
Lack of Effect of Biowish Multibio 3PS Probiotic on Bacterial Cold-Water Disease-Induced Mortality in Rainbow Trout
*Corresponding Author(s):
Nathan HuysmanSouth Dakota Game, Fish, And Parks McNenny State Fish Hatchery 19619 Trout Loop Spearfish, South Dakota 57783, United States
Email:nathan.huysman@state.sd.us
Abstract
Flavobacterium psychrophilum, the causative agent of Bacterial Cold-Water Disease (BCWD) is prevalent in salmonid hatcheries worldwide. This study evaluated the potential impact of a commercially available probiotic (BiOWiSH MultiBio 3PS) on BCWD-induced mortality in Shasta strain rainbow trout Oncorhynchus mykiss. The experiment consisted of three consecutive trials. The first trial began at initial feeding and lasted for 31 days. The second trial began on day 32 and lasted for 29 days and the third trial began on day 62 and lasted for 61 days. Tanks of fish received either Bio-Oregon trout feed (control) or Bio-Oregon trout feed top-coated with the BiOWiSH probiotic at 0.5g per kg of feed. Percent mortality was not significantly different between the two treatments in each trial. Feed conversion ratios were not significantly different between the treatments in the first and third trial. However, feed conversion ratio was significantly lower in the control compared to the probiotic treatment in the second trial, at 0.84 and 0.90, respectively. Further studies should be performed with feeds that contain alternative protein sources or at different concentrations of probiotic.
Keywords
Bacterial cold-water disease; BCWD; Oncorhynchus mykiss; Probiotic; Rainbow trout
Introduction
Bacterial Cold-Water Disease (BCWD) causes substantial mortality in salmonid hatcheries worldwide [1]. The causative agent of BCWD is Flavobacterium psychrophilum [2]. Davis [3] first identified BCWD in rainbow trout Oncorhynchus mykiss at a hatchery in West Virginia USA and labelled it peduncle disease. Since then, BCWD has also been called fin rot disease, saddleback disease, fry mortality syndrome, rainbow trout fry syndrome, rainbow trout fry mortality syndrome, bacterial disease of cold water, and cold-water disease [4].
Clinical signs of BCWD are numerous, ranging from tissue erosion around the caudal peduncle [3,5], whitish material along a fin margin [6], pale gill tissue, epidermal hyperplasia, lethargy, spinal abnormalities, and spiral swimming behaviors [7-12]. Reported mortality rates have ranged from 90% in rainbow trout fry [13] to 1% in juvenile rainbow trout [14].
Cleghorn Springs State Fish Hatchery in Rapid City, South Dakota has an endemic strain of Flavobacterium psychrophilum and regular outbreaks of BCWD [15]. BCWD-induced mortality of Shasta strain rainbow trout at the hatchery has ranged from 1% to nearly 70% [16,17].
Probiotics are microbial feed additives that boost the immune response of host organisms by modulating the intestinal microbiota [18,19]. Specific probiotic bacterial species and strains have been shown to reduce BCWD-associated mortality [20-22]. For example, Burbank et al. [20] identified 16 bacterial isolates from rainbow trout digestive tracts as potential probiotic candidates for BCWD control. Subsequently, Ghosh et al. [23] reported that intraperitoneally-administered Enterobacter species C6-6 could protect rainbow trout fry from Flavobacterium psychrophilum infection.
The commercially-available probiotic BiOWiSH MultiBio 3PS contains the probiotic bacterial species Pediococcus acidilacti, Pediococcus pentasaceus, Lactobacillus plantaum, and Bacillus subtillis. Bacillus spp. and Lactobacillus spp. are the known core microbiota of rainbow trout [24-26]. Multi-Bio 3PS has successfully been used to improve immune performance in pigs and calves [27,28], Nile tilapia Oreochromis niloticus [29], and Pacific whiteleg shrimp Pennaeus vannamei [30].
