
Improvement of Camel Milk Microbial Loads by Activation of Lactoperoxidase Enzyme System during Different Storage Temperature
*Corresponding Author(s):
IEM El ZubeirDepartment Of Dairy Production, Faculty Of Animal Production, University Of Khartoum, P.O. 321, Khartoum, Sudan
Email:Ibtisamelzubeir17@gmail.com / Ibtisam.elzubeir@uofk.edu
Abstract
This study was designed to investigate the effect of using the recommended FAO Lactoperoxidase Enzyme System (LPS) on improving the keeping quality and increasing the shelf life of raw milk from camels at different stages of lactations. Fresh milk samples were obtained after morning milking from Camel Research Center of Khartoum University. The milk samples were cooled immediately before transported to the laboratory, where they were divided into two groups (control and treated), then each group was subdivided into four sub-groups that kept at 5±2°C, 13±2°C, 25±2°C and 37±2°C temperatures. All samples were subjected to microbial examinations (total bacterial count, psychrotrophic bacterial count, coliform count and yeast and mould counts), acidity and clot-on-boiling test during the period of storage. The results showed significant (P<0.05) variations between the control and LPS treated milk samples on Total Bacterial Count (TBC), psychrotrophic bacterial count, coliform count and yeast and mould counts. Moreover the data revealed that camels’ milk samples from different stages of lactation treated with LPS showed longer shelf life and better keeping quality during all storage degrees of temperature compared with the control samples under the same conditions. The study concluded that although the LPS activity is affected by the storage temperature of the milk, it is useful in prolonging the shelf life of raw milk. Hence it could be applied in hot areas like Sudan in order to utilize the huge quantity of the milk produced in the traditional systems.
Keywords
Camel milk; Lactation stage; Lactoperoxidase enzyme system; Microbial load; Temperature
INTRODUCTION
Camel milk is an important source of essential nutrients, but beyond its nutritive value it has different functional and medicinal properties that make it a very useful choice as it is used in some parts of the world to improve health status [1]. Moreover the production of camel milk on commercial level would provide not only more food for camel herders, but also it is an additional source of income [2].
Despite the lack of refrigeration, camel’s milk remains unspoiled for several days, which might be due to the antibacterial activity of certain minor proteins contained in camel’s milk [3]. The shelf life of camels milk is longer compared to other milk animals since it contain antibacterial agent [4]. However the inhibition of bacterial growth during storage and transportation of camel raw milk is important in order to enhance its utilization [5].
In Sudan, the TBC (log10 4.6±0.08), coliform (log10 3.4±0.09) and psychrotrophic count (log10 0.8±0.1) of camel milk samples were found higher during summer season [6]. This situation necessitates the introduction of safe method of preservation suitable for the local conditions. Moreover, because of the recent awareness of the medicinal value of camel milk and the more commercially oriented attitude by camel herders, it has been recommended the use of Lactoperoxidase System (LPS) in Sudan after initiation and development of milk collection centers [7]. Because the camel milk is produced in areas with high ambient temperature that lack milk cooling facilities, the LPS could be one the methods to preserve freshness of milk until it is marketed or reaches where there is milk cooling facilities [8].
Lactoperoxidase is a glycoprotein that occurs naturally in colostrum, milk, and many other human and animal secretions [9]. For anti-microbial function, lactoperoxidase needs the presence of hydrogen peroxide and thiocyanate, which have been called together ‘lactoperoxidase system’. At present, this system is considered to be an important part of the natural host defense system in mammals [10]. Lactoperoxidase is found in milk, tears, and saliva contributes to the non-immune host defense system, exerting bactericidal activity mainly on Gram negative bacteria [11].
Lactoperoxidase system (LPS) is active against both Gram positive and Gram negative microbes to varying extents [12-14]. Activation of lactoperoxidase system was investigated as potential alternative method to sustain the safety of milk by inhibiting certain microorganisms with known pathogenic potential [13].
Lactoperoxidase activity is maintained at a high level throughout lactation; however, human lactoperoxidase is present only in colostrum and becomes undetectable within one week after parturition [15]. The aim of this study is to correlate the effect of LPS on the preservation time (shelf life) of camel’s milk of different stages of lactation in relation to different storage temperature.
MATERIALS AND METHODS
Sources and collection of milk samples
Camel milk was obtained from Camel Research Center of the University of Khartoum, where nutrition and environment were controlled. The samples (n= 36) of camel’s milk were obtained at morning milking from six healthy Arabi local she camels (Rashaida type) at the third parity. The same she camels are sampled six times during the entire period of milk production (early, medium and late stages of lactation; two samples were taken during each stage within intervals of 45-50 days). Two liters of the milk samples was collected separately for each animal after cleaning the udder carefully and drying it with clean towels. The samples were collected into clean, sterilized containers and transported to the laboratory in an ice box container. The pH was determined immediately after milking using pH meter (model 5A520, Rench Meter).
Treatment of milk samples
This study was conducted at the laboratory of microbiology, Department of Dairy Production, Faculty of Animal Production, University of Khartoum. The lactoperoxidase enzyme system kits were obtained from Ministry of Animal Resources, offered by the FAO for field trail of the LPS in Sudan).
The milk samples from each animal was divided into two equal portions, one portion was preserved with the lactoperoxidase enzyme system (concentration of 0.25 mM), while the second was kept as control. Each portion was further subdivided into four equal groups, the first was kept at 37±2ºC, the second was kept at 25±2ºC, the third was kept at 13±2ºC and the fourth was kept at refrigerator (5±2ºC).
