
Natural Spawning of Pigfish Orthopristis Chrysoptera in Captivity and Predictors of Egg and Larval Quality
*Corresponding Author(s):
Jason S. BroachWaddell Mariculture Center, South Carolina Department Of Natural Resources, 211 Sawmill Creek Road, Bluffton, SC 29910, United States
Email:BroachJ@dnr.sc.gov
Abstract
Pigfish Orthopristis chrysoptera are a popular aquaculture species for use as bait in the southeastern United States. Their captive spawning potential was assessed and relationships among spawning characteristics were determined. A single population of twenty pigfish spawned over an 86 day period producing 5.6 million eggs with an estimated absolute fecundity of 1,439 eggs/g body mass. Fertilization, hatching success, and larval survival to first feeding were relatively high throughout the observation period (93.59 ± 12.53%, 63.21 ± 27.63%, and 44.33 ± 27.92%, respectively), although late season variability in hatching success and larval survival was extreme. Of all egg and larval morphometric parameters, the diameter of the oil droplet in recently hatched larvae had the strongest correlation with hatching success and survival to first feeding. The fatty acid composition of eggs also revealed the strongest positive correlation was the relationship of c16:1 with survival to first feeding whereas the strongest negative correlation was the relationship of the docosahexaenoic acid (DHA): eicosapentaenoic acid (EPA) ratio with survival to first feeding. These findings highlight the potential of protracted volitional spawning of pigfish in captive settings and initial insights for refinement of spawning and nutritional protocols for broodfish and larvae.
Keywords
Aquaculture; Baitfish; Egg, Larvae; Lipids; Spawning
Introduction
Successful commercial production of many cultured fishes is reliant on captive spawning. Reproductive dysfunctions such as the failure of females to undergo Final Oocyte Maturation (FOM), ovulation, or to spawn often requires the use of hormonal aids to overcome such dysfunctions and induce spawning [1,2]. For many marine fishes such as European sea bass Dicentrarchus labrax (Linnaeus 1758) [3] and red snapper Lutjanus campechanus (Poey 1860) [4,5], the quality of the spawns produced after hormone induction is lower compared to natural spawns. A fish’s ability to spawn naturally without the use of hormonal aids can alleviate issues associated with hormone-induced spawning. Natural spawning can be especially beneficial for many marine fishes which are asynchronous batch spawners and capable of spawning multiple times over a prolonged period [5]. Nevertheless, understanding how reliable natural spawning is based on spawn frequency and quality throughout the spawning season for a given species is critical if production protocols are to be based on natural spawning.
Some marine fishes spawn naturally and consistently in captivity. This often occurs after many generations have been produced in a culture setting and/or broodfish are properly conditioned and environmental conditions are appropriate [6]. During the beginning stages of gilthead seabream Sparus aurata L. 1758 culture in the 1970s, hormonal aids were required to produce high quantities of eggs, whereas now, broodfish will spawn daily for over three months during their natural spawning season and when held under manipulated photo-thermal conditions [2]. Some species of angelfish (Centropyge spp.) are capable of natural spawning daily for an entire year [7]. Other species such as mangrove red snapper Lutjanus argentimaculatus (Forsskål 1775) will spawn over an extended period greater than 100 days although intervals between spawns may be as much as 22 days [8,9].
Even with successful natural spawning in captivity, the quantity and quality of the eggs produced per spawn may vary throughout the spawning season and can be influenced by several factors such as stress and maternal [10-13]. Spawning frequency and egg output per spawn can be very cyclical depending on the species and influenced by factors such as lunar periods [8,14,15]. Despite high egg output, the collection of only eggs which are unfertile has also been observed for marine fishes [16].
Egg quality has been defined by many morphometric and nutrient composition factors depending upon species. Common morphometric parameters often associated with egg quality are the overall egg size and size of nutrient stores (e.g. oil globules) which are presumed beneficial to the survival and growth of developing embryos based on the greater capacity for allocation of nutrient reserves into the egg and embryo [11,13,17]. Decreases in egg diameter during later periods of the natural spawning seasons have been observed in many fishes and is attributed to changes in temperature as well as diminishing nutritional resources of the females for allocation into eggs [11,18,19]. Lipids, especially the essential fatty acids eicosapentaenoic acid (EPA; c20:5(n-3)), docosahexaenoic acid (DHA; c22:6(n-3)), and arachidonic acid (ARA; c20:4(n-6)), are the most commonly investigated nutrients to assess their effects on spawn quality [12]. Low levels of these essential fatty acids within eggs from natural spawns have been associated with poor fertilization and larval survival of some marine fishes [19,20,21].
