prev 7: 439-443.
- Mocan T (2013) Hemolysis as expression of nanoparticles-induced cytotoxicity in red blood cells. Biotechnology Molecular Biology and Nanomedicine 1: 7-12.
- Zohra M, Fawzia A (2014) Hemolytic activity of different herbal extracts used in Algeria. International Journal of Pharma Sciences and Research 5: 495-500.
- Gaiser BK, Hirn S, Kermanizadeh A, Kanase N, Fytianos K, et al. (2013) Effects of silver nanoparticles on the liver and hepatocytes in vitro. Toxicol Sci 131: 537-547.
- Heydrnejad MS, Samani RJ, Aghaeivanda S (2015) Toxic effects of silver nanoparticles on liver and some hematological parameters in male and female mice (Mus musculus). Biol Trace Elem Res 165: 153-158.
- Mahyoub JA, Aldhahri M, Al-Saidi HM, Aljameeli MME, Assagaf AI, et al. (2016) Biological effects of Salicornia fruiticosa aqueous extract and its synthesized silver nanoparticles against Culex pipiens L. (diptera: Culicidae). Egypt J Biol Pest Control 26: 833-838.
- Elumalai D, Kaleena PK, Ashok K, Suresh A, Hemavathi M (2016) Green synthesis of silver nanoparticle using Achyranthes aspera and its larvicidal activity against three major mosquito vectors. Eng Agric Environ Food 9: 1-8.
- Fouad H, Hongjie L, Hosni D, Wei J, Abbas G, et al. (2017) Controlling Aedes albopictus and Culex pipiens pallens using silver nanoparticles synthesized from aqueous extract of Cassia fistula fruit pulp and its mode of action. Artif Cells Nanomed Biotechnol 46: 558-567.
- Zeng Q, Shao D, Ji W, Li J, Chen L, et al. (2014) The nanotoxicity investigation of optical nanoparticles to cultured cells in vitro. Toxicol Rep 1: 137-144.
- Khurgain AA, Dahan ZA, Gyananath G (2018) The GC-MS analysis of leaf extracts of a medicinal plant, Anisotes trisulcus (Forrsk.). Int J green Herb Chem 7: 1-9.
Citation: Ghramh HA, Ibrahim EH, Kilany M, Khan KA, El-Mekkawy HI (2019) Immunomodulatory, Antimicrobial and Insecticidal Properties of Anisotes Trisulcus (Forssk.) Leaf Extract Containing Silver Nanoparticles. J food Sci Nutr 5:050.
Copyright: © 2019 Hamed A Ghramh, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