The milk samples were examined to determine the following microbial load, the total viable bacterial count, coliform count, yeast count and psychotrophic bacterial count. The determination of coliform, yeast and psychotrophic bacterial counts were carried out after one hour after milking, after six hours after adding the enzyme and then after each 48 hours until the milk samples were spoiled. However the determination of the total viable bacterial count was done after one hour after milking, after six hours after adding the LPS enzyme and daily until the spoilage of the samples. Acidity and clot–on-boiling test were determined daily to assess the shelf life of the samples.
Examination of milk samples
Microbiological examination
All media were obtained in dehydrated forms and prepared according to the manufacturer’s instructions. The preparation of serial dilutions and preparation of the media were done as outlined by Houghtaby [16]. Sterilization of equipments was done according to standards methods [17]. Plate count agar medium (Scharlau Chemie S.A. Batch 15599 Barcelona, Spain European Union) was used to determine the total viable bacteria and psychrotrophic bacterial counts [16].
MacConkey agar medium (Scharlau Chemie S.A. Batch 15455 Barcelona, Spain, European Union) was used to determine the coliform count [18]. Potato dextrose agar medium (Hi media Laboratories Pvt. Ltd. Mumbai. 400086, India) was used to determine the total yeast count [19].
Chemical examinations
The acidity was determined according to commercial testing and public control in the dairy industry [20]. The clot on boiling test was performed as described by IDF [21]. Both tests were done every day until the milk samples were spoiled.
Statistical Analysis
The obtained results were analyzed statistically using factorial arrangement. Analysis of variance (ANOVA) test was used to evaluate the mean differences among different treatments at P≤0.05 significant level [22,23].
RESULTS
Effect of LPS, storage temperature and stages of lactation on camel’s milk
This study presented milk samples of camel’s at three stages of lactation (early, medium and late), stored at different degrees of temperature (5±2°C, 13±2°C, 25±2°C and 37±2°C) and either treated by the LPS or non-treated as shown in table 1.
|
Temperature ( C) |
Control milk |
LPS treated milk |
||||
|
Lactation stages |
||||||
|
Early |
Medium |
Late |
Early |
Medium |
Late |
|
|
5±2 C |
4.90±0.84hi |
5.10±0.39ef |
5.11±0.37ef |
4.49±1.02l |
4.73±0.57k |
4.78±0.32j |
|
13±2 C |
5.17±0.42c |
5.16±0.20cd |
5.18±0.19c |
4.90±0.58hi |
4.93±0.25h |
4.91±0.20hi |
|
25±2 C |
5.23±0.22b |
5.24±0.15b |
5.23±0.18b |
4.99±0.28gh |
5.01±0.19g |
4.98±0.21gh |
|
37±2 C |
5.35±0.21ab |
5.34±0.16ab |
5.37±0.12a |
5.12±0.25e |
5.12±0.16e |
5.14±0.15de |
|
P-value |
0.0391* |
|||||
|
SE± |
0.0072 |
|||||
Table 1: Effect of LPS enzymes, temperature and lactation stages on total bacterial count (log) of camel’s milk.
The means sharing the same superscript letters are not significantly (P>0.05) different according to DMRT.
SE± = Experimental standard error
P-value = Level of significance (probability)
Effect of LPS, storage temperature and stages of lactation of camels on the total bacterial count of milk
The LPS treated camel’s milk samples revealed very low total bacterial count in comparison with the control samples at the same storage temperature and stages of lactation. The effect of the total bacterial count of camel’s milk samples were affected significantly (P≤0.05) by LPS, storage temperature and stages of lactation (Table 1).
The means and standard errors of log total bacterial count of LPS treated milk samples from camels at early, medium and late stages of lactation that stored at 5±2°C revealed 4.49±1.02, 4.73±0.57 and 4.78±0.32 respectively. However the non-treated milk samples under the same storage degrees of temperature showed 4.90±0.84, 5.10±0.39 and 5.11±0.39, respectively (Table 1). At 13±2°C, the means and standard errors of log total bacterial count of LPS treated milk samples were 4.90±0.85, 4.93±0.25 and 4.91±0.02 for camels at early, medium and late stages of lactation respectively, compared with 5.17±0.42, 5.16±0.20 and 5.18±0.19 for untreated milk samples, respectively (Table 1).
The means and standard errors of log total bacterial count were 4.99±0.28, 5.01±0.19 and 4.98±0.21 for the LPS treated milk samples of camels at early, medium and late stages of lactation respectively, compared with log 5.23±0.22, 5.24±0.15 and 5.23±0.18 for untreated (control) camel’s milk samples respectively, at 25±2°C (Table 1). Also the means and standard errors of log total bacterial count of LPS treated milk samples stored at 37±2°C showed log 5.12±0.25, 5.12±0.16 and 5.14±0.15 for camels at early, medium and late stages of lactation, respectively. However the control camel’s milk samples stored at the same degrees of storage temperature revealed log 5.35±0.21, 5.34±0.16 and 5.37±0.12, respectively (Table 1).
