
Level of Toxic Metals in Consumable Aquatic Plant Ledermanniella schlechteri from Congo River and Potential Risk Assessment through Plant Consumption
*Corresponding Author(s):
John W PotéDepartment F.-A, Forel For Environmental And Aquatic Sciences And Institute Of Environmental Sciences, Earth And Environmental Science Section, Faculty Of Science, University Of Geneva, Geneva, Switzerland, University Of Kinshasa, Faculty Of Sciences, Department Of Chemistry, Kinshasa XI, Congo, The Democratic Republic Of The
Tel:+41 223790321,
Fax:+41 223790329
Email:john.pote@unige.ch
Abstract
The accumulation of toxic metals in food consumption has been identified as a serious human health risk. Ledermanniella schlechteri (LDMSC) is a genus of Podostemaceae plant family and is well known to grow naturally on rocks of rapid waters. LDMSC is rich in nutrients and one of the most consumed aquatic vegetables in Africa. However, there are not any studies performed to assess the level of toxic metals in this aquatic plant. Consequently, the aim of this study is to assess the concentration of toxic metals in LDMSC and to evaluate the potential health risk to the consumers. Plant samples were collected in Congo River at the rapid of Kinsuka-Mimosa, in the vicinity of Kinshasa, Capital City of the Democratic Republic of the Congo (DRC). Toxic metal (Cr, Cu, Zn, As, Cd and Pb) concentrations in LDMSC were determined using Inductively Coupled Plasma Mass Spectrometry whereas Hg analysis was performed using atomic absorption spectrophotometry. Metal levels in LDMSC were compared with international regulation for human consumption set by the Food and Agriculture Organization (FAO) and World Health Organization (WHO). Metal concentrations in LDMSC varied significantly according to sampling sites. The average values (in mg kg-1) ranging from 0.44 -9.1 (Cr), 0.14-4.52 (Ni), 5.5-78.4 (Cu), 336.14-1520.91 (Zn), 0.08-0.49 (As), 0.21-0.78 (Cd), 0.44-11.81 (Pb) and 0.02-0.24 (Hg). In all sampling sites, the average concentration of Zn, As, Cd and Hg in plant samples exceeded the FAO/WHO permissible limits for human consumption. According to FAO/WHO regulation and the calculated values of targeted risk quotient, the impact on human health is likely to occur. On other hand, according to the metal levels in LDMSC, this plant can be used as bioindicator to evaluate the state of river pollution by toxic metals.
Keywords
Bioaccumulation; Human health risks; Ledermanniella schlechteri; Plant contamination; River pollution; Toxic metals
GRAPHICAL ABSTRACT
INTRODUCTION
The presence of toxic metals in food chain is of worldly concern to human health due to its toxicity, ubiquity, persistence, non-degradability and bioaccumulation. Consumption of food contaminated by toxic metals leads to their accumulation in the kidney and liver of humans and can cause several diseases including cancer, anemia, infertility to males, cardiovascular, nervous, kidney and lung diseases [1-3]. Vegetables are vital and provide essential nutrients for the human healthy diet to maintain normal physiological functions, antioxidants, dietary fiber metabolites and prevention of several diseases [4-7]. However, exposure to toxic metals by the consumption of contaminated vegetables can leads to human serious health risks [3,8,9]. It is therefore very important and recommended to consume the non-contaminated vegetables by organic and inorganic pollutants (such as toxic heavy metals) to avoid the human health risks [1,2,4,10].
The Congo River is the most important river of DRC. The river basin is the second largest drainage basin in the world after the Amazon and has a great economic importance [11]. It has important more diverse species than of any other freshwater system in Africa, providing benefits to humans both directly, such as through livelihoods from fisheries and indirectly through services such as the purification of water for drinking [11,12]. According to its economic situation, some studies have been performed to assess the water and sediment quality in the Congo River Basin [11,13-15] and accumulation of toxic metals in fish species from the river [16]. These studies strongly recommended further researches to evaluate the quality of this important river; e.g., the accumulation of toxic metals in consumable aquatic plants. To our best knowledge, no data is available on the toxic metal accumulation in aquatic plants of the Congo River in the vicinity of the city of Kinshasa.
