
Neurofilament Light (NF-L) Chain Protein from a Highly Polymerized Structural Component of the Neuronal Cytoskeleton to a Neurodegenerative Disease Biomarker in the Periphery
*Corresponding Author(s):
Walter J LukiwLSU Neuroscience Center, Louisiana State University Health Science Center, New Orleans LA 70112, United States
Email:wlukiw@lsuhsc.edu
Abstract
Neurofilaments (NFs) are critical scaffolding components of the axoskeleton of healthy neurons interacting directly with multiple synaptic-phosphoproteins to support and coordinate neuronal cell shape, cytoarchitecture, synaptogenesis and neurotransmission. While neuronal presynaptic proteins such as synapsin-2 (SYN II) degrade rapidly via the ubiquitin-proteasome pathway, a considerably more stable neurofilament light (NF-L) chain protein turns over much more slowly, and in several neurological diseases is accompanied by a pathological shift from an intracellular neuronal cytoplasmic location into various biofluid compartments. NF-L has been found to be significantly elevated in peripheral biofluids in multiple neurodegenerative disorders, however it is not as widely appreciated that NF-L expression within neurons undergoing inflammatory neurodegeneration exhibit a significant down-regulation in these neuron-specific intermediate-filament components. Down-regulated NF-L in neurons correlates well with the observed axonal and neuronal atrophy, neurite deterioration and synaptic disorganization in tissues affected by Alzheimer’s disease (AD) and other progressive, age-related neurological diseases. This Review paper: (i) will briefly assess the remarkably high number of neurological disorders that exhibit NF-L depolymerization, liberation from neuron-specific compartments, mobilization and enrichment into pathological biofluids; (ii) will evaluate how NF-L exhibits compartmentalization effects in age-related neurological disorders; (iii) will review how the shift of NF-L compartmentalization from within the neuronal cytoskeleton into peripheral biofluids may be a diagnostic biomarker for neuronal-decline in all cause dementia most useful in distinguishing between closely related neurological disorders; and (iv) will review emerging evidence that deficits in plasma membrane barrier integrity, pathological transport and/or vesicle-mediated trafficking dysfunction of NF-L may contribute to neuronal decline, with specific reference to AD wherever possible.
Keywords
Aging; Alzheimer’s disease (AD); AD biomarkers; AD diagnostics; Degradation; Neurofilament light (NF-L) chain protein; Neuronal atrophy; Synapsin-2 (SYN-II)
Abbreviations
Aβ: Amyloid beta (peptide)
AD: Alzheimer’s Disease
CSF: Cerebrospinal Fluid
ECF: Extracellular Fluid
ELISA: Enzyme-linked Immunoassay
FTD: Frontotemporal Dementia
GI: Gastrointestinal
IF: Intermediate Filament
NF: Neurofilament
NF-L: Neurofilament Light Chain Protein
PD: Parkinson’s Disease
PNS: Peripheral Nervous System
SYN II: Synapsin 2
VaD: Vascular Dementia
Introduction
Neurofilaments (NFs) are 10 nm diameter, neuron-specific filaments belonging to the type IV intermediate filament (IF) family of structural cytoskeletal polymers in neurons. NFs consist of 3 major classes referred to as the NF triplet: the neurofilament light (NF-L; ~ 60 kDa) chain, the neurofilament medium chain (NF-M; ~90 kDa) and the neurofilament heavy chain (NF-H; ~115 kDa). The stoichiometry of these 3 NF subunits varies slightly, but is approximately 5:3:1 for NF-L:NF-M:NF-H in large neurons of the human brain neocortex; large neocortical layer 3 and 5 pyramidal neurons of the neocortex are specifically targeted and devastated by the Alzheimer’s disease (AD) process [1-4]. Together with the ~ 25 nm diameter microtubules and ~ 7 nm diameter microfilaments and accessory filament proteins such as α-internexin and peripherin, the NF triplet forms a highly dynamic neuronal cytoskeleton contributing to the cytoarchitecture of the neuron [2,3,5-8]. The NF-L chain protein is the most abundant NF in axons, forming the core of the NF bundle, and is a critical scaffolding component of neurite extensions, the primary regulator of the radial diameter of axons and the overall shape of the neuronal cytoskeleton. In doing so NFs support synaptic function and organization by enabling electrical signal transmission along axons and across dendrites and synapses. IF-bound NF-L is therefore an important integral contributor to the structural organization of the ~90+ billion neurons of the brain and spinal cord of the central nervous system (CNS), and also appears to contribute in part to the heterogeneous axonal structures of the sensory and motor neurons of the peripheral nervous system (PNS) [3,4,8-12].