There have been no studies examining the efficacy of a non-species-specific, commercially available probiotic such as Bio-Wish MultiBio 3PS on the control of BCWD. Thus, the objective of this study was to evaluate the effect of dietarily-administered Bio-Wish MultiBio 3PS on BCWD-induced mortality and growth of rainbow trout.
Materials And Methods
All experimentation occurred at Cleghorn Springs State Fish Hatchery, Rapid City, South Dakota, USA using degassed, and aerated spring water at a constant temperature of 11°C (total hardness as CaCO3, 360 mg L-1; alkalinity as CaCO3, 210 mg L-1; pH, 7.6; total dissolved solids, 390 mg L-1). Shasta strain rainbow trout in the study were incubated as eggs and subsequently reared similarly prior to the start of the experiment. Eight flow-through semi-square 190-liter tanks with partial (~50%) overhead covers [31] were used. Four tanks received a diet of BioVita Fry extruded feed (Bio-Oregon, Longview, Washington, USA) top-coated with BiO-WiSH MultiBio 3PS (BiO-WiSH Technologies, Cincinnati, Ohio, USA) probiotic at 0.5g per kg of feed. BiO-WiSH MultiBio 3PS contained the probiotic bacteria Pediococcus acidilacti (≥ 1 x 108 cfu/g), Pediococcus pentasaceus (≥ 1 x 108 cfu/g), Lactobacillus plantaum (≥ 1 x 108 cfu/g), and Bacillus subtillis (≥ 1 x 107 cfu/g). Fish in the other four tanks were fed only Bio-Oregon BioVita Fry (N=4). Inflow to each tank was 7.57 l/min and water velocities were negligible (< 0.1 m/s).
This experiment was divided into 3 trials (Table 1). Beginning on 23 February 2022, each tank received 153 g (approximately 1,860) of pre-swim-up Shasta strain rainbow trout alevins (mean ± SE; initial weight = 0.082 ± 0.00g; total length = 21.7 ± 0.2 mm; n=30). Trial 1 began at first feeding of swim-up fry on 27 February 2022, and continued for 31 days, ending on 29 March. Trial 2 began the next day and loadings were reduced to approximately 1,000 fish/tank (exactly 650 g). Trial 2 ran for 29 days, ending on 28 April. Trial 3 began the next day, and loadings were reduced to 500 fish/tank (exactly 1,110 g). Trial 3 ended on 29 June, lasting 61 days.
|
Trial |
Days |
Fish/tank |
|
1 |
31 |
1860 |
|
2 |
29 |
1000 |
|
3 |
62 |
500 |
Table 1: Length of time (days) and number of fish per tank for three trials with rainbow trout fed diets either with or without Bio-Wish MultiBio 3PS probiotic.
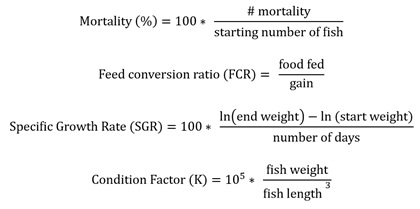
At the conclusion of each trial, the total biomass in each tank was weighed to the nearest g. In addition, 10 fish were individually weighed (0.01 g) and measured (0.1 mm). Fish were fed according to the Hatchery Constant (HC) method [32] with a planned feed conversion rate of 1.1 and a maximum growth rate of 0.075 cm/day. Vibratory feeders (Pentair Aquatic Eco-Systems, Inc., Apopka, FL, USA) connected to a timer (Sweeney Enterprises Inc., Boerne, TX, USA) delivered feed at 60-minute intervals. Mortalities were removed and recorded daily. The following formulas were used:
Data was analyzed using the SPSS (9.0) statistical program (SPSS, Chicago, Illinois, USA), with significance predetermined at P < 0.05. Individual and total tank metrics were analyzed using a one-way analysis of variance. If treatments were determined to be significantly different a post hoc means separation test was performed using the Tukey HSD test.