Ledermanniella schlechteri is a genus of plant Podostemaceae family. The plant grows naturally and fixed in the rocks of tropical freshwater falls, mainly in African Rivers. In the Congo River at the vicinity of Kinshasa, Ledermanniella is well known to be exclusively associated to rapid waters at Kinsuka section and grows during the middle-dry (march) and dry season [17]. LDMSC does not have a great economic value and is mostly consumed by the poorest population established at the Western part of the city of Kinshasa. However, no study was performed to assess the level of toxic metals in this aquatic plant. Consequently, the aim of this study is to assess the concentration of toxic metals including Cr, Cu, Zn, As, Cd, Pb and Hg in LDMSC collected from 7 sites in Congo River and to evaluate the consumer health risks.
MATERIALS AND METHODS
Study site and sampling procedure
This research was conducted in the Congo River, Kinsuka section in the vicinity of Kinshasa, the capital city of DRC (Figure 1). Congo River in Kinsuka section is characterized by large waterfalls and the presence of rocks. The site is well known for the artisanal exploitation of sand and rubbles for building houses and for both collection and selling of LDMSC during the low water period. The plant is totally or partially submerged and stirred by a strong river current (Figure 2A).
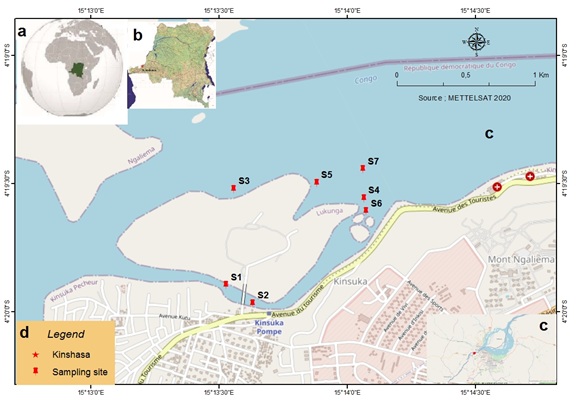
Figure 1: Adapted Map showing (a) Democratic Republic of the Congo (DRC) in African continent map, (b) Kinshasa City in DRC and (c) Sampling sites in Congo River at Kinsuka-Mimosa Section.

Figure 2: Photos taken by Henry Mata, in March 2019 indicating (A) Ledermanniella schlechteri in Congo River; (B) Plant sampling procedure; (C and D) Plant samples washed in river before stored in an icebox and transported to the laboratory.
Sampling took place in March 2019 during the low water period considered as a small dry season. Seven sites were chosen to collect plant samples. The sites are labelled S1-S7 and sampling site coordinates are reported in table 1. From each sites, about 800-900 g of edible plant part (n=5, biological replicates) were collected at a distance of about 60-100 cm between sampling points. Plant samples were collected manually in water depth less than 1 m (Figure 2B). After collection, samples were washed with river water (Figure 2 (C, D)) stored in an icebox and transported to the laboratory for different treatments within 24 h. After preliminary treatments (washing with deionized water and weighted), the samples were frozen and sent to the Department F.-A. Forel of the University of Geneva for analysis.