The pigfish Orthopristis chrysoptera (Linnaeus 1766) is a commonly used baitfish throughout most of the south-eastern United States and has strong market potential as a cultured product to supply recreational anglers. Orthopristis chrysoptera are asynchronous batch spawners that spawn in winter months [22]. Several investigations for induced spawning with hormonal aids have indicated Ovaprim® [23], catfish pituitary extract [24], and cGnRH IIa [25] to be effective for controlled spawning in O. chrysoptera. Protracted spawning for over 30 days has also been demonstrated using repeated Ovaprim® injections [26] and naturally using recently collected broodfish [27]. Orthopristis chrysoptera have spawned volitionally under both ambient, natural conditions and photo-thermally manipulated natural conditions, for even longer extended periods once they have been in captivity for a year and have previous year [28]. Such natural spawning could be beneficial in extensively supplying O. chrysoptera larvae during larval culture and developing production protocols. However, seasonal characteristics of natural spawns during extended periods in captivity should be described in detail to determine how reliable natural spawning would be for obtaining high quality eggs and larvae. Therefore, the objectives of this study were to describe spawning characteristics including production, morphometric, viability and fatty acid parameters during a full, natural, non-induced spawning season. Additionally, possible relationships among those parameters were determined with respect to hatching success and larval survival.
Methods and Materials
Broodfish husbandry
All procedures were in compliance with the Guide for the Care and Use of Laboratory Animals under the University of Florida's Institutional Animal Care and Use Committee protocol #201004993.
Broodfish were collected via hook and line from the Indian River Lagoon near Sebastian, Florida, and held in recirculating aquaculture systems for two years. A 10-day quarantine period in flow-through seawater systems was used prior to stocking fish into recirculating systems at the Indian River Research and Education Center (IRREC) in Fort Pierce, Florida. Collected fish were stocked into 2,536-L quarantine tanks and subjected to static 1-h, 250 mg/L formalin baths (Parasite-S, Syndel) every other day during the 10 days (five baths total) while also being fed daily an oxytetracycline artificial diet (Terramycin® 200, 5.5 mg/g, (Phibro Animal Health; www.pahc.com)) to satiation. Broodfish were then transported to IRREC and stocked into 1,600- L round tanks filled with sanitized (chlorination with sodium hypochlorite (c. 150 mg/L), followed by neutralization with sodium thiosulfate), natural seawater (salinity c. 34 g/L). System components of each tank included a single, trickle biological filter, bag filter, UV sterilizer, a heat pump for thermal control, single-point aeration, 500 µm mesh external egg collector, and a conical lid enclosure with a single incandescent light inside for photoperiod control. Ambient temperatures were applied during non-spawning months from July through early December, and the photoperiod was also periodically (c. monthly) adjusted to match ambient conditions.
Photoperiod and temperature were slowly adjusted to maintain pre-determined, volitional spawning conditions of 12 h light:12 h dark and 20-22° C from mid-December to late June. A 2.0 mm slow-sinking pellet (50% protein, 15% fat, 2% fibre, 12% moisture, 8% ash: Zeigler Bros. Inc.; www.zeiglerfeed.com) was fed daily to broodfish to apparent satiation. Broodfish were fed daily a mixed diet of diced squid Loligo opalescens and krill Euphausia superba to satiation in the mornings from October through late June, followed by afternoon feedings of 2.0- mm pellets also fed to apparent satiation.
On November 21, twenty O. chrysoptera (1M:1F- 285.0 ± 28.89 (mean ± SD) mm; 392 ± 93.19g) were stocked into a single, round spawning tank (1,600-L) during their second year in captivity. Ovaprim® had been used in previous seasons to spawn all broodfish. Sex was determined by either the presence of milt after gentle abdominal pressure or via ovarian biopsies with Teflon tubing (1.27 mm external diameter; 0.97 mm internal diameter) to obtain oocytes. No mortalities occurred during the study. Satiation feedings with squid in the morning and 2.0 mm pellets in the afternoon occurred from October 1 through April 11. Krill was also incorporated (1:1) into morning squid feedings starting on April 12.
Data collection
Egg collectors were monitored daily beginning January 1 for the presence of eggs. After two successive spawns were obtained (February 9), data collection was initiated. Enumeration of floating and sinking eggs from each spawn was done volumetrically. Eggs were allowed to briefly (c. 5 minutes) settle within graduated cylinders (≤ 500 mL, depending on spawn size). Floating and sinking egg quantities for each spawn was calculated by utilizing four subsamples (0.25 mL) of concentrated floating eggs to determine the eggs/mL. Equal egg contribution from each female was assumed to estimate fecundity. Egg fertilization for floating and sinking eggs was determined by counting fertilized eggs in 100 egg subsamples that were also photographed on a known reference grid (Sedgewick rafter cell; 1.0 mm squares) to provide a scale for morphometric analyses. Fifty eggs of each type from each replicate spawn were photographed to measure egg diameter, oil globule diameter, and percentage of eggs containing a single oil droplet. A 1.0 mL subsample of floating eggs from each spawn was tripled-rinsed with deionized water and temporarily stored in a -80° C freezer for fatty acid analysis.
