
Isolation and Characterization of Lactic Acid Bacteria Strains from Raw Camel Milk for Potential Use in the Production of Yogurt
*Corresponding Author(s):
Imen FguiriLaboratory Of Livestock And Wild Life, Institute Of Arid Lands (IRA Medenine), Médenine, Tunisia
Tel:+216 23804007,
Fax:+216 75633006
Email:imen.fguiri@yahoo.com
Abstract
The objective of this work is to formulate a starter lactic seen application camel milk to prepare fermented product yoghurt. Lactic acid bacteria isolated from camel milk has different characterization tests and selection: morphological study, catalase test, gram stain, use of citrate, acidifying power, lipolytic power and proteolytic power. These tests have to choose the strains: 1, 4, 5, 6, 7, 8, 9 and 10 which has ΔpH ≥ 0.3U after 6h as the most acidifying and EPS producing strains. All the strains showed a proteolytic activity with zone diameters and proteolysis was between 15 and 21mm. In addition, these lactic acid bacteria were considered low lipolytic but all having antimicrobial activity against 11 pathogenic strains. Then freeze-dried lactic acid bacteria were prepared from these strains starters (1, 4, 5, 6 and 9). These were used to inoculate three types of milk after pasteurization, using each time a combination of two strains. These strains were applied to goat, camel and cow’s milk for the preparation of yoghurt. The monitoring of these fermented products shows the combinations of strain nº1 with strain nº6 in the goat milk and cow milk certainly give us the desired product (yogurt). Because of pH, titratable acidity and viscosity seem so similar to those of natural yoghurt
Keywords
INTRODUCTION
In arid regions, camel milk is considered as one of the most important source of dairy products for human diet with potential therapeutic effects. Recent studies showed that camel milk is a natural source for probiotics [1]. The dominant and beneficial microflora in camel milk represented by LAB is a potential source of biological materials to be used in dairy technology [2]. Today, LAB are a focus of intensive international research for their essential role in most fermented food, for their ability to produce various antimicrobial compounds promoting probiotic properties [3], including antitumoral activity [4,5], reduction of serum cholesterol [6,7], alleviation of lactose intolerance [8], stimulation of the immune system [9] and stabilization of gut microflora [10]. LAB strains that produce Exopolysaccharide (ESP) are employed in the manufacture of fermented milk to improve its texture and viscosity [11,12]. Dairy products traditionally made are usually preserved due to spontaneous fermentation. However, modern large-scale production techniques generally make use of starter systems with defined strains so as to guarantee uniformity, safety and quality in the final product [13].
Camel milk has been used fresh or fermented in different regions of the world. Traditional fermented camel’s milk is widely consumed in Africa and in Middle Eastern countries [14].
It is produced by spontaneous souring of camel’s milk. In Tunisia, some practices of camel milk fermentation were known [15]. Development of new fermented functional camel milk in Tunisia can be promised. In fact, during the last decade, the interest of industries and consumers for functional foods has been exponentially increasing. The use of milk with particular nutritional properties such as camel milk, alone or in combination with bacterial strains having probiotic properties and/or producing physiologically active metabolites, represents one of the technology options for manufacturing dairy functional beverages [16]. The traditional method of milk fermentation results in a product with varying taste and flavor and often of poor hygienic quality.
Transformation of camel’s milk into traditional Tunisian Yoghurt is achieved in addition of the yogurt starters Streptococcus thermophilus and Lactobacillus Bulgaricus. Unfortunately, some of strains of Lactobacillus delbrueckii subsp. Bulgaricus and Streptococcus thermophilus did not produce EPS or produce only low yields of EPS, which may affect the end products quality [17,18]. Therefore, screening LAB from natural sources has been one of the powerful means to obtain strains for the food industry. Thus, the objectives of this work are to isolate and characterize Lactic Acid Bacteria (LAB) from raw camel milk and to study their potential use in the production of fermented Tunisian dairy products like yoghurt.