|
|
Coordinates |
Cr |
Ni |
Cu |
Zn |
As |
Cd |
Pb |
Hg |
|
S1 n=5 |
S 4°19.918’ E 15°13.585’ |
0.44-9.10 |
0.46-4.52 |
5.50-14.85 |
336.14-789.18 |
0.12-1.15 |
0.29-0.59 |
0.81-11.81 |
0.03-0.05 |
|
S2 n=5 |
S 4°19.922’ E 15°13.617’ |
0.68-2.51 |
0.43-1.38 |
11.81-16.10 |
459.98-1077.89 |
0.15-0.49 |
0.32-0.78 |
1.33-2.8 |
0.03-0.06 |
|
S3 n=5 |
S 4°19.665’ E 15°13.524’ |
0.74-1.79 |
0.57-1.55 |
12.80-78.40 |
596.66-1331.45 |
0.14-0.31 |
0.48-0.68 |
2.07-3.17 |
0.03-0.04 |
|
S4 n=5 |
S 4°19.613’ E 15°14.019’ |
0.53-1.53 |
0.46-1.3 |
7.82±75.70 |
752.61-1520.91 |
0.1-0.26 |
0.25-0.63 |
0.74-3.57 |
0.02-0.15 |
|
S5 n=5 |
S 4°19.543’ S E 15°13.572’ |
0.48-1.36 |
0.14-1.84 |
6.24-12.30 |
436.85-689.32 |
0.08-0.22 |
0.21-0.49 |
0.44-1.26 |
0.02-0.03 |
|
S6 |
S 4°19.614’ E 15°14.002’ |
0.64-2.65 |
0.35-0.96 |
8.96-13.98 |
576.99-1223.86 |
0.08-0.24 |
0.27-0.44 |
0.61-1.66 |
0.02-0.03 |
|
S7 n=5 |
S 4°19.605’ E 15°14.012’ |
0.73-0.98 |
0.53-0.77 |
9.51-16.61 |
416.81-1280.67 |
0.14-0.25 |
0.24-0.59 |
0.61-2.06 |
0.07-0.01 |
Table 1: Sampling site coordinates and concentration range (mg kg-1) of toxic metals in Ledermanniella schlechteri.
Plant samples digestion
The digestion of plant samples was performed as described by Larras et al. [18], but with some modification. Briefly, plant samples were washed with deionized water, weighted, lyophilized and water content was calculated. About 0.5 g of ground plant samples (with liquid nitrogen) were digested with 8 mL of HNO3 (65%, Suprapur®, Merck KGaA, Darmstadt Germany) and 2 mL of H2O2 (30%, Merck KGaA, Darmstadt Germany) in Teflon bombs heated during 16 hours at 105°C in a Salvis Lab oven (Salvis AG, Luzern, Switzerland). The digested solution was then centrifuged at 20°C during 15 min at 4000 rpm. The digestion liquid (supernatant) was diluted 50 times with 1% HNO3 (Suprapur®, Merck KGaA, Darmstadt Germany) and then analyzed by ICP-MS.
Metals analysis in plant samples by ICP-MS
Toxic metals (Cr, Ni, Cu, Zn, As, Cd and Pb) in digested plant samples were measured using ICP-MS (model 7700 series, Agilent, Santa Clara, CA, USA). The standard solutions at different concentrations (0, 0.2, 1, 5, 10, 20, 50 and 100 μg L−1) were prepared from ICP multi-element standard solution Merck IV, 1000 mg L−1 (Merck IV, KGaA, Darmstadt Germany) and a mono element solution (As, Merck KGaA, Darmstadt Germany) for ICP-MS analysis and were used for calibration. A collision/reaction cell (Helium mode) and interference equations were utilized to remove spectral interferences that might otherwise bias results. The Limit of Detection (LOD) was calculated as 3xSD (Standard Deviation) of the blanks and was less than 0.001 µg L-1 for all analyzed elements. The results are expressed in mg kg-1 wet weight calculated with values of water content in plant samples.
Mercury analysis plant samples
Total Hg analysis in plant samples was carried out using the Atomic Absorption Spectrophotometer (AAS) for mercury determination (Advanced Mercury Analyser; AMA 254, Altecs.r.l., Czech Rep.) following the method described by Larras et al. and Roos-Barraclough et al. [18,19]. The method is based on sample combustion, gold amalgamation and AAS. The results are expressed in mg kg-1 wet weight calculated with values of water content in plant samples.
Quality control and statistical analysis
The accuracy of the method was checked by analysis of the certified reference material (BCR®-414, for aquatic plant, EU Commission-JRC, Geel, Belgium), prepared and analyzed in the same conditions as the plant samples, for both ICP-MS and AMA analyses. Triplicate measurements were made for all analyses. Statistical processing of data (Pearson Correlation Matrix) was performed using Sigma Stat 12.5 (Systat Software, Inc., USA).