Subsamples (n = 6) of 50 floating eggs from each spawn were stocked into screen-bottom (55 µm), 1.0-L containers that were supported (c. 750 mL water capacity) in a 325 L temperature- controlled water bath filled with sanitized natural seawater. Three of the replicate containers stocked were used to determine hatching percentage and three were used for determining survival to first feeding (2 days post hatch, dph). Approximately 24-36 h after spawning, live larvae which fully emerged from eggs were counted in to determine hatching percentage, and a 10 larvae subsample from each replicate container was photographed on a known reference grid (Sedgewick rafter cell; 1.0 mm squares) for larval morphometric analyses. Notochord length, oil droplet diameter, yolk width, yolk height, and yolk volume of 0 dph larvae were measured. Yolk volume was calculated based on an assumed volume of a prolate sphere according to Avila and Juario (1987): V=π(6LH2)-1, where V is volume, L is length, and H is height. At 2 dph, larval survival was determined for each replicate container and 10 larvae subsamples from each container were photographed to measure length. All morphometric measurements were obtained by using SigmaScan Pro 5.0 (www.systatsoftware.com) software. A YSI 556 meter (www.ysi.com) was used to record the daily water quality (temperature, salinity, dissolved oxygen (DO), and pH) of the spawning tank and the water bath used for egg stocking. At least weekly, a Hach DR 4800 spectrophotometer (www.hach.com) was also used to deter mine total ammonia nitrogen (TAN) and nitrite nitrogen in water samples.
Fatty acid analysis
All floating egg samples from spawns were temporarily stored in a -80° C freezer before bulk shipment on dry ice to Roger Williams University, Bristol, Rhode Island. Total lipids were extracted following lyophilization utilizing methods developed by Folch, Lees, & Sloane-Stanley [29]. Methods developed by Drillet, Iversen, Sorensen, Hans, Torben, & Hansen [30], were used to prepare fatty acid methyl esters (FAME) via transesterification. Gas chromatography- mass spectrometry were used to determine concentrations (relative %) of total fatty acids (TFA) by comparing aliquots of FAMEs for each spawn with known standards.
Statistical analysis
Overall means (± SD) of the production, morphometric, viability and fatty acid composition parameters were recorded and plotted over time. To determine possible relationships among viability parameters with other production, morphometric, and fatty acid composition parameters, Pearson’s correlation coefficients (r) were generated. Percentage values were transformed (arcsine-square-root) prior to determination of coefficients. Correlations (positive or negative) ≥ 0.5 were considered highly correlated and moderate correlations were those where 0.3 < r < 0.5.
Results
Water quality in the spawning tank was: temperature, 21.57 ± 0.67° C; salinity, 33.48 ± 0.72 g/L; DO, 6.21 ± 0.6 mg/L; pH, 7.71 ± 0.3; TAN, 0.025 ± 0.024 mg/L; nitrite, 0.286 ± 0.115 mg/L. Water quality in the water bath used for egg hatching and larval survival and morphometrics was: temperature, 22.64 ± 0.05° C; salinity, 33.27 ± 1.44 g/L; DO, 6.87 ± 0.04 mg/L; pH, 8.11 ± 0.03; TAN, 0 ± 0 mg/L; nitrite, 0.0025 ± 0.0017 mg/L.
A total of 50 spawns were collected from O. chrysoptera over an 86 day period during February 9 through May 4. The calculated spawning frequency was one spawn every 1.72 days; as many as 11 spawns were observed consecutively and the largest amount of time between spawns was 18 days (Figure 1). Over 5,600,000 eggs were produced with an estimated absolute fecundity of 564,000 eggs/female or 1,439 eggs/g body mass. Floating eggs (107,562.60 ± 86,504.72 eggs/spawn) were produced in every spawn while small amounts of sinking eggs (9,989.19 ± 20,493.14 eggs/spawn) were observed in only 23 spawns (Figure 1). Three distinct peaks in total egg production were observed on March 16, March 30, and April 14, which corresponded closely (within one day) to the last quarter, new quarter, and last quarter moon phase during those months (Figure 1). Total egg production peaked to nearly 350,000 eggs/spawn on March 30, but fewer than 50,000 eggs/spawn were also observed in some spawns (Figure 1). Fecundity (floating eggs and total eggs/spawn) was also not well correlated with either hatching success or larval survival to first feeding (Table 1).
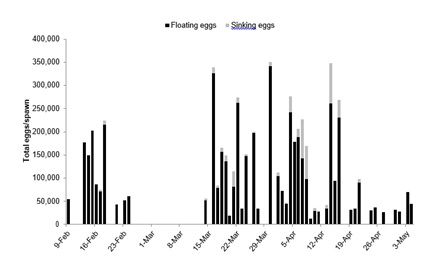 Figure 1: Egg production from the 50 Orthopristis chrysoptera spawns throughout the observation period.
