
Impact of Salt Concentrations and Storage Temperatures on Microbial Safety of Paddlefish Roe
*Corresponding Author(s):
Ravi JadejaDepartment Of Animal Science, Oklahoma State University, Stillwater, OK, 74078, United States
Tel:405-744-3922,
Email:Ravi.jadeja@okstate.edu
Abstract
In this study, authors have investigated the impacts of salt concentrations and storage temperatures on the microbial safety of paddlefish roe. Fresh caught fish raw samples were obtained from the Paddlefish Research Center in Miami, Oklahoma. Fish raw samples with the final salt concentrations 3, 2.5, and 2% (W/W) were prepared and inoculated with L. monocytogenes to achieve the final bacterial concentration of approximately 4 log CFU/g. Each inoculated sample was stored at -0.5oC, 3.3oC, and 10oC for 15 days. Each samples was analyzed for pathogen presence at 0, 5, 10, and 15 days of storage. Results indicated that salt concentrations have a significant impact on microbial growth, especially in product stored at an elevated temperature. For the samples stored at 10oC, no salt sample had the most L. monocytogenes growth of 4.92 log CFU/g compared to the 3% salt sample, which had a 2.52 log CFU/g increase over 15 days. The salt concentrations did not have a significant impact on the bacterial growth for the samples stored at–0.5oC. The research indicated that precise storage temperature control is the key to safety produce low salt-containing fish roe products.
Keywords
Caviar; Egg; L. monocytogenes; Salt Paddlefish
Introduction
Caviar and Fish Roe products are aquatic animals' salt-cured and preserved eggs [1]. The word “caviar” originates from the Persian expression “Mahi Khaviari,” which translates to “egg generating fish” [2]. Sturgeon and other fish roe-producing fish have been around for millions of years. The eggs of fish have been preserved and eaten in many ancient cultures [3]. The first references to the products most like Caviar today are found in the works of Homer and Aristotle. In those times, Caviar was served on platters garnished with flowers. Today, Caviar is served on lightly buttered or dry toast. For the past 200-300 years, caviar has widely been regarded as a ‘luxury product’ and has been coveted by elites for many years. Russian tsars implemented a caviar tax, adding to rarity of the product (Van Uhm & Siegel, 2016). Because of this, fish roe products have historically had a large black market [4].
Fish Roe and Caviar products are becoming increasingly popular in markets such as America, Russia, and Iran [1]. The market is projected to increase in the years 2020-2024 by 8%. Caviar and fish roe products are salt-cured unfertilized fish eggs. Caviar only comes from sturgeon fish; meanwhile, fish roe refers to all fish eggs. The terms are often used interchangeably by consumers. Caviar and fish roe products are ready-to-eat products that do not require further cooking but can be heat treated.
In the last decade, it was observed that there was a significant surge in the production of fish roe as the demand for the product increased steeply. In 2014, the United States exported two metric tons of fish roe prized at three hundred euros per kilogram. The U.S. export of fish roe increased from two matric tons in 2014 to almost ten metric tons in 2018 [5]. The increase in the fish roe production in the U.S. matches the worldwide trend of increased production, which observed over almost 300% increase in fish roe production between 2014 to 2018 [5].
Since 2020, COVID-19 has severely impacted food production worldwide. But, the pandemic has not slowed down the growth of the caviar market. Caviar and fish roe products are projected to have a high growth rate compared to the global GDP growth [6]. The compound annual growth rate of the caviar and fish roe markets is estimated to be between 7%-9% in the next five years, and the growth for the fiscal year 2021 was at 6.26% [6].