Estimation of health potential risks for Ledermanniella schlechteri consumption
The human health risks for LDMSC consumption was performed by comparing the metal concentrations in LDMSC with the permissible levels for human consumption set by Food and Agriculture Organization and World Health Organization [5]. Additionally, the Targeted Risk Quotient (THQ) were calculated [16,20-22], to estimate the health risks associated with the consumption of LDMSC by the local population.
RESULTS AND DISCUSSION
Quality control and certified reference material values of metal concentrations
The obtained values of analyzed metals by ICP-MS for the reference material BCR®-414 were in the certified range. The results are reported in table 2. The recovery values ranged from 86.8 to 96.8% for ICP-MS analysis and 95.8% for Hg analysis by AMA. The good recoveries of metal concentrations from certified reference material BCR®-414 demonstrated the accuracy of the used protocol for analysis of plant samples.
|
Metal |
Certified Value |
Measured Value (n=3) |
Recovery (%) |
|
Cr |
23.80±1.20 |
22.75±3.61 |
95.58 |
|
Ni |
18.80±0.80 |
17.82±2.29 |
94.78 |
|
Cu |
29.5±1.30 |
27.87±3.42 |
94.47 |
|
Zn |
111.6±2.50 |
107.23±6.13 |
96.08 |
|
As |
6.82±0.28 |
6.27±1.8 |
91.93 |
|
Cd |
0.38±0.01 |
0.33±0.06 |
86.84 |
|
Pb |
3.97±0.19 |
3.78±0.42 |
95.21 |
|
Hg |
0.28±0.02 |
0.26±0.03 |
92.85 |
Table 2: Certified and observed values of metal concentration of reference material BCR®-414 (in mg kg-1 dry weight).
Concentration of metals in Ledermanniella schlechteri
The concentration of toxic metals including Cr, Ni, Cu, Zn, As, Cd, Pb and Hg in LDMSC is reported in table 1.The toxic metal concentrations in LDMSC varied significantly according to sampling sites (P?0.05). The values (in mg kg-1) ranged from 0.44-9.10 (Cr), 0.14-4.52 (Ni), 5.5-78.4 (Cu), 336.14-1520.91 (Zn), 0.08-0.49 (As), 0.21-0.78 (Cd), 0.44-11.81 (Pb) and 0.02-0.07 (Hg).
Except for the site S1 (with following order Zn>Cu>Pb>Cr>Ni>As>Cd>Hg), the order of average concentration of toxic metals in LDMSC was similar amongst sampling sites (S2-S7) and in the following order: Zn>Cu>Pb>Cr>Ni>Cd>As>Hg. The results of this study indicate that the LDMSC present high metal concentration, especially for Zn, As, Cd and moderately the Hg. In Congo River basin in the vicinity of Kinshasa City, the metal concentrations in different sites can reach the values of 107.2, 111.7, 88.6, 39.3, 15.4, 6.1 and 4.7 mg kg-1 for Cr, Ni, Zn, Cu, Pb, As and Hg, respectively [13,14]. The Congo river pollution in the vicinity of Kinshasa can be explained by several sources including commercial and domestic activities, presence of uncontrolled landfills in the banks of river, urban runoff and untreated industrial wastewaters discharge into the river and the boats and automobile exhausts [13,14]. All of the potential sources of river contamination are located upstream of our study site (Kinsuka-Mimosa). Additionally, in the studied area, there are many commercial and touristic activities generating different waste materials such as batteries, plastics, mobile phone, scrapped vehicles and metals with old paints, which were observed during sampling. These aspects can be considered as potential sources of LDMSC contamination by investigated toxic metals. However, the accumulation from natural/localmatrix (sediments/rocks) cannot be excluded and should be explored in depth. Aquatic plants (e.g. Macrophytes, Elodea nuttallii, Podostemaceae) are susceptible to accumulate toxic metals from different sources/local matrix and can be considered as bioindicator to determine the distribution and monitoring of river contamination by heavy metals [18,23-25]. The results from this study indicate that LDMSC can be considered as bioindicator and bioaccumulator of metals, especially Zn, Cd, As and Hg (Figures 3 and 4).