MATERIALS AND METHODS
Sampling
Milk composition
Physicochemical analysis:
The complex viscosity (in Pa s) was determined by applying a shear stress of 0.1Pa at an oscillation frequency of 1Hz for 1min with a Brookfield type viscometer (model DV-E, MA, USA).
Dry matter expressed in grams per litter milk is calculated after weighing the sample at 105ºC for 24h of its dry residue. The sample is 5g, Ash content, expressed in g/l of milk was determined after drying at 505ºC [19].
The fat content was measured by an acid-butyrometric method using a "Neusol solution" cited by Farah. This method is a direct reading on a butyrometer the amount of fat contained in 12ml of sample after centrifugation in the presence of amyl alcohol. The direct reading of graduations determines the amount of fat in g/l.
Microbiological analysis:
Milk samples (1ml) were diluted in buffered peptone saline (10-1 to 10-3), mixed in stomacher bag. In order to quantify the various microbial groups, appropriate dilutions were surface plated:
Aerobic Total Plate Count (ATPC) (Sharlau Chemie S.A) was carried out on Plate Count Agar (PCA), incubated at 32ºC for 72h [21]. Yeast and moulds on Sabouraud Chloramphenicol (Pronadisa Micro & Molecular Biology) and incubated at 25ºC for 3 to 5 days. Total coliform were grown in Violet Red Bile Agar (VRBA) (AppliChem - Biochemica.Chemica Services) in double layer. After solidifying of the agar, the plates were incubated at 30ºC for 22h [22]. The lactic acid bacteria on MRS [23] are shown on the surface and then incubated 30ºC for 48h.
Isolation and identification of strains
Strains conservation
Technological characterization
Proteolytic activity:
Lipolytic activity:
Biomass production:
Exopolysaccharides production:
Antibacterial effect:
Lyophilization survival rate
Survival rate (%) = Ln N / Ln N0 * 100
With:
N: Number of viable cells after concentration,
N0: number of viable cells before concentration.
Yogurt preparation
Sensory evaluation
Statistical analysis
RESULT AND DISCUSSION
Milk composition
|
Parameter |
Cow Milk |
Goat Milk |
Camel Milk |
|
pH |
6.77±0.00 |
6.63±0.06 |
6.736±0.005 |
|
Acidity (ºD) |
17.1±0.9 |
16.2±0.9 |
13.8±0.5 |
|
Density |
1.032±0.000 |
1.028±0.000 |
1.027±0.000 |
|
Viscosity |
3.03±0.10 |
3.84±0.06 |
3.3±0.04 |
|
Ash (g/l) |
8.43±0 .72 |
8.73±0.15 |
9.1±0.72 |
|
Matter fat (g/l) |
20.33±4.61 |
60.33±7.50 |
23.66±11.01 |
|
Dry matter (g/l) |
108.1±17 |
162.2±5.5 |
133.7±1.2 |
|
|
Aerobic Total Plate Count (UFC/ml) |
Total Coliform (UFC/ml) |
Yeast and Molds (UFC/ml) |
Lactic Acid Bacteria (UFC/ml) |
|
Camel milk |
4.7×104 |
0 |
0 |
8×102 |
|
Cow milk |
2.09×106 |
2.15×105 |
8.3×104 |
6.5×103 |
|
Goat milk |
2.39×105 |
2.67×104 |
3×102 |
1.15×103 |
Table 2: Microbiological characteristics of the 3 types of milk (cow, goat and camel).