Cytoskeletal disorganization and synaptic degeneration are early and widespread pathogenic events in neurodegenerative disorders with reduced levels of neurofilament and pre- and postsynaptic proteins being recognized as a critical feature of AD pathophysiology. NF-L and synaptic proteins in peripheral biofluids provide valuable diagnostic, prognostic and disease-monitoring biomarkers [8,10,12-20]. While SYN-II and in particular NF-L are found to be abundant in all healthy neurons of the CNS and PNS, diseases that exhibit neuronal degeneration with axonal and synaptic damage display increases in the CSF- and serum-levels of NF-L and other disease-associated proteins. It has been known for at least 25 years that patients with AD, amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS) slowly release NF-L filament proteins from diseased and atrophied neurons into the CSF [21-23]. AD and ALS patients exhibit increased levels of NF-L protein in their CSF as verified using ELISA, from about ~ 2.5-15-fold over age-matched controls, thus providing a peripheral biofluid indicator of axonal damage and neuronal cell demise. This finding has been suggested to serve as a useful biomarker and indicator for the degree of neurodegeneration as well as a monitor for the progression of both all-cause and AD-type dementia [12,19-22,24]. In tissue biofluids such as the CSF, blood serum and vitreous, well outside of the actual neuronal cell body, the widely observed elevations in NF-L in biofluids strongly suggest a very active neuropathology-related NF-L mobilization, translocation and trafficking beyond the confines of the neuronal plasma membrane and neuronal cytoarchitectural compartments.
NF-L And SYN-II Expression And Function
In our laboratory the expression of NF-L and SYN-II at both the mRNA and protein level, and the translocation and trafficking of NF-L and SYN-II have been studied together for multiple, highly interactive and sometimes overlapping reasons over the last ~ 35 years. NF-L and SYN-II: (i) are among the most abundant neuron-specific transcripts within healthy neurons of the human association neocortex and are especially abundant in the human hippocampal CA1 and limbic system which are the same anatomical regions targeted by Alzheimer’s disease (AD) neuropathology [8,10,12,25-30]; (ii) both play roles in adult neurogenesis, neuronal development and synaptic physiology that are in-dispensable in nervous system development [3,31,32]; (iii) NF-L and SYN-II expression are found to be hyper-sensitive to AD-type change; as two of the most abundantly expressed neuron-specific phosphoproteins in pyramidal layer 5 neocortical neurons, the largest neurons in the brain (control neocortical layer 5 neurons have normal perikaryal volumes averaging up to 999 μm3 in control neocortex); these are very large neurons highly susceptible to AD-induced neuropathology both in human AD and in stressed human neurons (transplantation grade) in primary co-culture [30,33,34]; (iv) are respectively key axonal and presynaptic components involved in the neurotransmission mechanism and the neurotransmitter release cycle [30,35]; (v) NF-L and SYN-II are key supporters of the cytoskeleton synaptic cytoskeletal system in neural development and in both neuronal structure and function and utilize the common transcription factor activators zif268/egr-1/Krox-24 in their promotors (see below); both NF-L and SYN-II may be co-regulated in neuronal gene expression during development and appear to be deregulated together during neurodegeneration [5,30,36,37]; (vi) are approximately the same size and mass (NF-L size 543 amino acids; NF-L molecular mass: 61517 Da; SYN-II size: 582 amino acids; SYN-II molecular mass: 62996 Da) and their mRNAs and protein can be analyzed, quantified and compared with extremely high sensitivity using immunocytochemistry and Western gel analysis, and also by using ELISA, RT-PCR, Northern blots, RNA sequencing and array-based technologies (www.genecards.org/cgi-bin/carddisp.pl?gene=NEFL; www.genecards.org/cgi-bin/card disp.pl?gene=SYN2#; last accessed 28 September 2021; [30]; (vii) are significantly down regulated in progressive neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS) and AD [11,30,33,38,39]; (viii) are two of the most intensively studied of neuron-specific, neurotransmission related phosphoproteins in health and disease, in brain aging, ALS, MS and AD and a considerable literature exists for both NF-L and SYN-II in neurodevelopment, synaptic maturation and molecular and genetic neurobiology [5,15,24,26,33]; www.uniprot.org/uniprot/p07196#ptm_processing; last accessed 28 September 2021); (ix) are two of the most down-regulated neuron-specific phosphoproteins