In the U.S., Paddlefish (Polyodon spathula) has been identified as one of the significant sources of fish roe. Paddlefish are found throughout the U.S., including Oklahoma. Paddlefish produce a fish roe that is sensitive to overexploitation because it is an expensive source of caviar [7]. The overexploitation of Paddlefish roe comes from overfishing. Paddlefish meat is highly regarded; additionally, the roe is held to a very high standard for a caviar product [8]. Overfishing was occurring because Caviar and Fish roe products are increasing in demand. Fish roe products are seen as luxurious and have seen a sharp increase in consumption. To curb this, other states across America have implemented measures such as bag limits, harvest caps or quotas, and restrictive licensure. To address the issue of Paddlefish overfishing in Oklahoma, the Oklahoma Department of Wildlife Conservation banned the commercial fishing of Paddlefish [7]. The Paddlefish conservation efforts in Oklahoma were further strengthened by establishing the Paddlefish Research Center (PRC) by the Oklahoma Department of Wildlife Conservation near Miami, Oklahoma (Scarnecchia et al., 2013). The PRC engages in research related to Paddlefish and promotes conservation efforts for the fish. The center supports the anglers by cleaning and processing their Paddlefish for a donation of fish roe. The PRC further processes and salts the fish roe and sells the product worldwide.
L. monocytogenes infections can cause serious foodborne illnesses. The foodborne illness caused by L. monocytogenes is known as Listeriosis. The food contaminated with L. monocytogenes is the primary source of Listeriosis (Madjunkov et al., 2017). The mortality rate of L. monocytogenes infection is estimated to be around 20% to 30% [9]. This pathogenic agent is estimated to be the third-largest cause of death among infections caused by 31 known foodborne pathogens [10]. In the United States, the Centers for Disease Control and Prevention estimates that L. monocytogenes is responsible for approximately 260 deaths and 1500 illnesses each year [11]. Ready-to-eat food products including meat and seafood along with unpasteurized milk products are major sources of Listeria infection. Table 1 summarizes L. monocytogenes related foodborne illnesses and implicated foods worldwide.
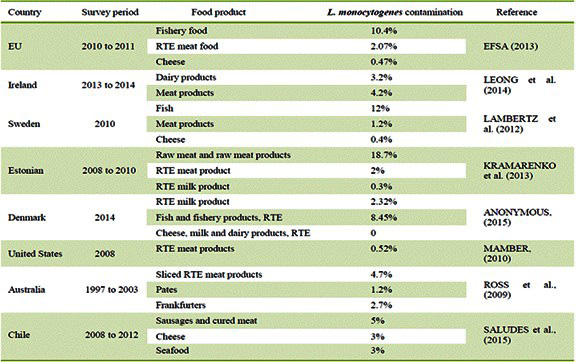 Table 1: L. Monocytogenes contamination by product type.
Table 1: L. Monocytogenes contamination by product type.
Caviar and fish roe products could be potential reservoirs of L. monocytogenes contamination. Table 2 shows L. monocytogenes contamination by fish species. Recognizing that there are L. monocytogenes in fish, there is also a potential for contamination in fish roe and caviar [12]. Some caviar producers utilize a heat process to pasteurize fish roe, but the processed fish roe is less attractive to the consumer and experiences a significant reduction in monetary value. Therefore, many fish roe producers rely on salt (at least 3%) and low-temperature storage to control pathogen growth (Huss et al., 2000; Farber, 1991).
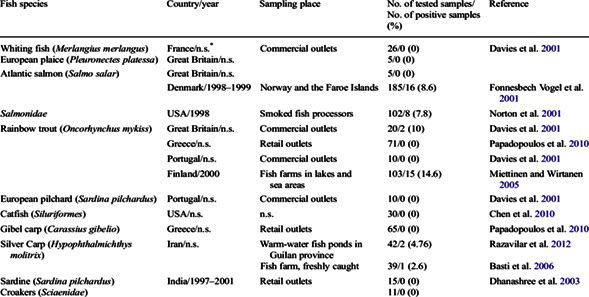 Table 2: L. Monocytogenes contamination by fish species.
Table 2: L. Monocytogenes contamination by fish species.
Consumers are shifting toward products with less salt, and Caviar is no exception. Hypertension is one of the leading causes of death in America, and consuming less salt reduces the risk of high blood pressure [13]. But, there is a complete lack of research on the impact of varying levels of salt concentrations and storage temperatures on L. monocytogenes growth in Paddlefish roe. Therefore, this study measures the microbial risks associated with the low salt Paddlefish roe stored at varying temperatures.