Effect of LPS, storage temperature and different stages of lactation of camels on the psychrotrophic bacterial count milk samples
There were no variations between psychrotrophic bacterial count at 13±2°C and 25±2°C of control camel’s milk samples, but all control samples contained higher load of psychrotrophic bacteria than the LPS treated samples. The effect of lactoperoxidase enzyme system, storage temperature and stages of lactation of camels on the psychrotrophic bacterial count of milk samples were affected significantly (P≤0.05) by LPS, storage temperature and stages of lactation (Table 2).
|
Temperature °C |
Control milk |
LPS milk |
||||
|
Lactation stages |
||||||
|
Early |
Medium |
Late |
Early |
Medium |
Late |
|
|
5±2°C |
4.60±0.58l |
4.80±0.38g |
4.67±0.38j |
4.07±0.91o |
4.41±0.36n |
4.40±0.48n |
|
13±2°C |
4.83±0.34f |
4.96±0.15b |
4.85±0.19e |
4.52±0.51m |
4.59±0.22l |
4.53±0.25m |
|
25±2°C |
4.91±0.28c |
4.97±0.11b |
4.94±0.17bc |
4.65±0.48jk |
4.74±0.18h |
4.71±0.22i |
|
37±2°C |
4.91±0.16c |
5.12±0.12a |
5.10±0.17ab |
4.88±0.39d |
4.90±0.12c |
4.85±0.19e |
|
P-value |
0.0375* |
|||||
|
SE |
0.0094 |
|||||
Table 2: Effect of LPS enzymes, temperature and lactation stages of camels on psychrotrophic bacterial count (log) milk.
The means sharing the same superscript letters are not significantly (P>0.05) different according to DMRT.
SE± = Experimental standard error
P-value = Level of significance (probability)
The means and standard errors of log psychrotrophic bacterial count of LPS treated milk samples from camels at early, medium and late stages of lactation that stored at 5±2°C were 4.07±0.91, 4.41±0.36 and 4.40±0.48, respectively. However the non-treated milk samples under the same storage temperature revealed log 4.60±0.58, 4.80±0.38 and 4.67±0.38, respectively (Table 2). At 13±2°C, the means and standard errors of log psychrotrophic bacterial count of LPS treated milk samples were 4.52±0.51, 4.59±0.22 and 4.53±0.25 for camels at early, medium and late stages of lactation respectively, compared with log 4.83±0.34, 4.96±0.15 and 4.85±0.19 for untreated milk samples, respectively (Table 2).
The means and standard errors of log psychrotrophic bacterial count were 4.65±0.48, 4.74±0.18 and 4.71±0.22 for the LPS treated milk samples from camels at early, medium and late stages of lactation respectively. However log 4.91±0.28, 4.97±0.11 and 4.94±0.17 were found for the untreated (control) camel’s milk samples at the same stage of lactation at 25±2 °C as shown in table 2. Also the means and standard errors of log psychrotrophic bacterial count of LPS treated samples stored at 37±2°C were 4.88±0.39, 4.90±0.12 and 4.85±0.19 for milk samples from camels at early, medium and late stages of lactation respectively, while the control milk samples from camels of the same stages of lactation and stored at the same degrees of storage temperature revealed log 4.91±0.16, 5.12±0.12 and 5.10±0.17, respectively (Table 2).
Effect of LPS, storage temperature and different stages of lactation of camels on log coliform count milk samples
The LPS treated camel’s milk samples showed lower log coliform count at all degrees of storage temperature compared with the control samples. The effect of lactoperoxidase enzyme system, storage temperature and stages of lactation on log coliform count of camel’s milk samples were affected significantly (P≤0.05) by LPS, storage temperature and stages of lactation (Table 3).
|
Temperature (°C) |
Control milk |
LPS milk |
||||
|
Lactation stages |
||||||
|
Early |
Medium |
Late |
Early |
Medium |
Late |
|
|
5±2°C |
4.55±1.27m |
4.57±0.96kl |
4.59±0.29k |
4.21±1.43p |
4.23±1.18o |
4.20±0.27p |
|
13±2°C |
4.75±1.24ij |
4.79±0.42h |
4.77±0.16i |
4.53±1.18mn |
4.52±0.50n |
4.53±0.18mn |
|
25±2°C |
5.00±1.69e |
5.04±0.43d |
5.11±0.19c |
4.75±1.56ij |
4.80±0.55h |
4.77±0.38i |
|
37±2°C |
5.16±0.36bc |
5.18±0.35b |
5.23±0.18a |
3.91±2.05g |
4.93±0.50f |
5.01±0.23e |
|
P-value |
0.0276* |
|||||
|
SE± |
0.0043 |
|||||
Table 3: Effect of LPS enzymes, storage temperature and lactation stages of camels on coliform count (log) milk.
The means sharing the same superscript letters are not significantly (P>0.05) different according to DMRT.
SE± = Experimental standard error
P-value = Level of significance (probability)
The means and standard errors of log coliform count of LPS treated milk samples of camels at early, medium and late stages of lactation that stored at 5±2°C were 4.21±1.43, 4.23±1.18 and 4.20±0.27, respectively. However the non-treated camel’s milk samples under the same degrees of storage temperature revealed higher values (log 4.55±1.27, 4.57±0.96 and 4.59±0.29, respectively) as shown in table 3. At 13±2°C, the means and standard errors of log coliform count of LPS treated milk samples were 4.53±1.18, 4.52±0.50 and 4.53±0.18 for camels at early, medium and late stages of lactation respectively, compared with log 4.75±1.24, 4.79±0.42 and 4.77±0.16 for untreated milk samples, respectively (Table 3).
The means and standard errors of log coliform count were found as 4.75±1.56, 4.80±0.55 and 4.77±0.38 for the LPS treated milk samples of camels at the early, medium and late stages of lactation, respectively. However higher values (log 5.00±1.69, 5.04±0.43 and 5.11±0.19) were found for the untreated (control) milk samples, respectively at 25±2°C (Table 3). The means and standard errors of log coliform count of LPS treated milk samples stored at 37±2 °C showed 3.91±2.05, 4.93±0.50 and 5.01±0.23 for the camels at early, medium and late stages of lactation respectively, while the control milk samples stored at the same degree of temperature obtained from the same stages of lactation revealed log 5.16±0.36, 5.18±0.35 and 5.23±0.18, respectively (Table 3).