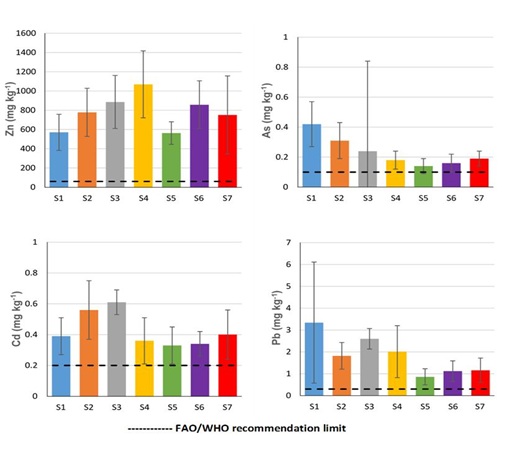 Figure 3: Average concentration (in ppm (mg kg-1 dry weight)) of Zn, As, Cd and Pb in Ledermanniella schlechteri comparing to regulation for human consumption set by the Food and Agriculture Organization (FAO) and World Health Organization (WHO) (FAO/WHO, 2007).
Figure 3: Average concentration (in ppm (mg kg-1 dry weight)) of Zn, As, Cd and Pb in Ledermanniella schlechteri comparing to regulation for human consumption set by the Food and Agriculture Organization (FAO) and World Health Organization (WHO) (FAO/WHO, 2007).
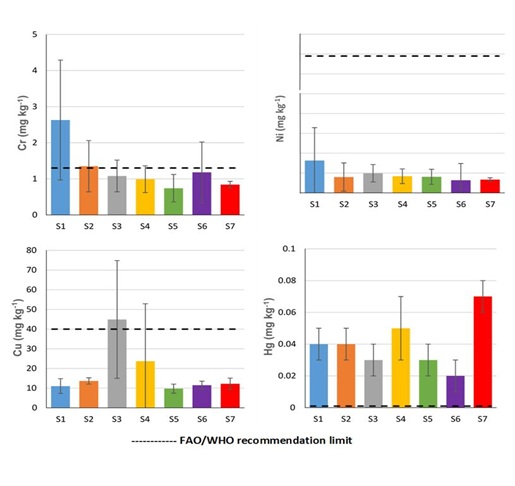 Figure 4: Average concentration (in ppm (mg kg-1 dry weight)) of Hg, Cu, Cr and Ni in Ledermanniella schlechteri comparing to regulation for human consumption set by the Food and Agriculture Organization (FAO) and World Health Organization (WHO) (FAO/WHO, 2007).
Figure 4: Average concentration (in ppm (mg kg-1 dry weight)) of Hg, Cu, Cr and Ni in Ledermanniella schlechteri comparing to regulation for human consumption set by the Food and Agriculture Organization (FAO) and World Health Organization (WHO) (FAO/WHO, 2007).
Potential health risk assessment for the consumption of Ledermanniella schlechteri
The average concentration of toxic metals in LDMSC from each site was compared with international regulation for human consumption set by FAO/WHO [5]. The FAO/WHO maximum permissible level (mg kg-1) are 1.3(Cr), 66.9(Ni), 40(Cu), 60(Zn), 0.1(As), 0.2(Cd), 0.3(Pb) and 0.001(Hg). The results demonstrate that the average concentrations (mg kg-1) of Zn, As, Cd, Pb and Hg exceeded respectively, about 6-25, 1-5, 1-4, 1-39, 20-70 times the maximum level recommended by Joint FAO/WHO Expert Committee on Food Additives [5]. The average concentrations of Cr, Ni and Cu in LDMSC from all sampling sites were under the permissible levels for human consumption set by FAO/WHO, except for the site S1 (Cr: 2.6 mg kg-1) and the site S3 (Cu: 44.9 mg kg-1). In addition, the Target Hazard Quotients (THQ) were performed to evaluate the human health risks associated with LDMSC contamination by selected toxic metals. The results are presented in table 3. Results indicate that, except for Zn (in the sites S1-S6) and As (in S1 and S2), the obtained values of THQ for individual metal were less than 1 indicating negligible human health risks for intake through consumption of LDMSC.