Figure 1: Egg production from the 50 Orthopristis chrysoptera spawns throughout the observation period.
|
|
|
Hatching success |
Survival to first feeding |
|
Fecundity |
Floating eggs/spawn |
-0.05285 |
-0.16562 |
|
|
Total eggs/spawn |
-0.14842 |
-0.22701 |
|
Floating egg morphometrics |
Egg diameter |
-0.03984 |
-0.05924 |
|
|
Oil droplet diameter |
0.14175 |
0.05445 |
|
|
Eggs with single oil globule |
-0.24906 |
-0.20479 |
|
Larval morphometrics |
Length at hatch |
0.06649 |
0.14580 |
|
|
Oil droplet diameter† |
0.38830 |
0.40057 |
|
|
Yolk volume |
0.25198 |
0.12397 |
|
|
Length at first feeding† |
0.27881 |
0.48601 |
|
Floating egg fatty acids |
c14:0† |
0.46558 |
0.49335 |
|
|
c15:0 |
-0.05035 |
-0.08491 |
|
|
c16:0 |
0.09152 |
-0.05175 |
|
|
c17:0 |
0.03575 |
0.02725 |
|
|
c18:0 |
0.13745 |
0.15688 |
|
|
c22:0 |
-0.00961 |
0.11376 |
|
|
∑Saturated† |
0.34198 |
0.28944 |
|
|
c16:1† |
0.42280 |
0.53107 |
|
|
c18:1n-9trans |
0.18527 |
0.21902 |
|
|
c18:1n-9cis† |
-0.28133 |
-0.33144 |
|
|
c20:1† |
0.35017 |
0.33391 |
|
|
c24:1† |
0.23989 |
0.32693 |
|
|
∑Monounsaturated |
0.04867 |
0.07588 |
|
c18:2n-6cis |
-0.03357 |
-0.01267 |
|
c20:4n-6 (ARA) |
0.20847 |
0.23184 |
|
∑n-6 PUFA |
-0.00463 |
0.02123 |
|
c18:3n-3† |
0.29293 |
0.35056 |
|
c20:5n-3 (EPA)† |
0.28129 |
0.35146 |
|
c22:6n-3 (DHA)† |
-0.31045 |
-0.32453 |
|
∑n-3 PUFA |
-0.17488 |
-0.15144 |
|
∑PUFA |
-0.22602 |
-0.18531 |
|
n-3:n-6 |
-0.05917 |
-0.07659 |
|
DHA:EPA† |
-0.36184 |
-0.40736 |
|
DHA:ARA† |
-0.33645 |
-0.36488 |
Table 1: Pearson correlation coefficients (r) for fecundity, floating egg parameters, larval morphometrics, hatching success, and survival to first feeding for Orthopristis chrysopter a natural spawning.
PUFA, polyunsaturated fatty acids; AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ARA, arachidonic acid. †Indicates at least a medium correlation (0.3 < r < 0.5, or −0.3 > r > −0.5) between variables (n ≥ 43). Values greater than a positive or negative value of 0.3, P < 0.05
The overall mean diameter of floating eggs was 0.900 ± 0.039 mm whereas oil droplets were 0.180 ± 0.019 mm. Floating egg diameter did appear more variable around the end of the spawning period (Figure 2). Floating egg oil diameter appeared to have peaks around the beginning and end of the spawning period (Figure 2). Overall mean fertilization of floating eggs throughout the observation period was 93.59 ± 12.53% whereas only one of the 23 spawns of sinking eggs contained a small amount (10%) of fertile eggs. Fertilization of floating eggs was high (> 75%) throughout the spawning period with the exception of two spawns at the beginning of the season (Figure 3). The percentage of floating eggs with a single oil globule (60.13 ± 21.15%) was extremely variable throughout the spawning period (Figure 3). Hatching success (63.21 ± 27.63%) and survival to first feeding (44.33 ± 27.92%) both experienced late season variability and also reached some of their lowest levels in late March to mid-April (Figure 4). None of the egg morphometric variables were well correlated with either hatching success or larval survival to first feeding (Table 1).
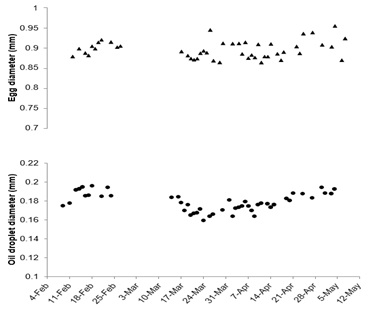 Figure 2: Mean diameters of floating eggs and oil droplets from the 50 Orthopristis chrysoptera spawns throughout the observation period.
Figure 2: Mean diameters of floating eggs and oil droplets from the 50 Orthopristis chrysoptera spawns throughout the observation period.
 Figure 3: Floating egg fertilization and percentage of floating eggs with a single oil globule from the 50 Orthopristis chrysoptera spawns throughout the observation period.
Figure 3: Floating egg fertilization and percentage of floating eggs with a single oil globule from the 50 Orthopristis chrysoptera spawns throughout the observation period.
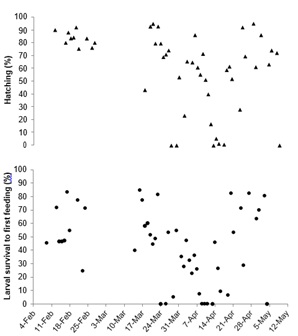 Figure 4: Means of hatching success of floating eggs and larval survival to first feeding from the 50 Orthopristis chrysoptera spawns throughout the observation period.