Effect of LPS, storage temperature and different stages of lactation of camels on log yeast count of milk samples
Yeast count was found to increase with increasing the storage temperature for both the LPS treated and control camel’s milk samples, moreover the control camel milk samples had higher load of yeast count compared with LPS treated samples. The effect of lactoperoxidase enzyme system, storage temperature and stages of lactation on log yeast count of camel’s milk samples revealed significant (P≤0.05) variations (Table 4).
|
Temperature (°C) |
Control milk |
LPS milk |
||||
|
Lactation stages |
||||||
|
Early |
Medium |
Late |
Early |
Medium |
Late |
|
|
5±2°C |
2.77±1.98op |
2.94±1.93l |
2.78±2.06o |
2.12±1.93s |
1.97±1.98t |
2.12±1.93s |
|
13±2°C |
3.29±1.84j |
3.75±1.12g |
3.23±1.83k |
2.60±2.00pq |
2.61±1.82p |
2.59±2.12r |
|
25±2°C |
3.87±2.11f |
4.13±0.32b |
3.94±2.08d |
2.94±1.82l |
2.91±1.78n |
2.93±1.81lm |
|
37±2°C |
3.95±2.23d |
4.43±0.34a |
3.99±1.69c |
3.38±1.97i |
3.41±1.61h |
3.39±2.24i |
|
P-value |
0.0441* |
|||||
|
SE± |
0.0036 |
|||||
Table 4: Effect of LPS enzymes, storage temperature and lactation stages camels on yeast count (log) of milk.
The means sharing the same superscript letters are not significantly (P>0.05) different according to DMRT.
SE± = Experimental standard error
P-value = Level of significance (probability)
Yeast count was found to increase with increasing the degrees of storage temperature for both the LPS treated and control camel’s milk samples, moreover the control camel milk samples had higher load of yeast count compared with LPS treated samples. The effect of lactoperoxidase enzyme system, storage temperature and stages of lactation on log yeast count of camel’s milk samples were affected significantly (P≤0.05) as shown in table 4.
The means and standard errors of log yeast count of LPS treated milk samples of camels at early, medium and late stages of lactation that stored at 5±2°C were 2.12±1.93, 1.97±1.98 and 2.12±1.93 respectively, while the non-treated milk samples under the same degree of storage temperature revealed log 2.77±1.98, 2.94±1.93 and 2.78±2.06, respectively (Table 4). At 13±2°C, the means and standard errors of log yeast count of LPS treated milk samples showed lower values (log 2.60±2.00, 2.61±1.82 and 2.59±2.12) for camels at early, medium and late stages of lactation respectively, compared with untreated milk samples (log 3.29±1.84, 3.75±1.12 and 3.23±1.18), respectively (Table 4).
The means and standard errors of log yeast count were 2.94±1.82, 2.91±1.78 and 2.93±1.81 for the LPS treated milk samples of camels at early, medium and late stages of lactation, respectively. However untreated milk samples from camels at the same stages of lactation revealed log 3.87±2.11, 4.13±0.32 and 3.94±2.08 respectively, at 25±2°C as shown in Table 4. The means and standard errors of LPS treated milk samples stored at 37±2°C were 3.38±1.97, 3.41±1.61 and 3.39±2.24 for camels at early, medium and late stages of lactation respectively, while the control camel’s milk samples stored at the same storage temperature revealed log 3.95±2.23, 4.43±0.34 and 3.99±1.69, respectively (Table 4).
The means and standard errors of log yeast count were found as 2.94±1.82, 2.91±1.78 and 2.93±1.81 compared to log 3.87±2.11, 4.13±0.32 and 3.94±2.08 for the LPS treated and control milk samples of camels at early, medium and late stages of lactation respectively at 25±2°C (Table 4). The means and standard errors of LPS treated milk samples stored at 37±2°C were 3.38±1.97, 3.41±1.61 and 3.39±2.24 for camels at early, medium and late stages of lactation respectively, while the control camel’s milk samples stored at the same storage temperature revealed log 3.95±2.23, 4.43±0.34 and 3.99±1.69, respectively (Table 4).
Effect of LPS on the acidity of camels’ milk samples kept at different storage temperature
This result presented the means and standard errors of titratable acidity (expressed as the lactic acid percent) of milk samples treated with lactoperoxidase enzyme system that stored at different storage temperature (5±2°C, 13±2°C, 25±2°C and 37±2°C) as shown in table 5 and figure 1.
|
Temperature (°C) |
Control milk |
LPS milks |
|
5 2°C |
0.44±0.51e |
0.20±0.11h |
|
13 2°C |
0.51±1.77d |
0.30±0.13g |
|
25 2°C |
0.63±5.47b |
0.41±0.02f |
|
37 2°C |
0.98±8.04a |
0.62±0.43c |
|
P-value |
0.0205* |
|
|
SE± |
0.0036 |
|
Table 5: Effect of LPS enzymes and storage temperature on the acidity (%) of camel’s milk.
The means sharing the same superscript letters are not significantly (P>0.05) different according to DMRT.
SE± = Experimental standard error
P-value = Level of significance (probability)
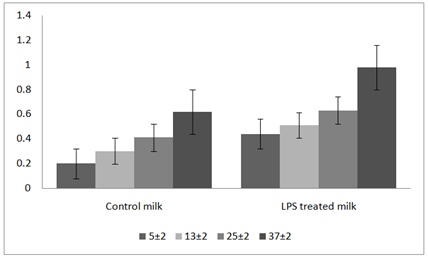 Figure 1: Effect of LPS enzymes and storage temerature on the acidity (%) of camel milk.