Isolation and identification of strains
The ten strains encoded SCC1-2, SCC1-6, SCC1-7, SCC1-8, SCC1-13, SCC1-15, SCC1-24, SCC1-33, SLch6 and SLch14 are characterized by their ability to grow at different temperatures (10, 39 and 45ºC) at different salt concentrations (4, 6.5) while growth of these strains at 8% of salts concentration were not observed. All strains grow only at pH = 9.6 (Table 3).
|
Parameter |
Cow Milk |
Goat Milk |
Camel Milk |
|
pH |
6.77±0.00 |
6.63±0.06 |
6.736±0.005 |
|
Acidity (ºD) |
17.1±0.9 |
16.2±0.9 |
13.8±0.5 |
|
Density |
1.032±0.000 |
1.028±0.000 |
1.027±0.000 |
|
Viscosity |
3.03±0.10 |
3.84±0.06 |
3.3±0.04 |
|
Ash (g/l) |
8.43±0 .72 |
8.73±0.15 |
9.1±0.72 |
|
Matter fat (g/l) |
20.33±4.61 |
60.33±7.50 |
23.66±11.01 |
|
Dry matter (g/l) |
108.1±17 |
162.2±5.5 |
133.7±1.2 |
Table 3: Biochemical criteria of presumptive lactic species isolated from raw camel milk.
Technological properties of LAB isolates
Acidifying activity
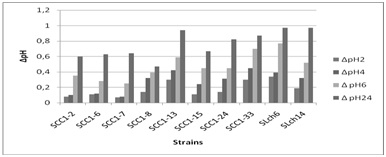 Figure 1: Evolution of ΔpH during the fermentation of camel milk after 2H (?), 4H (?), 6H and 24H (?? inoculated with different lactic strains and incubated at 30ºC.
Figure 1: Evolution of ΔpH during the fermentation of camel milk after 2H (?), 4H (?), 6H and 24H (?? inoculated with different lactic strains and incubated at 30ºC.The difference observed from one lactic acid bacteria species to another were explained by Badis et al. [26]. In fact, the acidifying activity of each strain is related to its specific capacity to break down the substances in the medium and render the capability of assimilation. On occasion, differences are also due to the presence or absence of nutrient transport systems [45].
Proteolytic activity
The proteolytic activity of dairy lactic acid bacteria is essential for the bacterial growth in milk and involved in the development of organoleptic properties of different fermented milk products [46,47]. The production of high quality fermented dairy products is dependent on proteolytic systems of starter bacteria, since peptidase and amino acids formed have a direct impact on flavor or serve as flavor precursors in these products.
Lipolytic activity:
|
Strains |
Lipolytic Diameter Zone (mm) |
Proteolytic Activity |
||
|
1% tween 80 |
3% tween 80 |
5% tween 80 |
Diameter Zone mm |
|
|
SCC1-2 |
11,375 |
9 |
9 |
15±1.4 |
|
SCC1-6 |
8,5 |
9 |
9,125 |
15±0.0 |
|
SCC1-7 |
9,5 |
10 |
9,875 |
18±1.41 |
|
SCC1-8 |
9,25 |
9 |
9,125 |
16±0.0 |
|
SCC1-13 |
13,5 |
9,75 |
9,5 |
21±0.0 |
|
SCC1-15 |
9,625 |
10,5 |
11,5 |
16.5±3.53 |
|
SCC1-24 |
8,875 |
9,5 |
10,25 |
16.5±3.53 |
|
SCC1-33 |
9,5 |
8,75 |
9,5 |
16.5±3.53 |
|
SLch6 |
8,625 |
9,5 |
11 |
19±0.0 |
|
SLch14 |
11,125 |
9 |
9,5 |
18±0.0 |
Table 4: Proteolytic and lipolytic activity of lactic acid bacteria.
Biomass production and growth rate:
The fermentation broth was centrifuged and the pellet was dried in order to determine biomass. The difference between the initial Optical Density (OD600) and the OD600 at which cells were collected (ΔOD600) as well as the dry weight of strains were used to reflect the growth amount (Table 5). Based on the biomass, cultures were divided into 3 groups: major yields when biomass ≥ 1.30mg/L, an average yield when the formed biomass ranged from 0.6 to 1.29mg/L, poor performance when the biomass was <0.6mg/L [35]. Strains SCC1-6, SCC1-15, SCC1-33 and SLCch14 were characterized by a high value of ΔOD600 and an important growth rate. The strains SCC1-24, SCC1-2, SCC1-13 and SCC1-13 presented a weak biomass and growth rate.