Results
There was no significant difference in mortality between treatments in each of the three trials (Table 2). Trial 1 had the highest mortality rate at 7.43 ± 0.89% in the probiotic treatment and 9.44 ± 1.47% in the control. Mortality was greatly reduced to less than 0.34% for both treatments during trial 2 and less than 0.08% in trial 3. Ending tank weights and feed conversion ratio were not significantly different between the treatments in trials 1 and 3. However, in trial 2, ending tank weight was significantly higher in the control tanks and feed conversion ratio was significantly lower compared to the probiotic treatment tanks No significant differences were found for individual fish lengths, weights, SGR, or K for any trial of the experiment (Table 3).
|
Trial |
Probiotic |
Initial Weight (kg) |
End Weight (kg) |
FCR |
% Mortality |
|
1 |
Yes |
0.153 |
1.1 ± 0.07 |
1.05 ± 0.04 |
7.43 ± 0.89 |
|
No |
0.153 |
0.98 ± 0.04 |
1.17 ± 0.05 |
9.44 ± 1.47 |
|
|
2 |
Yes |
0.65 |
2.1 ± 0.03b |
0.90 ± 0.01b |
0.27 ± 0.08 |
|
No |
0.65 |
2.3 ± 0.03a |
0.84 ± 0.01a |
0.34 ± 0.04 |
|
|
3 |
Yes |
1.11 |
6.03 ± 0.11 |
0.98 ± 0.02 |
0.04 ± 0.01 |
|
No |
1.11 |
6.09 ± 0.08 |
0.97 ± 0.01 |
0.08 ± 0.05 |
Table 2: Total tank initial weight (kg), ending weight (kg), FCR, and % mortality for three consecutive trials with rainbow trout Oncorhynchus mykiss receiving a diet either with or without BioWish MutliBio 3PS as a probiotic.
Columns with a superscript letter are significantly different from each other (p ≤ 0.05).
|
Period |
Probiotic |
Start length (mm) |
End length (mm) |
Start weight (g) |
End weight (g) |
SGR |
K |
|
1 |
Yes |
22 ± 0.2 |
40 ± 0.3 |
0.08 ± 0.0 |
0.65 ± 0.03 |
6.76 ± 0.12 |
1.01 ±0.04 |
|
No |
22 ± 0.2 |
41 ± 1.1 |
0.08 ± 0.0 |
0.64 ± 0.02 |
6.68 ± 0.11 |
0.95 ± 0.07 |
|
|
2 |
Yes |
40 ± 0.3 |
59 ± 0.7 |
0.65 ± 0.03 |
2.21 ± 0.11 |
11.44 ± 0.18 |
1.08 ± 0.02 |
|
No |
41 ± 1.1 |
60 ± 0.2 |
0.64 ± 0.02 |
2.30 ± 0.03 |
11.59 ± 0.04 |
1.06 ± 0.01 |
|
|
3 |
Yes |
59 ± 0.7 |
111 ± 3 |
2.21 ± 0.11 |
14.74 ± 1.4 |
3.10 ± 0.15 |
1.06 ± 0.05 |
|
No |
60 ± 0.2 |
109 ± 1 |
2.30 ± 0.03 |
14.61 ± 0.86 |
3.10 ± 0.09 |
1.12 ± 0.04 |
Table 3: Individual fish starting length (mm), end length (mm), start weight (g), end weight (g), SGR, and K for three consecutive trials with rainbow trout Oncorhynchus mykiss receiving a diet either with or without BioWish MutliBio 3PS as a probiotic.
Discussion
The BiO-WiSH MultiBio 3PS probiotic used in this study did not significantly impact survival or growth of rainbow trout. While this is the first study to use MultiBio 3PS on cold water fish, it has been shown to be effective on other species. For example, MultiBio 3PS has been shown to increase disease resistance to Streptococcus iniae in Nile tilapia [29] and improve immune performance in pigs and calves [27,28] Contrarily, MultiBio 3PS did not increase immune gene expression in channel catfish Ictalurus punctatus [33]. It also did not improve gut or intestinal bacterial profiles on Pacific White shrimp [34,35].