observed in degenerating neurons in inflammatory neurodegeneration both in vitro and in vivo such as in AD brain and in amyloid over-expressing transgenic murine models for AD (TgAD; [30,33]; (x) as functionally related abundant neuronal components NFs and SYN-II co purify in neuronal extracts; interestingly however the similarly sized NF-L and SYN-II have differential decay schemes and kinetics with NF-L accumulating in the periphery while SYN-II appears to be locally degraded [30,40,41]; (xi) deficits in NF-L, SYN-II and other related neuron-specific elements are not only associated with atrophied or degenerating neurons but are also colocalized within the core and periphery of the senile plaque (SP) and/or neurofibrillary tangles (NFT), two key lesions that characterize AD brain neuropathology [25, 30,42-47]; (xii) disruption in NF-L and SYN-II expression has been associated with over ~200 neurological disorders including ALS, AD, MS, schizophrenia, autism, bipolar disorder, epilepsy, several different types of brain cancer and many other human diseases with a neurological component; (www.malacards.org/search/results/NEFL; www. malacards.org/search/results?query=SYN2; last accessed 28 September 2021); and (xiii) both NF-L and SYN-II expression are under transcriptional control by the CNS-abundant zif268/egr-1/Krox-24 family of transcription factors that are known to be involved at the coordinated transcription level in a variety of higher order neurological processes within the human CNS; these include learning, memory, synaptic network formation and plasticity in the mammalian brain. Again both NF-L and SYN-II expression are observed to be down-regulated in multiple neuropsychiatric disorders involving progressive axonal and neuronal atrophy and neurodegeneration [11,30,36,37,39,48] Largely because of the high stability and long half-life of NF-L compared to SYN-II the major focus of this short review will be on NF-L and its translocation from a highly polymerized structural component of the neuronal cytoskeleton to a neurodegenerative disease biomarker in multiple biofluids of the periphery.
NF-L Dynamics and Trafficking
Under normal homeostatic conditions and in the presence of physiological calcium all NF proteins form relatively insoluble, tight filamentous bundles [3-5,25] and are in constant dynamic motion with continual assembly and disassembly [2,8,24,49]. All NF proteins are substrates of multiple proteolytic systems; for example when axoplasm is exposed to the extracellular fluid (ECF) elevated calcium concentrations activate calpains, calcium-dependent, non-lysosomal cysteine proteases that readily cleave NFs and promote neurofilament degradation [3,50].
Within the NF bundle, human NF-Ls are extremely stable filamentous structures with half-lives in the order of ~ 21 to ~ 55 days or longer [3,31,45]. As mentioned earlier, low levels of NF-L are constantly released from neuronal cell bodies and axons into the ECF that surrounds neurons and this ECF is contiguous with the CSF. Normally neuronal axon-integral NF-L filaments are depolymerized and released in an age-dependent manner, with low levels of NF-L appearing in circulating biofluids even during the healthy aging of the human CNS [12,51-53]. While the significantly increased appearance of NF-L in the CSF and other biofluids is thought to be an IF-mediated signal for dysfunctional neurons or neurons undergoing age-related demise or atrophy, the dynamics and patho-mechanism of NF triplet bundle depolymerization and NF-L liberation remains incompletely understood. NF-L proteins appear to be ‘secreted’, ‘liberated’ or ‘mobilized’ from neurons and subsequently delivered into the interstitial space and ECF surrounding neurons, and next compartmentalized into the CSF, followed by transport into the lymphatic and systemic circulation and blood serum where NF-L concentrations can be detected and analyzed. In the CSF and blood serum NF-L is typically detected with extreme precision using ELISA or other related fluorescent or chemiluminescent techniques to low picogram per milliliter (pg/ml) sensitivity (see https://www.mybiosource.colm/human-elisakits/neurofilament-light-chain-nfl/9399603; last accessed 28 September 2021; https://www.abbexa.com/ humanneuro-filament-light-polypeptide-nefl-clia-kit-2; last accessed 28 September 2021). Interestingly, while neuronal presynaptic proteins such as SYN II degrade rapidly via the ubiquitin-proteasome pathway, a considerably more stable NF-L turns over much more slowly, and in neurological disease is accompanied by a pathological shift from an intracellular neuronal cytoplasmic location across multiple membrane barriers into various biofluid compartments [16,20,54].