|
Strains |
ΔOD600* |
Biomass (g/l) |
μmax (h-1) |
EPS |
OD600 Supernatant |
|
SCC 1-2 |
0.44 |
0.53 |
0.05 |
- |
0.003 |
|
SCC1-6 |
1.13 |
0.81 |
0.13 |
- |
0.008 |
|
SCC1-7 |
0.67 |
0.69 |
0.07 |
- |
0.01 |
|
SCC1-8 |
0.86 |
0.20 |
0.08 |
+ |
0.10 |
|
SCC1-13 |
0.73 |
0.06 |
0.07 |
+ |
0.11 |
|
SCC1-15 |
1.32 |
0.79 |
0.12 |
+ |
0.02 |
|
SCC1-24 |
0.73 |
0,06 |
0,07 |
+ |
0.04 |
|
SCC1-33 |
1 .32 |
0.79 |
0,12 |
+ |
0.05 |
|
SLch6 |
0.68 |
0.887 |
0.14 |
+ |
0.06 |
|
SLCch14 |
1.93 |
0.98 |
0.13 |
+ |
0.02 |
Exopolysaccharide production:
Antagonism Effect:
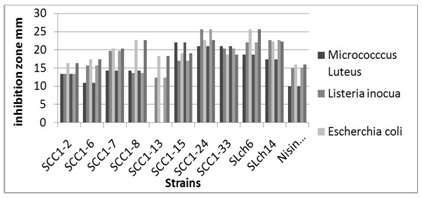 Figure 2: Antagonism effect of strains against Micrococcus luteus (?), Listeria inocua (?) and Escherichia coli (?).
Figure 2: Antagonism effect of strains against Micrococcus luteus (?), Listeria inocua (?) and Escherichia coli (?).Stability of lyophilized cultures:
|
Strains
|
Survival Rate Before Lyophilization |
Survival Rate After Lyophilization |
||
|
CFU/ml |
% |
CFU/ml |
% |
|
|
SCC1-2 |
8 108 |
100 |
2.3 108 |
28.75 |
|
SCC1-6 |
7.6 108 |
100 |
5.6 108 |
73.68 |
|
SCC1-7 |
6.5 108 |
100 |
5.3 108 |
81.53 |
|
SCC1-8 |
8.9 107 |
100 |
6.7 106 |
75.28 |
|
SCC1-13 |
5.7 108 |
100 |
5.9 108 |
103.5 |
|
SCC1-15 |
9.6 107 |
100 |
5.3 108 |
55.21 |
|
SCC1-24 |
8 .1 108 |
100 |
5.1 106 |
62.96 |
|
SCC1-33 |
9.2 107 |
100 |
5.2 106 |
56.52 |
|
SLch6 |
7.2 108 |
100 |
6.3 108 |
87.50 |
|
SLch14 |
5.6 108 |
100 |
7.2 106 |
128.5 |
Application of strains in the preparation of yoghurt from goat, cow and camel milk:
Camel, cow and goat milk were inoculated with these selected strains to prepare the pre-culture. After preparing dairy product, pH, acidity, and microbial count were measured. The results were shown in figure 3, 4 and table 7.
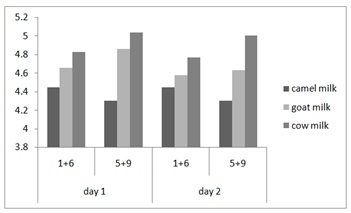 Figure 3: Strains effect on the pH in yoghurt from camel, cow and goat milk. Strains 1+6: SCC1-2+SCC1-15 and Strains 5+9: SCC1-13+SLch6.