Probiotics used in rainbow trout culture have been shown to improve water quality [36], stimulate an improved immune response [37-39], reduce mortality [40], and stimulate respiratory burst activity [41]. Furthermore, after compiling the first meta-analysis looking at probiotic effects on rainbow trout, Rahimi et al. [42] found that probiotic use enhanced most of the components of the immune systems of rainbow trout in most studies. The benefits of probiotics have also been studied for disease resistance in other fish and crustaceans. Pirarat et al. [43] demonstrated that after feeding tilapia Oreochromis niloticus with Lactobacillus rhamnosus ATCC 53103 for 2 weeks, stimulation of cellular immunity was detected as demonstrated by an increase in phagocytic activity. Similar observations have been described by Balcazar et al. [44], who demonstrated a positive effect on humoral immune response following probiotic administration in brown trout Salmo trutta. Studies have shown that the use of Bacillus sp. in cropping systems has improved the water quality, survival, growth rate and health status of juvenile giant prawn Penaeus monodon and reduced the number of pathogenic vibrios [45].
The overall mortalities in this study were consistent with the 10% BCWD-induced mortality typically observed at Cleghorn Springs Hatchery [16]. Other studies have observed rainbow trout mortality rates as low as 1% [14] and as high as 90% [13] due to BCWD.
Most of the mortality in this study occurred in the first trial when the trout only had innate immunity. Martin et al. [17] suggested that diets leading to faster growth and more rapid development of the secondarily developed adaptive immunity may impact fish responses to future BCWD outbreaks. Prior to the development of adaptive immunity, young fish are more susceptible to opportunistic environmental pathogens such as F. psychrophilum [46-49].
The BioOregon starter diet used in this trial contains beta-glucans, nucleotides and a high level of vitamins designed to stimulate the immune system. It is possible that the positive effects of the probiotic might be suppressed with such a nutritionally complete diet with other immune-stimulating ingredients [50-52].
Burbank et al. [20] suggested that bacterial strains specific to rainbow trout GI tracts should be used as potential probiotic candidates. Bacillus spp. and Lactobacillus spp. are found naturally at high levels in the core microbiota of rainbow trout [24-26]. These bacterial species closely correspond to two of the four listed bacterial species in MultiBio 3PS; Lactobacillus plantaum, and Bacillus subtillis. As long as the concentrations of probiotic were sufficient for rainbow trout diets, and the water temperature was not too low to activate the bacteria, the species present in MultiBio 3PS would have been expected to stimulate an immune response in the fish.
The probiotic was prepared and applied to the feed according to manufacturer’s specifications at 0.5g per kg of feed. It is unknown if the probiotic dissolved off the feed or diluted into the water before ingestion, although the fish were feeding readily at the water’s surface. The concentration of bacterial cells used should have been sufficient since the 107-8 cfu/g-1 of each probiotic species contained in the MultiBio 3PS was the same concentration of bacterial cells used in most other trials utilizing similar strains with rainbow trout [20,53-55].
In conclusion, while MultiBio probiotics have a record of success in warmwater fish, shrimp, pigs, and cows, its effect was negligible in this study. Future tests should examine the use of different concentrations, different temperatures, different delivery methods, and different species, strains, and sizes of fish.
Acknowledgements
The authors would like to thank all their co-workers at Cleghorn Springs State Fish Hatchery for assisting with carrying out this experiment. Authors would also like to thank Alexis Gerber and Breah Rosner for assisting with formatting of this manuscript.
References
- Antaya C (2008) Current Eco-Economical Impacts of Flavobacterium psychrophilum. MMG 445 Basic Biotechnol J 4: 7-12.
- Starliper CE (2011) Bacterial coldwater disease of fishes caused by Flavobacterium psychrophilum. J Adv Res 2: 97-108.
- Davis HS (1946) Care and diseases of trout. Research Report No. 12; United States Fish and Wildlife Service, Washington DC.