Figure 4: Means of hatching success of floating eggs and larval survival to first feeding from the 50 Orthopristis chrysoptera spawns throughout the observation period.
Overall mean length at hatch (2.52 ± 0.22 mm), length at first feeding (3.17 ± 0.24 mm), and yolk volume (0.1500 ± 0.0019 mm3) did not vary greatly throughout the spawning period (Figure 5). Oil droplet diameter of larvae (0.186 ± 0.017 mm) appeared to have peaks around the beginning and end of the study period (Figure 5). Oil droplet diameter of larvae and length at first feeding were also positively correlated (r > 0.40) with larval survival to first feeding (Table 1).
The sum totals of major fatty acid groups as well as individual fatty acids and ratios generally experienced a greater amount of variation from mid-March to the beginning of May than at the beginning of the spawning period. The sum total of saturated (32.72 ± 1.41% of total fatty acids (TFA)) and monounsaturated fatty acids (19.89 ± 1.52% TFA) were lower and less variable than that of polyunsaturated fatty acids (PUFA) (49.63 ± 2.32% TFA) throughout the spawning period (Figure 6). The sum total of n-6 PUFA (7.29 ± 1.05% TFA) was also lower and less variable than the n-3 PUFA (42.34 ± 2.62% TFA; Figure 6). Linolenic acid (c18:3n-3) concentrations (1.11 ± 0.29% TFA) were lower and much less variable than linoleic acid (c18:2n-6) concentrations (5.21 ± 1.04% TFA) throughout the spawning period (Figure 7).
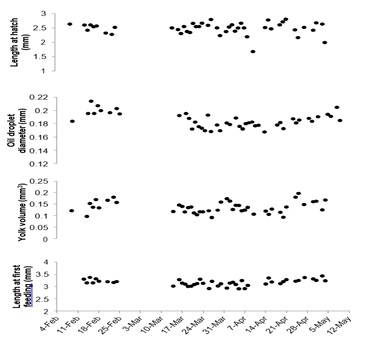 Figure 5: Means of notochord length, oil droplet diameter, and yolk volume of larvae at hatch and notochord length of larvae at first feeding from the 50 Orthopristis chrysoptera spawns throughout the observation period.
Figure 5: Means of notochord length, oil droplet diameter, and yolk volume of larvae at hatch and notochord length of larvae at first feeding from the 50 Orthopristis chrysoptera spawns throughout the observation period.
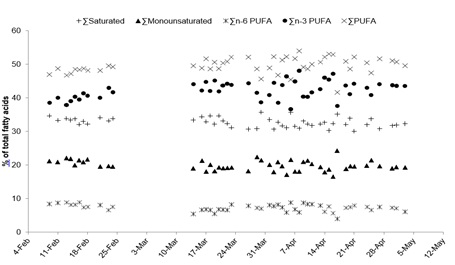 Figure 6: Total fatty acids (%) represented by major groups of fatty acids within floating eggs from the 50 Orthopristis chrysoptera spawns throughout the observation period.
Figure 6: Total fatty acids (%) represented by major groups of fatty acids within floating eggs from the 50 Orthopristis chrysoptera spawns throughout the observation period.
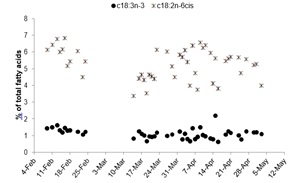 Figure 7: Total fatty acids (%) represented by linolenic (c18:3n-3) and linoleic (c18:2n-6) acid within floating eggs from the 50 Orthopristis chrysoptera spawns throughout the observation period.
Figure 7: Total fatty acids (%) represented by linolenic (c18:3n-3) and linoleic (c18:2n-6) acid within floating eggs from the 50 Orthopristis chrysoptera spawns throughout the observation period.
Docosahexaenoic acid (DHA) concentrations (32.49 ± 2.7% TFA) were higher and much more variable than eicosapentaenoic acid (EPA) (8.78 ± 1.08% TFA) and arachidonic acid (ARA) (2.08 ± 0.16% TFA) (Figure 8). The ratio of DHA:ARA was higher (15.71 ± 2.03) and more variable than the DHA:EPA ratio (3.76 ± 0.59; Figure 9). The overall mean of the ratio of n-3:n- 6 fatty acids was 5.97 ± 1.19.
Multiple fatty acids were moderately correlated with either hatching success or larval survival to first feeding (Table 1). The strongest positive correlation observed (r = 0.53107) was the relationship of c16:1 with larval survival to first feeding whereas the strongest negative correlation (r = -0.40736) was the relationship of the DHA:EPA ratio with larval survival to first feeding (Table 1). Other moderately positive correlations were observed between hatching success and larval survival to first feeding with c14:0 and c20:1, and negative correlations with DHA and DHA:ARA ratio. The sum total of saturated fatty acids and c16:1 were also positively correlated with hatching success, and c24:1, linolenic acid, and EPA were positively correlated with larval survival to first feeding. The DHA:EPA ratio was also negatively correlated with hatching success, and concentrations of c18:1n-9cis were negatively correlated with larval survival to first feeding.