Figure 3: Strains effect on the pH in yoghurt from camel, cow and goat milk. Strains 1+6: SCC1-2+SCC1-15 and Strains 5+9: SCC1-13+SLch6.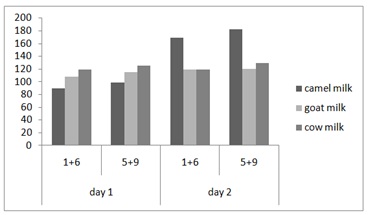 Figure 4: Strains effect on the acidity in yoghurt from camel, cow and goat milk. Strains 1+6: SCC1-2+SCC1-15 and Strains 5+9: SCC1-13+SLch6.
Figure 4: Strains effect on the acidity in yoghurt from camel, cow and goat milk. Strains 1+6: SCC1-2+SCC1-15 and Strains 5+9: SCC1-13+SLch6.|
|
Day 1 |
Day 2 |
||
|
(1+6) |
(5+9) |
(1+6) |
(5+9) |
|
|
Camel milk |
172×107 |
8×1010 |
4×107 |
4.1×1010 |
|
Cow milk |
3.16×109 |
5.36×109 |
>300×109 |
>300×109 |
|
Goat milk |
>300×107 |
>300×107 |
>300×107 |
>300×107 |
The fermentation profiles obtained for the three types of milk (camel, cow and goat) are similar. The acidity increases rapidly over time in different dairy products; this is explained by the importance the inoculum (2106) and its adaptation to the fermentation because the pre-culture is carried out on the same type of milk. Indeed, the fermentation of lactose into lactic acid lowers the pH and promotes the proteolysis of proteins [57]. The increase in acidity is accompanied by a decrease in pH to a final value 4.45, 4.83 and 5.04 respectively for camel, goat and cow milk in day 1 and this decrease continue after 24h (Figure 3 and 4).
Thus, it is observed that the pH of the fermented milks for the combination of strain 1 with strain 6 appear closest to being included in the pH range of plain yogurt (4.2 to 4.3) cited by Maurice M [58].
In some countries the yoghurt-equivalent product may contain others organisms beside Lb. bulgarius and S. thermophilus. For example, in India, other bacteria are used (Lactobacilus plantarum and Lactococcus lactis subsp lactis; Marshall) [59], while Chander et al., [60] have used only mixed cultures comprising Lac.lactis biovar diacetylactis and cremoris. Lactobacilus plantarum is a heterofermentative lactobacillus producing acetate in addition to lactate, and the lactococci produce only diacetyl and no acetataldehyde.
Sensory evaluation
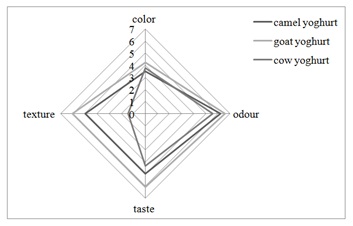 Figure 5: Sensory value for different starter fermented milk.
Figure 5: Sensory value for different starter fermented milk.CONCLUSION
The present study described the technological potential of combination of 2 selected strains of Lactic Acid bacteria isolated from camel milk and their use as starters for yoghurt preparation.
Based on the overall evaluation of the obtained results, the strains selected have high acidifying activity, high proteolytic activity and sensitive reaction to antibiotics. Among 10 strains, only 2 which have high acidification rate and high yield of biomass at the end of fermentation were applied to prepare dairy product and these dairy product present lowest pH values, highest acidity and lowest microbial cell count. This work may have important implication to put in the market yoghurt based of camel, cow and goat milk using a combination of lactic acid bacteria other than Streptococcus thermophilus and Lactobacillus delbrueckii subsp. Bulgaricus. After sensory evaluation, these implied that our own authority starter could produce the yogurt with similar or better quality compared with the commercial starters.
REFERENCES
- Al-Otaibi MM, Al-Zoreky NS, El-Dermerdash HA (2013) Camel's Milk as a Natural Source for Probiotics. Res J Microbiol 8: 70-80.
- Khedid K, Faid M, Mokhtari A, Soulaymani A, Zinedine A (2009) Characterization of lactic acid bacteria isolated from the one humped camel milk produced in Morocco. Microbiol Res 164: 81- 91.