The biomechanics of NF-L depolymerization, liberation and transport from the neuron’s axonal interior to the surrounding interstitial space and ECF, and through the CSF into the systemic circulation, including the suspected role of micro-vesicular transport of IF proteins is also not well understood in either its neurobiological or molecular complexity [55-59]. An augmentation or interference of axonal IF trafficking by disorganized or modified NFs, and deficits or alterations in NF transport or translocation across the neuronal plasma or cytoplasmic membranes has been proposed as one possible mechanism of NF-mediated neuropathology in both AD and ALS and perhaps other neurological disorders [5,39,60-65]. The increasing abundance of NF-L in biofluids including the ECF, CSF, blood serum and/or vitreous fluid of the eye may serve as a diagnostic or prognostic biomarker for evaluating disease progression in different nervous system disorders for neurodegenerative diseases and/or in transgenic animal models of progressive age-related neurodegeneration [13,30,39,57-59,66].The most recent research suggests that NF-L presence in peripheral biofluids may be a stronger biomarker for all cause generalized decline in neuronal structure and integrity, defective network signaling and progressive neurodegeneration and/or neurodegenerative dementia [4,18,24,29,67,68].
NF-L As A Biomarker And Indicator Of Neuropathology
NF-L presence and abundance in the CSF and/or peripheral blood serum may not only be useful diagnostically for detecting the earliest signs of neurodegeneration but also: (i) in evaluating the degree and severity of progressive neurodegenerative damage and pathological change in the aging nervous system; (ii) may be informative as an indicator of neuronal and/or axonal atrophy and related morphologic changes in the brain at both the microscopic and macroscopic level; and (iii) may be further useful as a general biomarker of neurological disease progression in multiple nervous system disorders, including the evaluation of treatment efficacy for traumatic brain injury (TBI), ischemic stroke and inflammatory neurodegeneration [4,47,69,70]. Importantly, deficits in NF-L abundance within the neuronal cytoskeleton and cytoarchitecture may not only have structural and functional implications for neuronal integrity and function but is also linked to excessive extra-neuronal NF-L presence in multiple neurodegenerative diseases exhibiting both progressive neurite degeneration and demyelination. The list of neurological disorders where NF-L has been found to be enriched in biofluids such as the CSF is both significant and remarkable. Such disorders include AD [16,24,71,72], amyotrophic lateral sclerosis (ALS; [24]); generalized brain atrophy [71]; frontotemporal dementia (FTD) [14,68]; idiopathic normal pressure hydrocephalus (iNPH); multiple sclerosis (MS) [13]; lysosomal storage diseases such as Gaucher’s disease (GD); spinal muscular atrophy (SMA); sub-cortical arteriosclerotic encephalopathy (SAE) [68,73]; hereditary sensory-motor neuropathy (HSMN); Parkinson's disease (PD); polyglutamine in CAG trinucleotide repeat disorders; both ischemic and hemorrhagic stroke; vascular dementia (VaD) and other relatively rare neurodegenerative disorders such as neuronal ceroid lipofuscinosis type 2 (CLN2 disease) and rare prion diseases that include Creutzfeld-Jacob disease (CJD) [4,10,12,14,24,40,53,68,74,75]. In fact defective NF-L and SYN-II signaling have been linked to over 200 human diseases and because each of these disease states involve these 2 neuron-specific functionally-linked cytoskeletal and synaptic phosphoproteins and the majority of these disorders have a neurological component (www.malacards.org/search/results/NEFL; www.malacards .org/search/results?query=SYN2; last accessed 28 September 2021).