Materials and Methods
Bacterial cultures
For this set of experiments, four different isolates of L. monocytogenes were used, V7-2, 39-2, ScottA-2, and 383-2. All strains were generously given by Dr. Peter Muriana, Oklahoma State University. To improve recovery from inoculated fish roe, all L. monocytogenes strains were pre-screened for Rifamycin S/V (10 ug/ml) and Streptomycin sulfate (100 ug/ml). The strains were grown individually in 6 mL of tryptic soy broth (TSB; Difco, Becton Dickinson, Sparks, MD) supplemented with Rifamycin S/V (10 ug/ml) and Streptomycin sulfate (100 ug/ml). The strains were allowed to grow for 20 hours at 32°C. Following incubation, 1 mL was taken from each strain and combined in a 15ml sterile centrifuge tube to form a four-strain pathogen mixture for inoculation. The pathogen cocktail was washed three times by centrifugation at 4000Xg and suspended in phosphate-buffered saline (PBS) solution. The strain cocktail was serially diluted in PBS to achieve approximately 4 log CFU/g final concentration L. monocytogenes in fish roe once mixed.
Fish roe sample preparation
The fish roe samples were obtained from the Paddlefish Research Center(PRC) in Miami, Oklahoma. Briefly, fresh-caught paddlefishes were processed to harvest fish roe at PRC, Miami, Oklahoma. The harvested fish roe (unsalted) were carefully packed in sterile Whirl-PAK bags (Model no S-22730- 69 Oz.) and transported to the food microbiology laboratory in a pre-cooled ice chest. The fish roe was stored at -20oC until used in the experiments in the laboratory. Before the experiment, the fish roe samples were thawed by holding at 4 ±1oC overnight. The next day, the fish roe was mixed with non-iodized salt (Sigma-Aldrich, MO) to obtain a finished product with the final salt concentrations of 3, 2.5, and 2% (W/W). No salt added fish roe samples were used as controls. After curing, four samples weighing 25g each were prepared from each corresponding fish roe sample with different salt levels. Each 25 g sample was placed in sterile Whirl-PAK (model S-19792- 4 Oz.) and inoculated with 1 ml of L. monocytogenes to achieve the final bacterial concentration of approximately 4 log CFU/g. The samples were stored at three different temperatures -0.5oC, 3.3oC, and 10oC and samples were analyzed for pathogen presence at 0, 5, 10, and 15 days of storage. The storage temperature and salt concentrations were selected based on PRC’s request. Salt intensity was measured using salt concentration and not water phase salt. Water Phase Salt is the amount of salt compared to the amount of moisture in the flesh. Moist fish products will require more salt than drier fish products and will taste saltier even with Water Phase Salt being the same. For this reason, measuring salt concentration is more accurate for consumer preference; salt concentration provides a more precise measure of how salty a product is [14].
Microbial enumeration
At the end of each storage period, each sample was mixed with 50 ml PBS and blended at a medium speed for 1 min (Seward Stomacher®, 80 Biomaster, Worthington, U.K.). The resulting slurry samples were then serially diluted, and appropriate concentrations were plated on tryptic soy agar supplemented with Rifamycin S/V (10 ug/ml) and Streptomycin sulfate (100 ug/ml). Plates were incubated at 32oC overnight, and bacterial colonies were counted the next day.
Statistical analysis
All experiments were repeated at least three times. The data are presented as the mean and standard deviation of multiple replications. The statistical analysis was performed using repeated measures, one-way analysis of variance, and the Holm-sidak test for pairwise multiple comparisons to determine significant differences (p < 0.05) among treatments.
Results
The growth of L. monocytogenes in the paddlefish roe containing salt concentrations ranging from 0 to 3% and stored at -0.5oC, 3.3oC, and 10oC for 15 days are presented in figures 3.1, 3.2, and 3.3 respectively. It was observed that L. monocytogenes was not impacted by the presence of salt when stored at -0.5oC (Figure 1). The minimum growth temperature for L. monocytogenes is -0.1oC to -0.4oC; therefore, the storage temperature alone was sufficient to prevent the growth of the target pathogen [15].