|
Metals |
Reference Dose (mg/bw kg day) |
Target Hazard Quotient (THQ)* |
||||||
|
S1 |
S2 |
S3 |
S4 |
S5 |
S6 |
S7 |
||
|
|
|
|
|
|
|
|
|
|
|
Cr |
0.0030 |
0.617 |
0.520 |
0.417 |
0.383 |
0.287 |
0.457 |
0.001 |
|
Cu |
0.0400 |
0.320 |
0.681 |
1.302 |
0.687 |
0.283 |
0.334 |
0.044 |
|
Zn |
0.300 |
2.205 |
3.007 |
3.424 |
4.132 |
2.174 |
3.315 |
0.395 |
|
As |
0.0003 |
1.633 |
1.200 |
0.933 |
0.700 |
0.533 |
0.633 |
0.000 |
|
Cd |
0.001 |
0.450 |
0.650 |
0.710 |
0.420 |
0.380 |
0.390 |
0.001 |
|
Pb |
0.004 |
0.968 |
0.053 |
0.753 |
0.583 |
0.250 |
0.325 |
0.001 |
|
Hg |
0.0001 |
0.500 |
0.500 |
0.300 |
0.600 |
0.200 |
0.300 |
0.000 |
Table 3: The Target Hazard Quotient of toxic metals through consumption of Ledermanniella schlechteri collected from Congo River, Kinsuka-Mimosa Section.
Zinc (Zn) is essential for many metabolic and enzymatic functions in humans such as growth and development, testicular maturation, neurological function, wound healing and immune competence [26]. It is one of the metals considered less toxic to humans and deficiency problems are more common and more severe than those of toxicity and deficiency of this metal can cause stunting, loss of taste and possible decrease of infertility [27,28]. Toxicity of Zn in human organism is rare, nevertheless, it may exert some toxicity at higher doses [26,28]. In the contrary, Arsenic (As) is not an essential element for humans. Arsenic and Arsenic compounds are classified by International Agency for Research on Cancer (IARC) as highly carcinogenic elements for humans. The contamination of aquatic plants and food chain by As can be explained by the presence of As in natural sources or through contaminated water, soil and sediments. According to IARC, human exposure to As via food consumption even in low concentration can have harmful effects on human health. Similar to As, even in low concentration, Hg is also very dangerous because of its high toxicity and its potential biomagnification and bioaccumulation in food chains [29,30]. The average concentration of Hg observed in LDMSC can also have harmful effects on human health.
Metal statistical correlations in Ledermanniella schlechteri
A Pearson correlation matrix was performed to track the behavior of the metal accumulation in the plant. The results are presented in table 4. Positive and negative correlation were observed between metals in LDMSC. These results indicate that accumulation of investigated metals in LDMSC may have originated from common/or different sources.
|
Cr |
Ni |
Cu |
Zn |
As |
Cd |
Pb |
Hg |
|
|
Cr |
1.00 |
0.89** |
-0.19 |
-0.39 |
0.91** |
0.04 |
0.80* |
-0.11 |
|
Ni |
|
1.00 |
0.05 |
-0.43 |
0.84* |
0.08 |
0.88** |
-0.07 |
|
Cu |
|
|
1.00 |
0.54 |
-0.03 |
0.68 |
0.41 |
-0.14 |
|
Zn |
|
|
|
1.00 |
-0.36 |
0.19 |
-0.01 |
0.09 |
|
As |
|
|
|
|
1.00 |
0.37 |
0.84* |
0.05 |
|
Cd |
|
|
|
|
|
1.00 |
0.41 |
-0.05 |
|
Pb |
|
|
|
|
|
|
1.00 |
-0.02 |
|
Hg |
|
|
|
|
|
|
1.00 |
Table 4: Pearson Correlation Matrix among the metals in Ledermanniella schlechteri.