Pathologically, NF-L filaments are further found within and/or associated with abnormal assemblies and disease-related accumulations and senile plaque lesions associated with amyloid beta (Aβ) peptide deposits in AD and synuclein in PD; NF-L proteins have also been found to directly associate with superoxide dismutase 1 (SOD1), TAR DNA-binding protein 43 (TDP43), neuronal RNA-binding FUS proteins, optineurin (OPTN), ubiquilin 2 (UBQLN2), dipeptide repeat protein (DRP), and synapsins and other pre-synaptic phosphoproteins [4,5,10,12,14,27,30,33,40,53,56,76]. Whether NF-L is covalently locked within these pathological lesions or can dissociate away and contribute to biofluid NF-L pools is not known. When axonal and dendritic damage, inflammatory, neurodegenerative, traumatic or vascular injury occurs in multiple neurological disorders such as AD, ALS, PrD, VaD there often appear to be increases in NF-L accumulation in the CSF which are directly proportional to the severity of these progressive age-related neurodegenerative disorders [4,12,24,38,49,56,77]. Interestingly in MS, CSF NF-L concentrations have been shown to correlate with clinical and radiological outcomes, making NF-L presence potentially useful for monitoring patient response to different MS therapies [11,22,49,51,57].
Compartmentalization of NF-L in Neurodegenerative Disease
While highly polymerized NF-L filaments are the major component of type IV IF scaffolding proteins in neurons and the most abundant neuron-specific filament class at the core of the NF triplet, their disease-related occurrence in circulating biofluids outside of the CNS may seem paradoxical. Normally large ~ 61.5 kDa proteins like the NF-L protein filaments exist as a highly organized polymer stably bundled and confined within the axonal cytoskelton of neurons, and have difficulty in passing through intact plasma membranes. However, damaged plasma membranes and leaky biophysiological barriers of the extensive neurovascular system of the human CNS appear to allow filamentous protein passage across biological membranes under both pathological conditions and with aging [62,65,78]. There is a strong correlation of brain endothelial neurovascular cell damage and leakiness of biological membrane structure associated with increased plasma membrane porosity and increased passage of normally excluded biomolecules, neurotoxins and multiple pro-inflammatory mediators such as lipopolysaccharide (LPS) and NF-L [49,62,65,78-82]. Recent reports indicate that higher serum NF-L levels are also associated with greater impairment of blood-brain barrier (BBB) integrity as measured by albumin quotient assay (at present one of the most reliable biomarkers for estimating the BBB permeability) coupled with brain MRI studies [49,83]. Moreover, as an index of neuronal degeneration and functional decline, higher concentrations of NF-L in both the CSF and plasma are associated with reduced [18F]FDG uptake and hypometabolism in AD brain and contribute to the diagnosis of neuronal atrophy and neurodegeneration in serum amyloid-positive individuals with AD [84]. There is evidence for a mechanistic link between inflammation and BBB integrity and function in both neurodevelopmental and neurodegenerative disease [85,86]. Another recent and related report provides evidence that in the vitreous fluid of the eye NF-L is positively associated with increased levels of Aβ42 peptide, inflammatory cytokines such as interleukin-15 (IL-15), monocyte chemoattractant protein-1 (MCP1), and vascular proteins such as vascular endothelial growth factor receptor-1 (VEGFR1), vascular cell adhesion molecule-1 (VCAM-1) and intracellular adhesion molecular-1 (ICAM-1) suggesting the clinical utility of obtaining vitreous samples as a possible source of protein array-based diagnostic testing for AD or other age-related, AD-like neurological disorders [86].
NF-L Depolymerization And Degradation In Neurological Disease
The NF-L chain IF protein monomer is the smallest of the NF triplet yet one of the most abundant and stable neuron-specific components critically essential for maintaining the cytoskeleton of healthy neurons, and thereby the organization of synaptic networks [5,11,13,45,57,76]. It follows that neurodegenerative disorders involving progressive neuronal atrophy and synaptic damage will involve alterations in NF-L and SYN-II neurobiology and abundant evidence continues to support this concept. NF-L and SYN-II proteins have a particularly high density in large pyramidal neurons and myelinated axons extending into the PNS, such as those found in motor neurons [5,10,87]. While SYN-II is rapidly degraded, NF-L release from a fully polymerized filamentous state significantly increases free NF-L concentration in the ECF and CSF in response to CNS axonal damage due to inflammatory, neurodegenerative, traumatic or neurovascular injury (Figure 1). Several studies provide evidence that NF-L concentrations in the CSF may be the highest in brain disorders with subcortical pathology, such as VaD, iNPH and white matter disease [87,88]. As aforementioned, significant free NF-L monomeric concentrations in the CSF have been reported in individuals with FTD, MS and CJD, and more closely correlate with rapidly progressing axonal deterioration and degeneration than in individuals with early stage AD [68,71,87]. It has been suggested that the serial detection of monomeric NF-L over time may be diagnostically useful to differentiate between multiple types of age-related neurological disorders with a similar or indistinguishable clinical presentation [29,67,68,71,87,88]. The most recent extensive correlative analysis on large numbers of neurodegenerative disease patients however indicates that NF-L does not appear to be a highly selective biomarker for any single neurodegenerative diseases or for AD at any stage, but rather an indicator for dendritic damage, axonal deterioration, overall neural cell demise, neurite regression and synaptic atrophy, especially in progressive pathological disorders where large neurons and myelinated axons are involved [47,68,87].