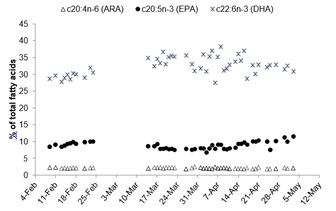 Figure 8: Total fatty acids (%) represented by ARA (c20:4n-6), EPA (c20:5n-3), and DHA (c22:6n-3) within floating eggs from the 50 Orthopristis chrysoptera spawns throughout the observation period.
Figure 8: Total fatty acids (%) represented by ARA (c20:4n-6), EPA (c20:5n-3), and DHA (c22:6n-3) within floating eggs from the 50 Orthopristis chrysoptera spawns throughout the observation period.
Discussion
The overall high spawning frequency observed in the present study supports previous observations of batch spawning in O. chrysoptera [22,26-28], and the frequency is near that of many other marine fishes [2,6,8,9]. The absolute fecundity observed in the present study was within the range reported for O. chrysoptera by DiMaggio et al. [26] (400,000 eggs/female) and DiMaggio [28] (1.1 million eggs/female). This high spawning frequency and egg production would be promising for the possibility of natural seasonal spawning to extensively provide eggs and larvae for growout, but the large period observed between some spawns and variation in eggs/spawn indicates stringent natural-spawning protocols may not be possible. The abrupt lack of spawning during the observed 18-day period after spawning had started nearly a month prior is likely indicative of an unknown stressor that was occurring. Inter-spawn intervals can be influenced by a variety of factors such as spawning hormones [31], nutrition [32], handling stress [33], and several environmental factors such as photoperiod, sex ratio, and stocking density [34,35]. Natural spawning of O. chrysoptera in this study may have been influenced by lunar period as observed with other marine fishes [14,15] such as L. argentimaculatus [8]. Further replicated research examining natural spawning in O. chrysoptera is needed to substantiate all findings related to spawning frequency and egg production data.
Egg and larval morphometric and viability parameters were similar to values reported in other studies for O. chrysoptera [23,26,27]. Observations of poor fertility in sinking vs. floating eggs supports the possibility of using egg buoyancy as a simple indicator in egg quality as proposed in other marine fishes [36]. The high fertilization success of floating eggs observed is a promising attribute for the possibility of natural seasonal spawning to extensively provide eggs and larvae.
However, the large amount of variation in hatching success and larval survival to first feeding and even the variation in oil droplet diameter of recently hatched larvae indicates possible difficulties in developing stringent natural-spawning protocols to provide higher quality larvae. Although egg morphometrics were not well correlated with hatching success or larval survival, the oil droplet diameter of eggs and recently hatched larvae were both positively correlated. Similar correlations of oil droplet size and egg viability has also been observed with California yellowtail Seriola dorsalis (Gill 1863) [19]. This relationship may have manifested from increased nutrient stores in larger oil droplets of larvae which conveyed growth and survival advantages as observed in capelin Malltous villosus (Müller 1776) [37], and other marine fishes [11,13,17]. As oil globules are comprised largely of monounsaturated fatty acids and selectively catabolized by embryos for energy and growth [38], it is also noteworthy that the strongest correlation of any fatty acid with hatching success and larval survival was a monounsaturated fatty acid (c16:1) and all but one of the monounsaturated fatty acids were positively correlated. Further replicated and detailed research where oil globules are isolated from other egg components and fatty acids analysed separately would help substantiate the importance of oil droplets and monounsaturated fatty acids to hatching and larval survival of O. chrysoptera.
Fatty acid concentrations in O. chrysoptera eggs adhered to established trends such as the higher concentrations of DHA followed by EPA and ARA observed in many other marine fishes such as Atlantic halibut Hippoglossus hippoglossus (L. 1758) [39], flame angelfish Centropyge loriculus (Günther 1874) [40], and S. dorsalis [19], and also higher levels of n-3 polyunsaturated fatty acids (PUFA) compared to n-6 PUFA [38,41]. The correlations of multiple fatty acids with hatching success and larval survival were expected based on observations in other marine fishes, but one unique observation in the O. chrysoptera data were the negative correlations with hatching success and larval survival for DHA and ratios of DHA to EPA and ARA, compared to positive correlations with EPA and ARA. This observation contrasts to the many positive correlations of DHA with egg and larval viability and hatching success which have been reported in other species including common snook Centropomus undecimalis (Bloch 1792) [41], and yellowfin seabream Acanthopagrus latus (Houttuyn 1782) [42], and the greater importance of DHA in marine fish larval nutrition compared to other essential fatty acids such as EPA [6,43]. The many positive correlations observed for short chain saturated fatty acids (c14:0) as well as multiple monounsaturated fatty acids may be indicative of high energy requirements that must be sustained for O. chrysoptera to successfully hatch and survive to first feeding and selective retention of long chain PUFA for other functions [38]. The negative correlation observed for O. chrysoptera hatching success and larval survival to first feeding with c18:1n-9cis is supportive of similar negative correlations observed in S. aurata [20]. However, the positive correlation of c18:3n-3 for O. chrysoptera contradicts the negative relationship reported for S. aurata [20], but similar to the positive relationship reported for S. dorsalis [19]. These observations in fatty acids patterns could be important considerations in the selection of O. chrysoptera broodfish diets, as maternal nutrition can strongly influence endogenous energy reserves of eggs and larvae [38,43-45], but more research is needed to substantiate these observations.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Mylonas CC, Fostier A, Zanuy S (2010) Broodstock management and hormonal manipulations of fish reproduction. General and Comparative Endocrinology 165: 516- 534.