Figure 1: Effect of LPS enzymes and storage temerature on the acidity (%) of camel milk.
Effect of LPS on the acidity of camel’s milk samples kept at different storage temperature
The means for titratable acidity and standard errors of LPS treated camel’s milk samples kept at 5±2°C, 13±2°C, 25±2°C and 37±2°C were 0.20±0.11%, 0.30±0.13%, 0.41±0.02% and 0.62±00.2%, respectively (Table 5). However, the titratable acidity of the control milk samples kept at the same storage temperature revealed 0.44±0.51%, 0.51±1.77%, 0.63±5.47% and 0.98±8.04%, respectively (Table 5). The data showed significant (P≤0.05) effect on titratable acidity of camel’s samples treated by LPS in comparison with the control samples kept at the same storage temperature (5±2°C, 13±2°C, 25±2°C and 37±2°C). However, an increase was observed in titratable acidity with increasing of the storage temperature in both LPS treated and control milk camels’ milk samples (Figure 1). The LPS treated camel’s milk samples kept at 5±2°C showed reduction in acidity in comparison with the acidity of control samples at the same degrees of storage temperature (Figure 1). The acidity of LPS treated and control camel’s milk samples kept at 13±2°C and the LPS treated samples that kept at 25±2°C were increased gradually with the increasing of the degree of storage temperature, while the control samples that kept at 25±2°C were increased sharply as shown in figure 1. However, the LPS treated milk samples kept at 37±2°C reached the maximum level (0.62±0.43%) after 24 hours. Similarly the acidity of control milk samples increase throughout the storage temperature and reached 0.98±8.04% at 37±2°C (Table 5 and Figure 1).
The shelf life of LPS treated and control camel’s milk samples
Means and standard errors of the shelf life of camel’s milk samples treated by LPS that stored at 5±2°C and 13±2°C were 10.78±1.77, 10.87±1.78 and 10.8±1.70 and 9.75±3.58, 9.70±52 and 9.71±3.53 days compared with control samples that revealed 8.52±4.65, 8.05±4.78 and 8.52±4.65 and 6.27±4.69, 6.27±4.69 and 6.20±4.75 days at early, medium and late stages of lactation, respectively (Table 6). The LPS treated camel’s milk samples stored at the same degree of temperature showed higher shelf life (10- 11 days and 9-10 days) than the control (8-9 days and 6-7 days, respectively) as shown in table 6. The range and means of the shelf life for the milk of camels at early, medium and late stages of lactation that treated with LPS and kept at 25±2 °C and 37±2 °C were 7-8 days (7.88±4.64, 7.95±4.71 and 7.93±4.69 days, respectively) and 6-7 days (6.14±3.69, 5.86±3.79 and 6.14±3.69 days, respectively). However, the control milk samples revealed 5-6 days (5.33±4.36, 5.29±4.38 and 5.67±4.61 days, respectively) and 2-3 days (2.43±1.11, 2.38±1.04 and 2.79±1.18 days, respectively) as shown in Table 6. The data also showed longer shelf life of all LPS treated milk samples of camels at the three stages of lactation compared with the control ones. Similarly the samples that stored at 5±2 °C and 13±2 °C showed longer shelf life compared with the others. The shelf life of camel’s milk samples were affected significantly (P≤0.05) by LPS, storage temperature and stages of lactation (Table 6).
|
Temperature (°C) |
Control milk |
LPS milk |
|||||
|
Lactation stages |
|||||||
|
Early |
Medium |
Late |
Early |
Medium |
Late |
||
|
5±2°C |
Range (days) |
8 - 9 |
8 - 9 |
8 - 9 |
10 -11 |
10 -11 |
10 -11 |
|
Mean± SD |
8.52±4.65 |
8.05±4.78 |
8.52±4.65 |
10.78±1.77 |
10.87±1.78 |
10.80±1.70 |
|
|
13±2°C |
Range (days) |
6 - 7 |
6 - 7 |
6 - 7 |
9 - 10 |
9 – 10 |
9 – 10 |
|
Mean± SD |
6.27±4.69 |
6.27±4.69 |
6.20±4.75 |
9.75 ±3.58 |
9.70±3.52 |
9.71 ±3.53 |
|
|
25±2°C |
Range (days) |
5 - 6 |
5 - 6 |
5 - 6 |
7 - 8 |
7 – 8 |
7 – 8 |
|
Mean± SD |
5.33±4.36 |
5.29±4.38 |
5.67±4.61 |
7.88±4.64 |
7.95±4.71 |
7.93±4.69 |
|
|
37±2°C |
Range (days) |
2-3 |
2-3 |
2-3 |
6-7 |
6-7 |
6-7 |
|
Mean± SD |
2.45±1.11 |
2.38±1.04 |
2.79±1.18 |
6.14±3.69 |
5.86±3.79 |
6.14±3.69 |
|
Table 6: The shelf life (days) of camel’s milk as affected by addition of LPS enzyme.
The means sharing the same superscript letters are not significantly (P>0.05) different according to DMRT.