- Barnes ME, Brown ML (2011) A review of flavobacterium psychrophilum biology, clinical signs, and bacterial cold water disease prevention and treatment. Open J Fish Res 4: 40-48.
- Borg AF (1960) Studies on myxobacteria associated with diseases in salmonid fishes. J Wildl Dis 8: 1-85.
- Martínez JL, Casado A, Enríquez R (2004) Experimental infection of Flavobacterium psychrophilum in fins of Atlantic salmon Salmo salar revealed by scanning electron microscopy. Dis Aquat Organ 59: 74-84.
- Rucker RR, Earp BJ, Ordahl EJ (1953) Infectious diseases of Pacific salmon. Trans Am Fish Soc 83: 297-312.
- Pacha RE (1968) Characteristics of Cytophaga psychrophila (Borg) isolated during outbreaks of bacterial cold-water disease. Appl Microbiol 16: 97-101.
- Holt RA (1987) Cytophaga psychrophila, the causative agent of bacterial cold-water disease in salmonid fish. Oregon State University Corvallis Oregon.
- Kent ML, Groff JM, Morrison JK, Yasutake WT, Holt RH (1989) Spiral swimming behavior due to cranial and vertebral lesions associated with Cytophaga psychrophila infections in salmonid fishes. Dis Aquat Organ 6: 11-16.
- Lorenzen E, Dalsgaard I (1991) Preliminary investigation of fry mortality syndrome in rainbow trout. Bull Eur Assoc Fish Pathol 11: 77-79.
- Santos Y, Huntly PG, Turnbull A, Hastings TS (1992) Isolation of Cytophaga psychrophila (Flexibacter psychorphilus) in association with rainbow trout mortality in the United Kingdom. Bull Eur Assoc Fish Pathol 12: 209-210.
- Nilsen H, Olsen AB, Vaagnes Ø, Hellberg H, Bottolfsen K, et al. (2011) Systemic Flavobacterium psychrophilum infection in rainbow trout, Oncorhynchus Mykiss (Walbaum), farmed in fresh and brackish water in Norway. J Fish Dis 34: 403-408.
- Post GP (1987) Textbook of fish health. TFH Publications Inc.: New Jersey USA.
- Neiger R, Thomas M, Das S, Barnes M, Fletcher B, et al. (2016) Draft genomes of three Flavobacterium psychrophilum strains isolated from cold water disease outbreaks at three production hatcheries. Am Soc Microbiol 4.
- Treft CE, Barnes ME, Vorhees JM, Martin TJ (2017) Impacts of feeding three commercial trout starter diets to rainbow trout on bacterial coldwater disease-induced mortality. J mar biol aquac 3: 1-5.
- Martin TJ, Voorhees JM, Treft CE, Fletcher B, Barnes ME (2018) Effects of four commercial diets on rainbow trout Oncorhynchus mykiss growth, feeding efficiency and mortality at a production hatchery with endemic bacterial coldwater disease. Insights Aquac Cult Biotechnol 1: 1-7.
- Fuller R (1989) Probiotics in man and animal. J App Bacteriol 66: 365-378.
- Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RTM, et al. E (2010) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302: 1-18.
- Burbank DR, Shah DH, LaPatra SE, Fornshell G, Cain KD (2011) Enhanced resistance to coldwater disease following feeding of probiotic bacterial strains to rainbow trout (Oncorhynchus Mykiss). Aquaculture 321: 185-190.
- Boutin S, Audet C, Derome N (2013) Probiotic treatment by indigenous bacteria decreases mortality without disturbing the natural microbiota of Salvelinus fontinalis. Can J Microbiol 59: 662-670.
- Ghosh B, Cain KD, Nowak BF, Bridle AR (2014) Microencapsulation of a Putative Probiotic Enterobacter Species, C6-6, to Protect Rainbow Trout, Oncorhynchus Mykiss (Walbaum), against Bacterial Coldwater Disease. J Fish Dis 39: 1-11.