- Zohar Y, Mylonas CC (2001) Endocrine manipulations of spawning in cultured fish: from hormones to genes. Aquaculture 197: 99-136.
- Fornies MA, Mananos E, Carrillo M, Rocha A, Laureau S, et al. (2001) Spawning induction of individual European sea bass females (Dicentrarchus labrax) using different GnRHa-delivery systems. Aquaculture 202: 221- 234.
- Papanikos N, Phelps RP, Ferry A, Maus D (2003) Egg and larval quality of natural and induced spawns of red snapper, Lutjanus campechanus. Fish Physiology and Biochemistry 28: 487-488.
- Murua H, Saborido-Rey F (2003) Female reproductive strategies of marine fish species of the north Atlantic. Journal of Northwest Atlantic Fisheries Science 33: 23-31.
- Tucker Jr, JW (1998) Marine Fish Culture. Boston, MA: Kluwer Academic Publishers. Watanabe, T. (1993). Importance of docosahexaenoic acid in marine larval fish. Journal of the World Aquaculture Society 24: 152-161.
- Bauer JA, Bauer SE (1981) Reproductive biology of pigmy angelfishes of the Genus Centropyge (Pomacanthidae). Bulletin of Marine Science 31: 495-513.
- Emata AC (2003) Reproductive performance in induced and spontaneous spawning of the mangrove red snapper Lutjanus argentimaculatus: A potential candidate species for sustainable aquaculture. Aquaculture Research 34: 849-857.
- Leu MY, Chen IH, Fang LS (2003) Natural spawning and rearing of mangrove red snapper, Lutjanus argentimaculatus, larvae in captivity. Israeli Journal of Aquaculture- Bamidgeh 55: 22-30.
- Bobe J (2015) Egg quality in fish: Present and future challenges. Animal Frontiers 5: 66-72.
- Brooks S, Tyler CR, Sumpter JP (1997) Egg quality in fish: what makes a good egg? Reviews in Fish Biology and Fisheries 7: 387-416.
- Izquierdo MS, Fernandez-Palacios H, Tacon AGJ (2001) Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 197: 25-42.
- Kjorsvik E, Mangorjensen A, Holmefjord I (1990) Egg quality in fishes. Advances in Marine Biology 26: 71-113.
- Gibson RN (1992) Tidally-synchronized behaviour in marine fishes. In M. A. Ali (editor), Rhythms in Fishes. New York, NY: Plenum Press 63-81.
- Lam TJ, Munro AD (1987) Environmental control of reproduction in teleosts: an overview. Proceedings of the Third International Symposium of Reproductive Physiology in Fish. Memorial University, St. John’s, Newfoundland: 279-288.
- Phelps R, Papanikos N, Bourque B, Bueno F, Hastey R, et al. (2009) Spawning of red snapper (Lutjanus campechanus) in response to hormonal induction or environmental control in a hatchery setting. Reviews in Fisheries Science 17: 149-155.
- Hunter JR (1981) Feeding ecology and predation of marine fish larvae. In R. Lasker (editor), Marine fish larvae, morphology, ecology and relation to fisheries Seattle WA: University of Washington Press: 32-77
- McEvoy LA, McEvoy J (1992) Multiple spawning in several commercial fish species and its consequences for fisheries management, cultivation and experimentation. Journal of Fish Biology 41: 125-136.
- Stuart KR, Armbruster L, Johnson R, Drawbridge MA (2020) Egg diameter as a predictor for egg quality of California yellowtail (Seriola dorsalis). Aquaculture 522:
- Martín MV, Almansa E, Cejas JR, Bolaños A, Jerez S, et al. (1999) Lipid and fatty acid composition of female gilthead seabream during their reproductive cycle: Effects of a diet lacking n−3 HUFA. Aquaculture 170: 323-336.
- Evans RP, Parrish CC, Brown JA, Davis PJ (1996) Biochemical composition of eggs from repeat and first-time spawning captive Atlantic Halibut (Hippoglossus hippoglossus). Aquaculture 139: 139-149.
- Sutter FC, McIlwain, TD (1987) Species profiles: Life histories and environmental requirements of coastal fishes and invertebrates (Gulf of Mexico)-pigfish. U.S. Fish and Wildlife Service Biological Report 82.
- DiMaggio MA, Broach JS, Ohs CL (2014) Evaluation of Ovaprim and human chorionic gonadotropin doses on spawning induction and egg and larval quality of Pigfish, Orthopristis chrysoptera. Journal of the World Aquaculture Society 45: 243- 257.