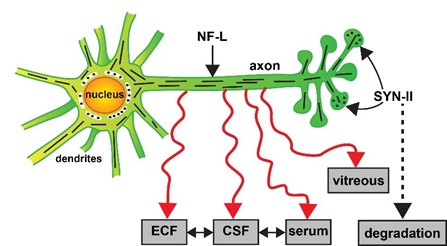
Figure 1: Alternate fate of the neuron-specific neurofilament light (NF-L) chain and synapsin 2 (SYN-II) proteins in degenerating neurons. Highly schematicized depiction of NF-L protein as an important intermediate filament (IF) component of the axon and cytoarchitecture that supports dendrites and synaptic structures of neurons and a key player in cytoskeletal dynamics; a critical pre-synaptic phosphoprotein SYN-II is involved in tethering synaptic vesicles to one another, in the regulation of synaptic vesicle release and trans-synaptic signaling neurons, in the linkage and association of synaptic vesicles to the cytoskeleton; in doing so SYN-II modulates neurotransmitter release across the presynaptic membrane in both the central and peripheral nervous system (CNS, PNS); NF-L is also an integral component and critical organizer of synapsin-enriched synapses that modulate neurotransmission, behavior and memory; NF-L and SYN synaptic proteins together serve highly interactive roles; both are significantly depleted in dystrophic and atrophied neurons in AD and in other related neurodegenerative disorders [3,30,31,40,76]. SYN-II proteins (dark black dots at the pre-synapse) are very labile (half-life ~ 24 hr), exhibit high rates of turnover, decomposition and decay, and are rapidly degraded via the ubiquitin-proteasome pathway [54]. On the other hand neuronal NF-L filament proteins (short black lines) are extremely stable filamentous proteins (half-life ~ 504 to ~ 1320 hr or longer; [3,45], normally insoluble, resist degradation, and can leak out of the plasma membrane of degenerating neurons to subsequently appear in peripheral biofluids in the ECF, CSF, blood serum and/or vitreous of the eye (red wiggly eyes); the mobility of NF-L between ECF, CSF and serum compartments is not well understood; elevated NF-L abundance in peripheral biofluids has been observed in AD, ALS, CJD, CLN2, FTD, GD, iNPH, MS, neuronal atrophy, VaD and other forms of progressive neurodegenerative disease (see manuscript text; [11,14,17,25,47,68,70,86,94]. The abundance of NF-L in biofluids appears to be an indicator of the degree of all-cause neurodegeneration, dendritic and axonal damage, overall neuronal atrophy and neuronal decline; the most recent evidence suggests that this may have diagnostic value to differentiate between AD and FTD or be useful in AD diagnosis and/or prognosis in combination with other biofluid biomarkers such as tau and Aβ peptides [12,19,29,47,67,91,93].