- Temmerman R, Pot B, Huys G, Swings J (2003) Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int J Food Microbiol 81: 1-10.
- De Vuyst L, Degeest B (1999) Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol Rev 23: 153-177.
- Østlie HM, Helland MH and Narvhus JA (2003) Growth and metabolism of selected strains of probiotic bacteria in milk. Int J Food Microbiol 87: 17-27.
- Desmazeaud M (1995)Growth Inhibitors of Lactic Acid Bacteria. In: Cogan TM, Accolas JP (eds.). Dairy Starter Cultures. Wiley-VCH, New Jersey, USA.
- Jackson MS, Bird AR, McOrist AL (2002) Comparison of two selective media for the detection and enumeration of Lactobacilli in human faeces. J Microbiol Methods 51: 313-321.
- De Vrese M, Stegelmann A, Richter B, Fenselau S, Lave C, et al. (2001) Probiotic-compensation for lactase insufficiency. Am J Clin Nutr 73: 421-429.
- Isolauri E, Arvola T, Sütas Y, Moilanen E, Salminen S (2000) Probiotics in the management of atopic eczema. Cli Exp Allergy 30: 1604-1610.
- Gibson GR, Saaverda JM, Macfarlane S, Macfarlane GT (1997) Probiotics And Intestinal Infections. In: Fuller R (ed.). Probiotics 2: Application and Practical Aspects. Chapman And Hall, London, UK.
- Curk MC, Hubert JC, Bringel F (1996) Lactobacillus paraplantarum nov., a new species related to Lactobacillus plantarum. Int J Syst Bacteriol 46: 595-598.
- Ruas Madiedo P, Hugenholtz J, Zoon P (2002) An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. International Dairy Journal 12: 163-171.
- Ross RP, Morgan S, Hill C (2002) Preservation and fermentation: past, present and future. Int J Food Microbiol 79: 3-16.
- Ashmaig A, Hasan A, El Gaali E (2009) Identification of lactic acid bacteria isolated from traditional Sudanese fermented camel’s milk (Gariss). African Journal of Microbiology Research 3: 451-457.
- Fguiri I, Ziadi M, Atigui M, Ayeb N, Arroum S, et al. (2015) Isolation and characterization of lactic acid bacteria strains from raw camel milk for potential use in the production of fermented Tunisian dairy products. Int J Dairy Tech 69: 103-113.
- Gomes AM, Malcata FX, Klaver FA (1998) Growth enhancement of Bifidobacterium lactis Bo and Lactobacillus acidophilus Ki by milk hydrolyzates. J Dairy Sci 81: 2817-2825.
- Vaningelgem F, Zamfir M, Mozzi F, Adriany T, Vancanneyt M, et al. (2004) Biodiversity of exopolysaccharides produced by Streptococcus thermophilusstrains is reflected in their production and their molecular and functional characteristics. Appl Environ Microbiol 70: 900-912.
- Grobben GJ, Smith MR, Sikkema J, De Bont JAM (1996) Influence of fructose and glucose on the production of exopolysaccharides and the activities of enzymes involved in the sugar metabolism and the synthesis of sugar nucleotides in Lactobacillus delbrueckii bulgaricus NCFB 2772. Applied Microbiology and Biotechnology 46: 279-284.
- AFNOR (1993) Contrôle de la qualité des produits alimentaires : lait et produits laitiers : analyses physicochimiques, (4th edn). AFNOR, La Défense, Paris.
- AFNOR (1996) Analyse microbiologique: contrôle de la qualité des produits alimentaires: Tome 1: méthodes horizontales. AFNOR, Paris.
- El-Ziney MG, Al-Turki AI (2007) Microbiological quality and safety assessment of camel milk (Camelus dromedaries) in Saudi Arabia (Quassim region). App Ecol Environment Res 5: 115-122.
- Federal Register (1992) Drinking water: national primary drinking water regulations; analytical techniques coliform bacteria Final rule 57: 24745-24746.