SE± = Experimental standard error
P-value = Level of significance (probability)
DISCUSSION
In this study, some microbial load was found associated with camel milk (Tables 1-4). Similarly camel milk was found to be contaminated with microorganisms in southwest Algeria [24], Saudi Arabia [25] and Sudan [6,26]. However the addition of lactoperoxidase enzyme system was reducing the microbial load of milk samples kept at different degrees of temperature (Tables 1-4). Similarly it was showed that the LPS treated camel milk samples kept at room temperature did not show any significant difference in total viable bacterial counts when compared to the samples kept in the refrigerator [27]. Also it was found that the viable counts increased significantly during storage at 10, 20 and 30°C by activation of the lactoperoxidase in raw camel milk at 8.5 ppm [28]. The inhibitory effect of LPS is found to depend on the storage temperature of treated milk samples [29]. The hygiene quality of raw cow milk at room and refrigerator temperatures showed significant (P≤0.001) differences for the total viable bacterial and coliform counts after preservation with LPS [30]. This is because milk is considered an important chain for transmission of pathogenic microorganisms to human beings unless it is produced and handled under good hygienic conditions [31].
The present result also supported the one which stated that lactoperoxidase system provide remarkable effect on decreasing total bacteria from 8 log cfu/ ml to 5 log cfu/ ml [32]. The LPS reduced the microbial load in milk stored under ambient temperature by more than one log cycle, after 8 hours of storage [33]. Reduction in total viable bacterial count, coliform, psychrotrophic bacteria and yeast and mould content of LPS treated goats’ milk samples were found with the reducing of degrees of temperature compared with the control samples kept under the same conditions [34]. Suppression in psychrotrophs population was observed for 5 days after activation of the lactoperoxidase system [28]. Moreover the samples kept at 37±2°C showed higher microbial load and shorter shelf life compared with that stored at 5±2°C [34]. Also significant (P<0.05) variations between the control and LPS treated cow’s milk samples on total bacterial count, psychrotrophic bacterial count, coliform count and yeast and mould counts were reported [35]. The coliform count and total bacterial count in LPS activated milk samples were significantly (P< 0.05) lower than their respective values in non LPS activated milk samples for both cow and camel milk, stored for 6, 12 and 24 hrs [8]. The reduction might be because the anti-bacterial action of the lactoperoxidase system is due to the effect of reaction products of thiocyanate oxidation, OSCN_ and HOSCN, which are able to oxidise free thiolate groups of various proteins that are important for the viability of pathogens, thus inactivating crucial enzyme and protein systems [36].
Table 6 illustrated long shelf life of treated LPS milk sample compared with those estimated for the control ones. Similarly it was found that the lactoperoxidase treatment prolongs the camel milk shelf life for 11-19 days at refrigeration temperature [30]. FAO/WHO stated that the inhibitory effect of lactoperoxidase system treatment depends on the storage temperature of LPS treated milk [37]. Moreover some authors suggested that the LPS can be applied to extend the shelf life of camel’s milk [27,38,39]. Because by activating the lactoperoxidase system, it is possible to extend the storage period of raw camel milk and that the effect of the lactoperoxidase system on the microbes varies with temperature of storage and levels of thiocyanate and H2O2 [28].
The present investigation revealed long shelf life for treated milk samples from camels at the three stages of lactation compared with the control ones. This might be because lactoperoxidase activity is maintained at a high level throughout lactation unlike human lactoperoxidase, which is present only in colostrum and becomes undetectable within a week after parturition [15]. The variations in the efficiency of enzyme in preserving milk were affected by many factors including stage of lactation, individual animal and sampling day [40].
The results during storage at 5±2°C, demonstrated that the activation with LPS system has the ability to extend the storage period of refrigerated raw camel’s milk samples for more than 76 hours. This result was concurred with the findings reported previously that a shelf-life extension of more than 72 hours by the activation of LP-system in raw cow’s milk stored at 7°C [41], similar result was also shown [42]. The LPS activation of both cow and camel milk helps to extend the shelf life of fresh milk up to 6 and 12 hours, respectively and enables milk producers to sell fresh milk within this time frame and reduce milk wastage. Also the LPS activation can be used in improving the microbiological quality and the shelf-life of raw camel and cow milk where milk cooling facilities are not available [8]. Moreover milk samples from cows in the different stages of lactation treated with LPS showed longer shelf life and better keeping quality during all storage degrees of temperature compared with the controlled samples under the same conditions [35]. Similarly the shelf life of sheep milk samples were significantly (P≤0.001) improved by the LPS addition using different storage temperature [29]. It was reported that activation of milk samples by the LPS extended the shelf life of milk by at least 8 hours at 37°C [43-44].
The shelf life of camel’s milk samples were affected significantly (P≤ 0.05) by LPS, storage temperature and stages of lactation (Table 6). With activating the LPS system, it is possible to extend the storage period of raw camel milk and that the effect of the LPS system on the microbes varies with temperature of storage (Tables 1-6). It was concluded that although the LPS activity is affected by the storage temperature of the milk, it is useful in prolonging the shelf life of raw milk [35]. Hence it could be applied in hot areas like Sudan in order to utilize the huge quantity of the milk produced in the traditional systems. The shelf life was extended by 19 hours during storage at 10 and 20°C and 4 hours at 30°C by activation of the LP system at 8.5ppm in raw camel milk [28]. This was also proven by the finding reported that, by activation of the LPS in raw milk, it is possible to store ovine, bovine and caprine milk at 4°C for several days [45]. Similarly 9 days and 3 days at refrigeration and room temperature respectively were reported, for camel milk [27]. Also highly significant (P≤ 0.001) effect of LPS on the shelf life, acidity and microbial content of sheep milk was reported [29]. Moreover it was shown that LPS have preservation effect by improving the keeping quality and the shelf life of camel’s milk without adverse effect on the chemical constituents [7,27,28,30].