- Broach JS, Ohs CL, DiMaggio MA, Green CC, Yant, DR (2015). Efficacy of varying doses of channel catfish pituitary extract on spawning performance of pinfish and pigfish. North American Journal of Aquaculture 77: 245-254.
- Broach JS, Ohs CL (2020) Efficacy of varying doses of cGnRH IIa on spawning performance of pinfish and pigfish. Aquaculture Research 51: 4541-4550.
- DiMaggio MA, DeSantis SM, Grabe SW, Beany AH, Ohs CL (2011) Protracted induced volitional spawning of pigfish Orthopristis chrysoptera, a common marine baitfish species. Proceedings of Aquaculture America New Orleans LA
- Kaiser JB, Faulk CK, Thompson KL, Fuiman LA (2018) Baitfish aquaculture: Spawning and juvenile requirements of pigfish. Word Aquaculture 49: 48-51.
- DiMaggio MA, Broach JS, Ohs CL, Grabe SW (2013) Captive volitional spawning and larval rearing of pigfish. North American Journal of Aquaculture 75: 109-113.
- Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry 226: 497- 507.
- Drillet G, Iversen MH, Sorensen TF, Hans R, Torben L, et al.(2006) Effect of cold storage upon eggs of a calanoid copepod, Acartia tonsa (Dana), and their offspring. Aquaculture 254: 714-729.
- Mylonas CC, Fatira E, Karkut P, Sigelaki I, Papadaki M, et al. (2015) Reproduction of hatchery-produced meagre Argyrosomus regius in captivity III. Comparison between GnRHa implants and injections on spawning kinetics and egg/larval performance parameters. Aquaculture 448: 44-53.
- Bobe J, Labbé C (2009) Egg and sperm quality in fish. General and Comparative Endocrinology 165: 535-548.
- Mileva VR, Gilmour KM, Balshine S (2011) Effects of maternal stress on egg characteristics in a cooperatively breeding fish. Comparative Biochemistry and Physiology Part A: Molecular and Integrative Physiology 158: 22-29.
- Campos-Mendoza A, McAndrew BJ, Coward K, Bromage N (2004) Reproductive response of Nile tilapia (Oreochromis niloticus) to photoperiodic manipulation; effects on spawning periodicity, fecundity and egg size. Aquaculture 231: 299-314.
- Grant B, Davie A, Selly SL, Picchi N, Bradley C, et al. (2016) Seasonal changes in broodstock spawning performance and egg quality in ballan wrasse (Labrus bergylta). Aquaculture 464: 505-514.
- Lahnsteiner F, Patarnello P (2003) Investigations on the metabolism of viable and nonviable gilthead sea bream (Sparus aurata) eggs. Aquaculture 223: 159-174.
- Chambers RC, Leggett WC, Brown JA (1989) Egg size, female effects, and the correlations between early life history traits of capelin, Mallotus villosus: an appraisal at the individual level. Fishery Bulletin 87: 515-523.
- Wiegand MD (1996) Composition, accumulation and utilization of yolk lipids in teleost fish. Reviews in Fish Biology and Fisheries 6: 259-286.
- Falk-Petersen S, Sargent JR, Fox C, Falk-Petersen IB, Haug T, et al. (1989) Lipids in Atlantic halibut (Hippoglossus hippoglossus) eggs from planktonic samples in Northern Norway. Marine Biology 101: 553-556.
- Callan CK, Laidley CW, Kling LJ, Breen NE, Rhyne AL (2014) The effects of dietary HUFA level on flame angelfish (Centropyge loriculus) spawning, egg quality and early larval characteristics. Aquaculture Research 45: 1176-1186.
- Yanes-Roca C, Rhody N, Nystrom M, Main KL (2009) Effects of fatty acid composition and spawning season patterns on egg quality and larval survival in Common Snook (Centropomus undecimalis). Aquaculture 287: 335-340.
- Zakeri M, Kochanian P, Marammazi JG, Yavari V, Savari A, et al. (2011) Effects of dietary n-3 HUFA concentrations on spawning performance and fatty acid composition of broodstock, eggs, and larvae in Yellowfin Sea Bream, Acanthopagrus latus. Aquaculture 310: 388-394.
- Rainuzzo JR, Reitan KI, Olsen Y (1997) The significance of lipids at early stages of marine fish: a review. Aquaculture 155: 103-115.
- Mylonas CC, Fostier A, Zanuy S (2010) Broodstock management and hormonal manipulations of fish reproduction. General and Comparative Endocrinology 165: 516- 534.
- Avila EM, Juario JV (1987) Yolk and oil globule utilization and developmental morphology of the digestive tract epithelium in larval Rabbitfish, Siganus guttatus (Bloch). Aquaculture 65L: 319-331.
Citation: Broach JS, Ohs CL, Breen NE, (2024) Natural Spawning of Pigfish Orthopristis Chrysoptera in Captivity and Predictors of Egg and Larval Quality. J Aquac Fisheries 8: 084.
Copyright: © 2024 Jason S. Broach, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