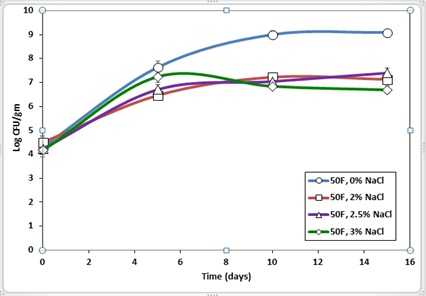 Figure 1: Survival of Listeria monocytogenes in Paddlefish roe with salt concentration ranging from 0 to 3% and stored at 10oC for 15 days.
Figure 1: Survival of Listeria monocytogenes in Paddlefish roe with salt concentration ranging from 0 to 3% and stored at 10oC for 15 days.
When fish roe samples were stored at 3.3oC the impact of salt was a little more noticeable. For the sample with no salt, over the 15 days of storage L. monocytogenes number increased by 4.22 log CFU/g, while the bacterial growth was significantly less (2.2 log CFU/g) in the sample with 3% salt. All salt-containing fish roe samples were equally effective in preventing L. monocytogenes growth from the fish roe stored at 3.3 oC during the first 10 days of storage. But, the 3% salt-containing sample was significantly better at slowing the target pathogen growth than 2 and 2.5% salt-containing samples at the end of the storage period (Figure 2). A study by Hwang (2007) observed that the salt concentrations above 2% can effectively reduce L. monocytogenes growth from smoked salmon when stored at 5 oC. The direct comparison of our research findings is not possible due to the difference in the food source used in the study by [16]. [17] noticed that the salt concentrations over 2% effectively prevent L. monocytogenes from chum salmon roe and caviar when stored at 3oC. In our study, we have observed the growth of the pathogen during the storage temperature. The difference in storage temperature (3oC Vs 3.3oC in our experiment) could be the reason for the microbial growth. In addition, no pathogen growth in Shin and Rasco’s study could be the function of selective media, modified oxford agar, to recover L. monocytogenes, which could /reduce the recovery of stressed microbes. [18] reported that modified oxford agar could reduce the recovery of heat-stressed L. monocytogenes by almost 3 log CFU/ml compared to tryptic soy agar.
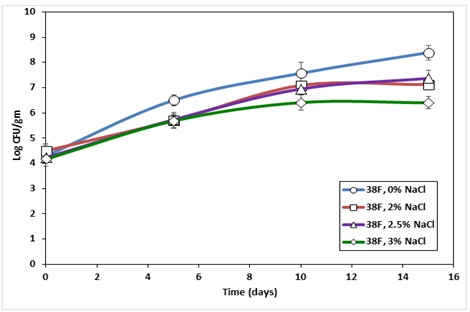 Figure 2: Survival of Listeria monocytogenes in Paddlefish roe with salt concentration ranging from 0 to 3% and stored at 3.3oC for 15 days.
Figure 2: Survival of Listeria monocytogenes in Paddlefish roe with salt concentration ranging from 0 to 3% and stored at 3.3oC for 15 days.
The Figure 3 lists L. monocytogenes recoveries from fish roe containing varying amounts of salt and stored at 10 oC. Significant growth of pathogens was observed among all samples, but the no salt sample had the most L. monocytogenes growth of 4.92 log CFU/g compared to the 3% salt sample, which had a 2.52 log CFU/g increase over 15 days. Our results are in agreement with the findings reported by [17]. In their study of the impact of salt concentration on L. monocytogenes survival in chum salmon roe and caviar, Shin and Rasco reported a significant growth of the target pathogen in caviar with more than 4.36% salt concentration when the product was stored at 7oC.
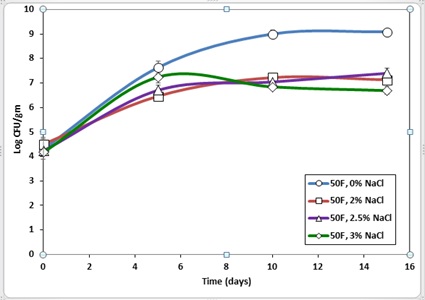 Figure 3: Survival of Listeria monocytogenes in Paddlefish roe with salt concentration ranging from 0 to 3% and stored at 10oC for 15 days.