Note: * Significant at the 0.05 level; ** Significant at the 0.01 level.
CONCLUSION
The present study constitutes the first evaluation of toxic metals in LDMSC from the Congo River. In general, high concentration of investigated metals was observed in LDMSC. The average concentrations of Zn, As, Cd, Pb and Hg exceeded the maximum level recommended by Joint FAO/WHO Expert Committee on Food Additives indicating the potential adverse effects to consumers. On other hand, except for Zn and As (for two sites), the values of target hazard quotient of toxic metals through consumption of LDMSC indicate negligible risks through the consumption of the vegetable. Thus, this research strongly recommends further studies for fully evaluate the potential human health risks through consumption of one of the most consumed aquatic vegetable.
Given the metal levels in LDMSC, this aquatic plant can be proposed as a bioindicator for monitoring of river pollution by toxic metals.
ACKNOWLEDGEMENT
We are grateful to financial support from the Swiss National Science Foundation (grant n° IZSEZO_188357 / 1). This research presents the collaboration between University of Geneva (Department F. A. Forel/ Institute of Environmental Sciences) and University of Kinshasa (DRC).
COMPLIANCE WITH ETHICAL STANDARDS
We confirm that the field studies did not involve endangered and protected species. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- FAO/WHO (2003) Diet, nutrition and the prevention of chronic diseases. Report of a Joint FAO/WHO Expert Consultation. Geneva, Switzerland.
- FAO/WHO (2004) Summary of evaluations performed by the joint FAO/WHO expert committee on food additives. ILSI Press International Life Sciences Institute. Washington, DC, USA.
- Manzoor D, Sharma M, Khursheed W (2018) Heavy metals in vegetables and their impact on the nutrient quality of vegetables: A review. J Plant Nutr 41: 1744-1763.
- Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, et al. (2012) Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur J Nutr 51: 637-663.
- FAO/WHO (2011) Codex committee on contaminants in foods. Expert Consultation. Geneva, Switzerland.
- Sgarbieri VC (2017) Food proteins and bioactive peptides, functional diets. HSOA J Food Sci Nutr 3: 023.
- Haddad R (2018) The secret of fruits and vegetables. HSOA J Food Sci Nutr 4: 039.
- Kayembe JM, Thevenon F, Laffite A, Sivalingam P, Ngelinkoto P, et al. (2018) High levels of faecal contamination in drinking groundwater and recreational water due to poor sanitation, in the sub-rural neighbor hoods of Kinshasa, Democratic Republic of the Congo. Int J Hyg Environ Health 221: 400-408.
- Kayembe JM, Sivalingam P, Salgado CD, Maliani J, Ngelinkoto P, et al. (2018) Assessment of water quality and time accumulation of heavy metals in the sediments of tropical urban rivers: Case of Bumbu River and Kokolo Canal, Kinshasa City, Democratic Republic of the Congo. J Afr Earth Scie 147: 536-543.
- Kumar S, Prasad S, Yadav KK, Shrivastava M, Gupta N, et al. (2019) Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches-A review. Environ Res 179: 08792.
- Dupré B, Gaillardet J. Rousseau D, Allègre CJ (1996) Major and trace elements of river-borne material: The Congo Basin. Geochimica et Cosmochimica Acta 60: 1301-1321.
- Brooks EGE, Allen DJ, Darwall WRT (2011) The Status and Distribution of Freshwater Biodiversity in Central Africa. IUCN, Switzerland.
- Mwanamoki PM, Devarajan N, Niane B, Ngelinkoto P, Thevenon F, et al. (2015) Trace metal distributions in the sediments from river-reservoir systems: Case of the Congo River and Lake Ma Vallée, Kinshasa (Democratic Republic of Congo). Environ Sci Pollut Res Int 22: 586-597.