- Burbank DR, LaPatra SE, Fornshell G, Cain KD (2012) Isolation of bacterial probiotic candidates from the gastrointestinal tract of rainbow trout, Oncorhynchus mykiss (Walbaum), and screening for inhibitory activity against Flavobacterium psychrophilum. J Fish Dis 35: 809-816.
- Wong S, Waldrop T, Summerfelt S, Davidson J, Barrows F, et al. (2013) Aquacultured rainbow trout (Oncorhynchus Mykiss) possess a large core intestinal microbiota that is resistant to variation in diet and rearing density. Appl Environ Microb 79: 4974-4984.
- Ricaud K, Rey M, Plafnes-Juan E, Larroquet L, Even M, et al. (2018) Composition of intestinal microbiota in two lines of rainbow trout (Oncorhynchus mykiss) divergently selected for muscle fat content. Open Microbiol J 12: 308-320.
- Parshukov AN, Kashunskaya EN, Simonov EP, Hlunov OV, Izvekonva KB, et al. (2019) Variations of the intestinal gut microbiota of farmed rainbow trout Oncorhynchus mykiss (Walbaum), depending on the infection status of the fish. J App Microbiol 127: 379-395.
- Ngoc TTB, Huyen NT, Oanh NC, Dang PK (2022) Effects of probiotics and organic acids and their mixture on nutrient digestibility, growth performance and fecal gas emission in grower-finisher pigs. Adv Ani Vet Sci 10: 480-487.
- Wang H, Yu Z, Gao Z, Li Q, Qiu X, et al. (2022) Effects of compound probiotics on growth performance, rumen fermentation, blood parameters, and health status of neonatal Holstein calves. J Dairy Sci 105: 2190-2200.
- Padeniya U, Davis DA, Liles MR, LaFrentz SA, Lafrentz BR, et al. (2023) Probiotics enhance resistance to Streptococcus iniae, in Nile tilapia (Oreochromis niloticus) reared in biofloc systems. J F Dis 46: 1137-1149.
- Hatchery Post Larvae Yield & Health in Vannamei Shrimp. Available online: (Accessed on 28 February 2023).
- Rosburg AJ, Fletcher BL, Barnes ME, Treft CE, Bursell BR (2019) Vertically suspended environmental enrichment structures improve the growth of juvenile landlocked fall Chinook salmon. Int j innov stud aquat biol fish 5: 17-24.
- Buterbaugh GL, Willoughby H (1967) A feeding guide for brook, brown, and rainbow trout. Prog Fish C 4: 210-215.
- Nguyen KQ, Bruce TJ, Afe OE, Liles MR, Beck BH, et al. (2022) Growth performance, survival, blood chemistry, and immune gene expression of channel catfish (Ictalurus punctatus) fed probiotic-supplemented diets. Vet Sci 9: 701.
- Landsman A, St-Pierre B, Rosales-Leija M, Brown M, Gibbons W (2019a) Investigation of the potential effects of host genetics and probiotic treatment on the gut bacterial community composition of aquaculture-raised pacific whiteleg shrimp, Litopenaeus vannamei. Microorganisms 7: 217.
- Landsman A, St-Pierre B, Rosales-Leija M, Brown M, Gibbons W (2019b) Impact of aquaculture practices on intestinal bacterial profiles of pacific whiteleg shrimp Litopenaeus vannamei. Microorganisms 7: 93.
- Hedayati A, Bagheri T (2009) The effect of probiotic (bacillus spp) on growth, survival, and innate immunity of rainbow trout (Onchorhynchus mykiss) fry during the first two months of feeding. J Comp Pathol 141: 289.
- Irianto A, Austin B (2002) Probiotics in aquaculture. J Fish Dis 25: 633-642.
- Panigrahi A, Kiron V, Kobayashi T, Puangkaew J, Satoh S, et al. (2004) Immune responses in rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet Immunol Immunop 102: 379-388.
- Balcazar JL, de Blas I, Ruiz-Zaezuela I, Cunningham D, Vendrell D, et al. (2006) The role of probiotics in aquaculture. Vet Microbiol 114: 173-186.