Concluding Remarks
Age-related neurodegenerative disease continues to present one of contemporary medicine's most intractable problems in its characterization, diagnosis, prognosis, disease monitoring and clinical management. There are a considerable number of dysfunctional biochemical, neurochemical, cytoskeletal and molecular genetic deficits across the entire spectrum of progressive, age-related degenerative brain diseases such as AD. In contrast to the rapidly degraded synaptic protein SYN-II, alterations in the compartmentalization of significantly more stable NF-L from fixed positions within neurons to their progressive appearance in multiple biofluids lends weight to the understudied idea that there may be transport, translocation and/or trafficking complications of neurofilaments and other essential neuron-specific and/or neuron- or synaptic-associated components which appear to contribute to the progressive and age-related nature of neuronal degeneration. The appearance of the neuron-specific NF-L filament protein in biofluid compartments outside of the neuron is: (i) an overall signal for the inception of the functional decline of large CNS neurons; (ii) the quantifiable abundance of NF-L in multiple biofluids appears to be an indicator of the degree of this neurodegeneration in both the CNS and/or PNS; and (iii) while absolute NF-L abundances are diagnostically useful NF-L presence alone does not appear to be a selective biomarker for any specific neurodegenerative disease. Alternately the appearance of NF-L in biofluids may simply be a consequence of a poorly understood natural decay mechanism for this abundant filament protein in progressive age-related neurological disorders in part due to NF-L’s notable stability and remarkably long half-life (Figure 1) [31,45,57].
Dysfunctional plasma membranes and altered biophysiological barriers including the neuronal plasma membrane-ECF interface and the BBB-systemic circulation endothelial cell barriers are strongly implicated in dysregulated biomolecular trafficking [65,78]. Biophysical barrier disruption may allow depolymerized NF-L mobilization from the neuronal axons and cytoplasm across biophysical interfaces into the ECF, CSF, throughout the systemic circulation, within the vitreous fluid of the eye and perhaps into other biofluid compartments. Multiple laboratories have reported disease-associated deficits in these biological membrane barriers, the BBB, gastrointestinal (GI)-tract intestinal lumen circulatory system barriers and other biophysical interfaces, and the channel and vesicle-mediated traffic passing across them [56,60,62,63,65,78,89,90]. Biofluids including ECF, CSF, blood serum and vitreous fluids contain microRNAs (miRNAs) both free and vesicle-bound that are known to regulate the expression and abundance of neuron-specific components including NF-L [56]. Further quantitative investigation of this vesicular traffic, vesicle content, specific globular and filamentous proteins, lipids, carbohydrates, small non-coding RNA (sncRNA) and other nucleic acids (both RNA and DNA) populating the systemic circulation (including the lymphatic, glymphatic and blood serum), CSF and other biofluids such as saliva and urine are important future goals of AD and neurodegenerative disease research.
Lastly, the detection and quantitation of NF-L in biofluids has become a widely researched, recognized and diagnostically valuable biomarker for the earliest detection and onset of all-cause neural decline in progressive, age-related neurodegeneration, stroke and traumatic brain injury [20,47,91]. It is becoming increasingly clear that NF-L presence in biofluids alone however, is neither specific or selective for AD or for any other progressive age-related neurological disorder [12,29,65,92].The highest value of measuring the abundance of NF-L as a biomarker in CSF may be to differentiate between complex neurological disorders with close and often overlapping clinical presentation, such as assisting the differentiation between FTD (the second most frequent cause of dementia, after AD in patients under the age of 65) from AD, and PD from atypical Parkinsonian syndromes [47,91,93]. Further studies on large age- and gender-matched genetically defined populations, including the application of a more personalized medicine approach for AD diagnosis and treatment [29,75]: (i) should provide an improved understanding of the disease mechanisms involved with NF-L liberation from neurons and NF-L accumulation in biofluids, brain dysfunction and neurodegeneration; and (ii) allow for the discovery of new biomarkers, or defined combinations of biomarkers, for disease prognosis and diagnosis that should engender equally novel therapeutic strategies [94-97].
Significance of the Research
An overwhelming abundance of research papers report the elevation of neurofilament light (NF-L) chain protein in various biofluids (CSF, blood serum, vitreous fluid and others) in Alzheimer’s disease (AD) and associated neurodegenerative diseases, and their potential role in providing valuable diagnostic, prognostic and disease-monitoring biomarkers. However, not one of these papers discusses the significant down-regulation and altered trafficking of NF-L in atrophied and dysfunctional neurons and in degenerating axons in AD and related degenerative disorders of the brain and CNS. This is Review paper is the first to address the hitherto unappreciated fate of NF-L from a highly polymerized neuron-specific structural component of the neuronal axoskeleton to a monomeric neurodegenerative disease biomarker in the periphery.