- Mwanamoki PM, Devarajan N, Thevenon F, Atibu EK, Tshibanda JB, et al. (2014) Assessment of pathogenic bacteria in water and sediment from a water reservoir under tropical conditions (Lake Ma Vallée), Kinshasa Democratic Republic of Congo. Environ Monit Assess 186: 6821-6830.
- Verhaert V, Covaci A, Bouillon S, Abrantes K, Musibono D, et al. (2013) Baseline levels and trophic transfer of persistent organic pollutants in sediments and biota from the Congo River Basin (DR Congo). Environ Int 59: 290-302.
- Mata HK, Sivalingam P, Konde J, Otamonga JP, Niane B, et al. (2019) Concentration of toxic metals and potential risk assessment in edible fishes from Congo River in urbanized area of Kinshasa, DR Congo. Hum Ecol Risk Assess: An Intern J 26: 1676-1692.
- Mansiangi PM (2014) Transmission d’Onchocerca Volvulus par simulium squamosum dans le foyer de Kinsuka à Thèse de doctorant, Université de Kinshasa. Kinshasa, DRC.
- Larras F, Regier N, Planchon S, Poté J, Renaut J, et al. (2013) Physiological and proteomic changes suggest an important role of cell walls in the high tolerance to metals of Elodea nuttallii. J Hazard Mater 263: 575-583.
- Roos-Barraclough F, Givelet N, Martinez-Cortizas A, Goodsite ME, Biester H, et al. (2002) An analytical protocol for the determination of total mercury concentrations in solid peat samples. Sci Total Environ 292: 129-139.
- Islam MS, Hoque MF (2014) Concentrations of heavy metals in vegetables around the industrial area of Dhaka city, Bangladesh and health risk assessment. Int Food Res Journal 21: 2121-2126.
- USEPA (1989) Risk assessment guidance for superfund: Volume I Human health evaluation manual (Part A).
- USEPA (2018) Regional Screening Level (RSL) Summary Table.
- Farias DR, Hurd CL, Eriksen RS, Macleod CK (2018) Macrophytes as bioindicators of heavy metal pollution in estuarine and coastal environments. Mar Pollut Bul128: 175-184.
- Martinez EA, Shu-Nyamboli C (2011) Determination of selected heavy metal concentrations and distribution in a southwestern stream using macrophytes. Ecotox Environm Saf 74: 1504-1511.
- Vardanyan LG, Ingole BS (2006) Studies on heavy metal accumulation in aquatic macrophytes from Sevan (Armenia) and Carambolim (India) lake systems. Environ Int 32: 208-218.
- Calabrese EJ, CanadaAT, Sacco C (1985) Trace elements and public health. Annu Rev Public Health 6: 131-146.
- Suami RB, Sivalingam P, Kabala CD, Otamonga JP, Mulaji CK, et al. (2018) Concentration of heavy metals in edible fishes from Atlantic Coast of Muanda, Democratic Republic of the Congo. J Food Comp Anal 73: 1-9.
- Walsh CT, Sandstead HH, Prasad AS, Newberne PM, Fraker PJ (1994) Zinc: Health effects and research priorities for the 1990s. Environmental health perspectives 102: 5-46.
- Li R, Wu H, Ding J, Fu W, Gan L, et al. (2017) Mercury pollution in vegetables, grains and soils from areas surrounding coal-fired power plants. Scientific Reports 7: 46545.
- Niane B, Moritz R, Guédron S, Ngom PM, Pfeifer HR, et al. (2014) Effect of recent artisanal small-scale gold mining on the contamination of surface river sediment: Case of Gambia River, Kedougou region, southeastern Senegal. Journal of Geochemical Exploration 144: 517-527.
Citation: Mata HK, Al Salah DMM, Konde JN, Kiyombo GM, Mulaji CK, et al. (2020) Level of Toxic Metals in Consumable Aquatic Plant Ledermanniella schlechteri from Congo River and Potential Risk Assessment through Plant Consumption. J Food Sci Nutr 6: 074.
Copyright: © 2020 Henry K Mata, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