The present result showed significant (P≤0.05) effect of LPS, degrees of storage temperature and stages of lactation of camels on lactic acid production for LPS treated milk samples. Similarly it was stated that the lag in lactic acid production in LP-activated raw camel’s milk was lower than the lag of untreated milk samples [39]. The percent of acidity were not significantly (P>0.05) different than that of the initial acidity level in LPS activated cow and camel milk up to 12 hours of storage [8]. Also an increase was observed in titratable acidity at increasing the degrees of temperature in both LPS treated and control goat’s milk samples [34]. The preservative effect of LPS on buffalo and cow’s milk at 30°C and 8°C caused a considerable slowing down in the rate of increase in titrable acidity during storage [46]. The inhibition of acid production by the LP-system was apparently more pronounced during storage at 20ºC and 30ºC compared to 10ºC, which could be due to the fact that the LP-system is more active at 30 ºC when the conditions are favorable for cell multiplication [28].
CONCLUSION
In conclusion, this study showed that the LP system, significantly (P≤0.05) reduced the bacterial multiplication and fungal in the milk of camel during the storage period of investigation.
ACKNOWLEDGMENT
The fund received from the Arab Foundation for Science and Technology (ASTF) is appreciated with thanks. Thanks should also goes to Ministry of Animal Resources and Fisheries, Sudan for providing the LPS kits.
REFERENCES
- Mati A, Senoussi-Ghezali C, Zennia SSA, Almi-Sebbane D, El-Hatmi H, et al. (2017) Dromedary camel milk proteins: A source of peptides having biological activities – A review. International Dairy Journal 73: 25-27.
- El Zubeir IEM, Nour EM (2006) Studies on some management practices in pre-urban areas of Khartoum State, Sudan. International Journal of Dairy Science 1:104-112.
- Elagamy EI, Rappanner R, Ismail A, Champagne CP, Assaf R (1996) Purification and characterization of lactoferrin, lactoperoxidase, lysozime and immunoglobulins from camels milk. International Dairy Journal 6: 129-145.
- Wernery U, Johnson B, Abrahm A (2005) The effect of short-term heat treatment on vitamin C concentrations in camel milk. Milchwissenschaft 60: 266-267.
- Farah Z, Mollet M, Younan M, Dahir R (2007) Camel dairy in Somalia: Limiting factors and development potential. Livestock Sciences 110: 187-191.
- Warsma LM, El Zubeir IEM (2015) Assessment of bacterial loads of camel milk from farms and sale points in Khartoum State, Sudan. Sudan Journal of Science and Technology 16: 118-123.
- El Zubeir IEM (2010) Improvement of keeping quality of raw camel’s and cow’s milk by lactoperoxidase enzymes system. Camels in Asia and North Africa. Interdisciplinary Workshop on their significance in past and present. 5th -7th October 2010. Austrian Academy of Sciences. Dr. Ignaz- Seipel- platz 2, Vienna, Austria, 2010.
- Bekele T, Hailu Y, Guya ME, Hansen EB (2017) Activation of lactoperoxidase system: Evaluation of the acidification rate, microbial quality, and shelf life of camel and cow milk. East African Journal of Sciences 11: 107-116.
- Conner GE, Salathe M, Forteza R (2002) Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am J Respir Crit Care Med 166: 57-61.
- Boots JW, Floris R (2006) Lactoperoxidase: From catalytic mechanism to practical applications. International Dairy Journal 16: 1272-1276.
- Elagamy EI (2009) Bioactive component in camel milk. In: Park YW. (ed.) Bioactive components of milk and dairy products. Wiley-Blackwell, Iowa, USA.
- Elagamy EI, Ruppaner R, Ismail A, Champagne CP, Assaf R (1992) Antibacterial and antiviral activity of camel milk protective proteins. J Dairy Res 59: 169-175.
- El-Zubeir IEM, El Zubeir SBO, Ahmed SOY (2006) Preservation of raw milk of Khartoum State (Sudan) by the lactoperoxidase systems. International Journal of Dairy Science 1: 155-160.
- Marks NE, Grandison AS, Lewis MJ (2001) Challenges testing of the lactoperoxidase system in pasteurized milk. J Appl Microbiol 91: 735-741.
- Ueda T, Sakamaki K, Kuroki T, Yano I, Nagata S (1997) Molecular cloning and characterization of the chromosomal gene for human lactoperoxidase. Eur J Biochem 243: 32-41.
- Houghtaby GA, Maturin LJ, Koenig EK (1992) Microbiological count methods. In: Standard Method for the Examination of Dairy Products. Marshall RT (Ed.). 16th Baltimore, Washington, DC, USA.
- Marshall RT (1992) Standard Methods for the Examination of dairy Products, 16th edition, Marshall RT (Ed.). American Public Health Association, Washington, DC, USA.
- Harrigan WF, MaCane ME (1976) Laboratory method. In: Food and Dairy Microbiology. Academic Press Inc., London, UK.
- Frank JF, Christen GL, Bullerman LB (1992) Test of group of microorganisms. In: Standard Methods for Examination of Dairy Products, 16th American Public Health Association, Washington, DC, USA. Pg no: 271-286.
- American Public Health Association (1976) Standard Method for the Examination of Dairy Products. Mer Publ Health Assoc Inc. Pg no: 510-517.
- International Dairy Federation (1990) Hand Book on Milk Collection in Warm Developing Countries. Bulletin of the International Dairy Federation, Brussels, Belgium.
- Dowdly S, Wearden S (1991) Statistical for Research. The 2nd John Wiley and Sons, New York, USA. Pg no: 629-633.