Figure 3: Survival of Listeria monocytogenes in Paddlefish roe with salt concentration ranging from 0 to 3% and stored at 10oC for 15 days.
Conclusion
In conclusion, it was observed that all salt concentrations used in the study were significantly more effective in controlling L. monocytogenes than the no-salt control samples. The study results indicate that precise low temperature control provides a more critical hurdle for preventing L. monocytogenes than salt alone.
Acknowledgements
Authors like to acknolwdge that the study was made possible through financial and materails provided by the Oklahoma Department of Wildlife Conservation and Virgil & Marge Jurgensmeyer Endowed Professorship, Oklahoma State University.
References
- Bledsoe GE, Bledsoe CD, Rasco B (2003) Caviars and Fish Roe Products. Critical Reviews in Food Science and Nutrition43: 317-356.
- Tavakoli S, Luo Y, Regenstein JM, Daneshvar E, Bhatnagar A, et al. (2021) Sturgeon, Caviar, and Caviar Substitutes: From Production, Gastronomy, Nutrition, and Quality Change to Trade and Commercial Mimicry. Reviews in Fisheries Science & Aquaculture 1-25.
- Shadrina E (2007) The Great Caspian Caviar Game. Security Index: A Russian. Journal on International Security 13: 55-78.
- Zabyelina YG (2014) The “fishy” business: A qualitative analysis of the illicit market in black caviar. Trends in Organized Crime 17: 181-198.
- European Union E (2021) The Caviar Market. In E. M. O. f. F. a. A. Products (Ed.) 10-24.
- Technavio (2021) Global Caviar Market to grow by almost $ 500 Million During 2021-2025 | Post COVID-19 Analysis.
- Schooley JD, Scarnecchia DL, Crews A (2014) Harvest Management Regulation Options for Oklahoma’s Grand Lake Stock of Paddlefish Journal of the Southeastern Association of Fish and Wildlife Agencies 1: 89-97.
- Jennings C, Zigler S (2009) Biology and life history of paddlefish in North America: An update 66: 1-22.
- Hernandez-Milian A, Payeras-Cifre A (2014) What Is New in Listeriosis? BioMed Research International 358051.
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, et al. (2011) Foodborne Illness Acquired in the United States-Major Pathogens. Emerging Infectious Disease Journal 17: 7-15.
- FDA US (2020) Get the Facts about Listeria.
- Jørgensen LV, Huss HH (1998) Prevalence and growth of Listeria monocytogenes in naturally contaminated seafood. International J of food microbiology 42: 127-131.
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ (2017) Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Pr. Hypertension 71: e13-e115.
- Centre for Disease Control BC (2013) Fish Safety Notes - Salting Fish.
- Walker SJ, Archer P, Banks JG (1990) Growth of Listeria monocytogenesat refrigeration temperatures. J Appl Bacteriol 68: 157-162.
- Hwang CA (2007) Effect of Salt Smoke Compound and Storage Temperatures on the Growth of Listeria monocytogenesin Simulated Smoked Salmon. J of Food Prot 70: 2321-2328.
- Shin JH, Rasco B (2007) Effect of Water Phase Salt Content and Storage Temperature on Listeria monocytogenes Survival in Chum Salmon Roe and Caviar. J Food Sci 72: M160-M165.
- Lavieri NA, Sebranek JG, Cordray JC, Dickson JS, Jung S, et al. (2014) Evaluation of the Thin Agar Layer Method for the Recovery of Pressure-injured and Heat-injured Listeria monocytogenes. J Food Prot 77: 828-831.
Citation: Washington M, Teng XM, Gainer C, Jadeja R (2023) Impact of Salt Concentrations and Storage Temperatures on Microbial Safety of Paddlefish Roe. J Food Sci Nutr 9: 156
Copyright: © 2023 Marcus Washington, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