- De Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Appl Microbiol 23: 130-135.
- Nguyen TD, Kang JH, Lee MS (2007) Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int J Food Microbiol 113: 358-361.
- Kopermsub P, Yunchalard S (2010) Identification of lactic acid bacteria associated with the production of plaa-som, a traditional fermented fish product of Thailand. Int J Food Microbiol 138: 200-204.
- Badis A, Guetarni D, Moussa-Boudjema B, Henni DE, Kihal M (2004) Identification and technological properties of lactic acid bacteria isolated from raw goat milk of four Algerian races. Food Microbiology 2: 579-588.
- Boumehira AZ, Mami A, Henni JE, Kihal M (2011) Identification and characterization of functional and technological Lactobacillus plantarum strains isolated from raw goat and camel milk collected in Algeria. Journal of Pure Applied Microbiology 5: 553-566.
- Kihal M, Prevost H, Lhotte ME, Huang DQ, Diviès C (1996) Instability of plasmid-encoded citrate permease in J Appl Microbiol 22: 219-223.
- Zarour K, Benmechernene Z, Hadadji M, Moussa-Boudjemaa B, Henni DJ, et al. (2012) Bioprospecting of Leuconostoc mesenteroides strains isolated from Algerian raw camel and goat milk for technological properties useful as adjunct starters. African Journal of Microbiology Research 6: 3192-3201.
- International Dairy Federation (1997) Lactic starters cultures of lactic acid bacteria. Standard composition. IDF Standard 149A Brussels, (In French), Belgium.
- Alonso-Calleja C, Carballo J, Capita R, Bernardo, A, Garcia-Lopez, ML (2002) Comparison of acidifying activity of Lactococcus lactis Lactis strains isolated from goat’s milk and valdeteja cheese. Lett Appl Microbiol 34: 134-138.
- Vuillemard JC (1986) Food microbiology. Evolution of proteolytic activity of the lactic bacteria (In French). Tec & Doc, Lavoisier Pg no: 3: 1-65.
- Simbert RM, Kierg (1981) Manual of methods for general bacteriology. In: Gerhardt P, Murray RGE American Society for microbiology (eds.). American Society for microbiology, Washington DC, USA.
- Smith JL, Haas MJ (1992) Lipolytic microorganisms. In: Vanderzant C, Splittstoesser DF (eds.). compendium of methods for microbiological examination of foods, (3rd edn). American Public Health Association, Washington DC, USA.
- Ayad EHE, Nashat S, El-Sadek N, Metwaly H, El-Soda M (2004) Selection of wild lactic acid bacteria isolated from traditional Egyptian dairy products according to production and technological criteria. Food Microbiology 21: 715-725.
- Van Geel-Schutten GH, Faber EJ, Smit E, Bonting K, Smith MR, et al. (1999) Biochemical and structural characterization of the Glucan and Fructan Exopolysaccharides synthesized by the Lactobacillus reuteri Wild-type strain and by mutant strains. Appl Environ Microbiol 65: 3008-3014.
- Knoshaug EP, Ahlgrent JA, Trempy JE (2000) Growth associated exopolysaccharide expression in Lactococcus lactis subspecies cremoris Ropy352. J Dairy Sci 83: 633-640.
- Sahan Y, Basoglu F, Gucer S (2007) ICP-MS analysis of a series of metals (namely: Mg, Cr, Co, Ni, Fe, Cu, Zn, Sn, Cd and Pb) in black and green olive samples from Bursa,Turkey. Food Chemistry 105: 395-399.
- El-Hatmi H, Khorchani T, Attia H (2006) Characterization and composition of camel’s (Camelus dromedarius) colostrum and milk. Microbiol Hyg Alim 18: 13-17.
- Farah Z, Rettenmayer R, Atkins D (1992) Vitamin content of camel milk. Int J Vitam Nutr Res 62: 30-33.