- SAS (1987) SAS User’s Guide: Statistics. SAS Institute Inc., Cary, North Carolina, USA.
- Benyagoub E (2013) Level of control of the hygienic quality of camel milk (Camelus dromedarius) in south west Algeria and its impact on security. Journal of Food Science and Technology 1: 53-60.
- El-Ziney M, Al-Turki AI (2007) Microbiological quality and safety assessment of camel milk (Camelus dromedaries) in Saudi Arabia (Quassim region). Applied Ecology and Environmental Research 5: 115-122.
- Mohamed IMA, El Zubeir IEM (2014) Effect of heat treatment on the keeping quality of camel milk. Annals of Food Science and Technology 15: 239-245.
- El Zubeir IEM (2012) Extending the shelf life of camel milk. In: Knoll EM and Burge P (Eds.). Camels in Asia and North Africa, Interdisciplinary Perspectives on their Past and Present Significance, Wissenschaften. Australia. Pg no: 219-224.
- Kamau PMN, Lamuka PO, Wangoh J (2010) Effect of lactoperoxidase-thiocyanate-hydrogen peroxide system and storage temperature on keeping quality of raw camel milk. African Journal of Food, Agriculture, Nutrition and Development 10: 4185-4201.
- Saad MSA, El Zubeir IEM, Fadel Elseed AMA (2013) Effect of lactoperoxidase enzyme system and storage temperature on the keeping quality of sheep milk. Livestock Research for Rural Development 25: 2013.
- El Zubeir IEM (2010a) Improvement of keeping quality of raw camel’s and cow’ milk by lactoperoxidase enzyme systems. University of Khartoum, Conference of Graduate Studies. Scientific Research-Pillar of Civilization Development, Friends-ship Hall, Khartoum, Sudan.
- Warsama LM, Mustafa NEM, El Zubeir IEM (2017) Physicochemical properties and microbial load of cow milk collected from milk supply chain during winter and summer in Khartoum State, Sudan. Journal of Veterinary Medicine and Animal Production 8: 41-52.
- Rasbawati BD, Ahmad N, Al-Baarri AML, Bintoro VP (2014) Total bacteria and pH of Dangke preserved using natural antimicrobial lactoferrin and lactoperoxidase from bovine whey. International Journal of Dairy Science 9: 116-123.
- Asaah NO, Fonteh F, Kamga P, Mendi S, Imele H (2007) Activation of the lactoperoxidase system as a method of preserving raw milk in areas without cooling facilities. African Journal of Food Agriculture Nutrition and Development 7: 1407-1413.
- Mohamed HMI, El Zubeir IEM, Fadlelmoula AA (2016a) Variation of microbial load of Sudanese Nubian goat milk as affected by lactoperoxidase enzyme system, sage of lactation and storage temperature. Research Journal of Microbiology 11: 64-69.
- Mohamed HMI, El Zubeir IEM, Fadlelmoula AA (2016) Influence of lactation stage and storage temperature on the activity of lactoperoxidase enzyme system on microbial load of raw cow’s milk. Annals of Food Science and Technology 17: 239-244.
- Sermon J, Vanoirbeek K, De Spiegeleer P, Van Houdt R, Aertsen A, et al. (2005) Unique stress response to the lactoperoxidase thiocyanate enzyme system in Escherichia coli. Res Microbiol 156: 225-232.
- FAO/WHO (2005) Benefits and potential risks of the lactoperoxidase system of raw milk preservation. Technical meeting. FAO, Rome, Italy.
- El Zubeir IEM, Hassan AK (2006) Improvement of the shelf life of camel milk using lactoperoxidase enzyme system. The 4th Conference on: Scientific Research Outlook and Technology Development in the Arab World, Damascus, Syrian Arab Republic.
- Kassa F, Yilma Z, Assefa G, Bekele T, Gojam Y, et al. (2013) Evaluation of Lactoperoxidase system as raw milk preservative at different storage temperature conditions in the central highlands of Ethiopia. Livestock Research for Rural Development 25: 2013.
- Augusta L, Andrea B (2010) The influence of lactation stage on lactation activity. Animal Science and Biotechnologies 67(: 231-234.
- Bjorck L (1978) Antibacterial effect of LPS on psychrotrophic bacteria in milk. J Dairy Res 24: 109-118.
- Bjorck L, Rosen CG, Marshall V, Reiter B (1975) Antibacterial activity of the lactoperoxidase system in milk against Psedomonase and other Gram-negative bacteria. Appl Microbiol 30: 199-208.
- Hamid OIA, Mohamed ZAB (2013) Effect of different level of sodium thiocyanate and percarbonate for activation of lactoperoxidase on keeping quality of raw milk. J Adv Sci Res 4: 27-30.
- Masud T, Khalid S, Maqsood S, Bilal A (2010) Preservation of raw buffalo’s milk by activation of lactoperoxidase system and its effect on yoghurt preparation. Journal of Food Processing and Preservation 34: 241-254.
- Boulares M, Mankai M, Hassoun M (2011) Effect of thiocyanate and hydrogen peroxide on the keeping quality of ovine, bovine and caprine raw milk. International Journal of Dairy Technology 64: 52-56.
- Elien S, Girgis A, Ismail S, El-dieb M, Zaky WM (2001) Application of LPS in milks and milk products. Egyptian Journal of Food Sciences 9: 233-256.
Citation: Mohamed HMI, El Zubeir IEM (2020) Improvement of Camel Milk Microbial Loads by Activation of Lactoperoxidase Enzyme System during Different Storage Temperature. J Dairy Res Tech 3: 021.
Copyright: © 2020 HMI Mohamed, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