- Nikoskelainen S, Salminen S, Bylund G, Ouwehand AC (2001) Characterization of the properties of human- and dairy-derived probiotics for prevention of infectious diseases in fish. Appl Environl Microb 67: 2430-2435.
- Nikoskelainen S, Ouwehand AC, Byland G, Salminen S, Lilius EM (2003) Immune enhancement in rainbow trout (Oncorhynchus mykiss) by potential probiotic bacteria (Lactobacillus rhamnosus). Fish and Shellfish Immunol 15: 443-452.
- Rahimi R, Mirahmadi SA, Hajirezaee S, Fallah AA (2021) How probiotics impact on immunological parameters in rainbow trout (Oncorhynchus mykiss): A systematic review and meta-analysis. Rev Aquacult 14: 27-53.
- Pirarat N, Kobayashi T, Katagiri T, Maita M, Endo M (2006) Protective effects and mechanisms of a probiotic bacterium Lactobacillus rhamnosus against experimental Edwardsiella tarda infection in tilapia (Oreochromis niloticus). Vet Immunol Immunop 113: 339-347.
- Balcazar JL, Rojas-Luna T, Cunningham DP (2007) Effect of the addition of four potential probiotic strains on the survival of pacific white shrimp (Litopenaeus vannamei) following immersion challenge with Vibrio parahaemolyticus. J Invertebr Pathol 96: 147-150.
- Dalmin G, Kathiresan K, Purushothaman A (2001) Effect of probiotics on bacterial population and health status of shrimp in culture pond ecosystem. Indian J Exp Biol 39: 939-942.
- Magnadóttir B (2006) Innate Immunity of Fish (Overview). Fish Shellfish Immunol 20: 137-151.
- Uribe C, Folch H, Enriquez R, Moran G (2011) Innate and Adaptive Immunity in Teleost Fish: A Review. Vet Med-Czech 56: 486-503.
- Smith NC, Rise ML, Christian SL (2019) A comparison of the innate and adaptive immune systems in cartilaginous fish, ray-finned fish, and lobe-finned fish. Front Immunol 10.
- Gomez E, Mendez J, Cascales D, Guijarro JA (2014) Flavobacterium psychrophilum vaccine development: Adifficult task. Microb Biotechnol 7: 414-423.
- Thompson I, Choubert G, Houlihan DF, Secombes CJ (1995) The effect of dietary vitamin A and astaxanthin on the immunocompetence of rainbow trout. Aquaculture 133: 91-102.
- Barnes ME, Fletcher B (2007) Effects of a proprietary yeast supplement during the rearing of two strains of juvenile rainbow trout and juvenile lake trout. Proceedings of the South Dakota Academy of Science 86: 89-96.
- Barros MM, Falcon DR, Orsi RO, Pezzato LE, Fernandes Jr. AC, et al. (2014) Non-specific Immune parameters and physiological response of Nile tilapia fed ß-glucan and vitamin C for different periods and submitted to stress and bacterial challenge. Fish shellfish Immunol 39: 188-195.
- Panigrahi A, Azad IS (2007) Microbial intervention for better fish health in aquaculture: the Idian scenario. Fish Physiol Biochem 33: 429-440.
- Sharifuzzaman SM, Austin B (2009) Influence of probiotic feeding duration on disease resistance and immune parameters in rainbow trout. Fish shellfish Immunol 27: 440-445.
- Schubinger CB, Orfe LH, Sudheesh PS, Cain KD, Shah DH, et al. (2015) Entericidin is required for a probiotic treatment (enterobacter sp. strain C6-6) to protect trout from cold-water disease challenge. Appl Enviro Microbiol 81: 658-665.
Citation: Hawkins M, Huysman N, Hodges B, Treft C, Barnes ME (2023) Lack of Effect of Biowish Multibio 3PS Probiotic on Bacterial Cold-Water Disease-Induced Mortality in Rainbow Trout. J Aquac Fisheries 7: 074.
Copyright: © 2023 Mack Hawkins, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