- Haddadin MS, Gammoh SI, Robinson RK (2008) Seasonal variations in the chemical composition of camel milk in Jordan. J Dairy Res 75: 8-12.
- Yagil R, Zagorski O, Van Creveld C (1994) Science and Camel’s Milk Production.
- Barbour K, Nabbut, NH, Frerichs WN, Al-Nakhli, HM (1984) Inhibition of Pathogenic Bacteria by Camel’s Milk; Relation to Whey Lysozyme and Stage of Lactation. Journal Food Protection 47: 838-840.
- Yagil R, Saran A, Etzion Z (1984) Camels’ milk: for drinking only? Comp Biochem Physiol A Comp Physiol 78: 263-266.
- Albenzio M, Corbo MR, Rehman US, Fox PF, De Angelis M, et al. (2001) Microbiological and biochemical characteristics of Canestrato Pugliese cheese made from raw milk, pasteurized milk or by heating the curd in hot whey. Int J Food Microbiol 67: 35-48.
- Axelsson L (1998) Lactic Acid Bacteria: Classification and physiology. In: Salimnen S, von Wright A (eds.). Microbiology and functional aspects, (2nd edn). Marcel Dekker Inc., New York, USA. Pg no : 1-72.
- Christensen JE, Dudley EG, Pederson JA, Steele JL (1999) Peptidases and amino acid catabolism in lactic acid bacteria. Antonie van Leeuwenhoek 76: 217-246.
- De Roissart H, Luquet FM (1994) Lactic Acid Bacteria (In French). Lorica 2: 1-220.
- Brennan NM, Ward AC, Beresford TP, Fox PF, Goodfellow M, et al. (2002) Biodiversity of the bacterial flora on the surface of a smear cheese. Appl Environ Microbiol 68: 820-830.
- Yadav H, Jain S, Sinha PR (2007) Production of free fatty acids and conjugated linoleic acid in probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei during fermentation and storage. International Dairy Journal 17: 1006 -1010.
- Smith DJ, Underwood GJC (1998) Exoploymer production by intertidal epipelic diatoms. Limnology Oceanography 43: 1578-1591.
- Ozer P, Erpicum M, Cortemiglia GC, Lucchetti G (1998) A dustfall event in November 1996 in Genoa, Italy. Weather 53: 140-145.
- Klayraung S, Viernstein H, Sirithunyalug J, Okonogi S (2008) Probiotic Properties of Lactobacilli Isolated from Thai Traditional. Sci Pharm 76: 485-503.
- Czulak J, Hammond LA (1953) Freeze-dried starter cultures. Aust J Dairy Technol 8: 89.
- De Valdez GF, de Giori GS, de Ruiz Holgado AP, Oliver G (1985) Effect of the rehydration medium on the recovery of freeze-dried lactic acid bacteria. Appl Environ Microbiol 50: 1339-1341.
- Fayed EO, Sultan N, Yassein NI, Shehata AE (1986) The effect of lyophilization on viability and activity of LAB cultivated in whey treated with certain proteolytic enzymes. Egypt J Food Sci 14: 313-322.
- De Roissart H (1986) Laits laitiers. Tec & Doc, Lavoisier-Apria, Paris.
- Malonga M (1985) Etude de la fabrication des yaourts en république populaire du Congo. Essais d’améliorations. Université de CLERMONT II, France.
- Marshal V (1986) The micro?ora and production of fermented milks. Progress in Industrial Microbiology 23: 1-44.
- Chander H, Batish VK, Mohan M, Chand R (1993) Effect of heat processing on bacterial quality of dahi: an Indian fermented dairy product [1992]. Food and Agriculture Organization of the United Nations 27: 8-9.
Citation: Fguiri I, Ziadi M, Rekaya K, Arroum S, Khorchani T (2017) Isolation and Characterization of Lactic Acid Bacteria Strains from Raw Camel Milk for Potential Use in the Production of Yogurt. J Food Sci Nut 3: 026.
Copyright: © 2017 Imen Fguiri, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