Funding
This research was supported in part through an unrestricted grant to the LSU Eye Center from Research to Prevent Blindness (RPB); The Brown Foundation, Joe and Dorothy Dorsett Innovation in Science Healthy Aging Award; the Louisiana Biotechnology Research Network (LBRN); the Louisiana Biotechnology Research Network (LBRN) and NIH grants NEI EY006311, NIA AG18031 and NIA AG038834 (WJL).
Acknowledgment
Sincere thanks are extended to Drs. Arthur J. Dalton, Christopher Eicken, Christopher Hebel, Wayne Poon and Piotr N. Alexandrov for short post-mortem interval (PMI) human brain, eye and/or retinal tissues or extracts, stabilized RNA and DNA probes, nucleic acid hybridization work and initial data interpretation, and to Aileen I. Pogue and Darlene Guillot for expert technical assistance and medical artwork. Thanks are further extended to the many neuropathologists, physicians and researchers of Canada and the USA who have provided high quality, short PMI human CNS, eye and retinal tissues or extracted total brain and eye DNA and RNA for scientific study and quantitative analysis. Research on human brain, eye and host cell transcriptomics in the Lukiw laboratory involving total human RNA analysis and gene expression, microRNA (miRNA) and messenger RNA (mRNA) sequencing and complexity and array-based quantitation, the innate-immune response in AD and in other forms of neurological or retinal disease, amyloidogenesis and neuroinflammation was supported through an unrestricted grant to the LSU Eye Center from Research to Prevent Blindness (RPB); The Brown Foundation, Joe and Dorothy Dorsett Innovation in Science Healthy Aging Award; the Louisiana Biotechnology Research Network (LBRN); the Louisiana Bio-technology Research Network (LBRN) and NIH grants NEI EY006311, NIA AG18031 and NIA AG038834 (WJL).
References
- Mann DM (1996) Pyramidal nerve cell loss in Alzheimer's disease. Neurodegeneration 5: 423-427.
- Lobsiger CS, Cleveland DW (2009) Neurofilaments: Organization and function in neurons. Encyclopedia of Neuroscience 6: 433-436.
- Yuan A, Rao MV, Veeranna, Nixon RA (2017) Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol 9: a018309.
- Lee Y, Lee BH, Yip W, Chou P, Yip BS (2020) Neurofilament proteins as prognostic biomarkers in neurological disorders. Curr Pharm Des 25: 4560-4569.
- Julien JP, Mushynski WE (1998) Neurofilaments in health and disease. Prog Nucleic Acid Res Mol Biol 61: 1-23.
- Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC (2007) Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res 313: 2098-2109.
- Laser-Azogui A, Kornreich M, Malka-Gibor E, Beck R (2015) Neurofilament assembly and function during neuronal development. Curr Opin Cell Biol 32: 92-101.
- Yuan A, Sershen H, Veeranna, Basavarajappa BS, Kumar Aet al. (2015) Neurofilament subunits are integral components of synapses and modulate neurotransmission and behavior in vivo. Mol Psychiatry 20: 986-994.
- Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti RE, et al. (2009) Equal numbers of neuronal and non-neuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513: 532-541.
- Gaetani L, Blennow K, Calabresi P, Filippo MD, Parnetti L, et al. (2019) NF-L chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 90: 870-881.
- Varhaug KN, Torkildsen Ø, Myhr KM, Vedeler CA (2019) Neurofilament light chain as a biomarker in multiple sclerosis. Front Neurol 10: 338.
- Palermo G, Mazzucchi S, Vecchia AD, Siciliano G, Bonuccelli U, et al. (2020) Different clinical contexts of use of blood NF-L protein in the spectrum of neurodegenerative diseases. Mol Neurobiol 57: 4667-4691.
- Brureau A, Blanchard-Bregeon V. Pech C, Hamon S, Chaillou P, et al. (2017) NF-L in cerebrospinal fluid and serum is a biomarker of neuronal damage in an inducible mouse model of neurodegeneration. Neurobiol Dis 104: 73-84.
- Goossens J, Bjerke M, Van Mossevelde S, Van den Bossche T, Goeman J, et al. (2018) Diagnostic value of cerebrospinal fluid tau, neurofilament, and progranulin in definite frontotemporal lobar degeneration. Alzheimers Res Ther 10: 31.
- Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, et al. (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 14: 577-589.
- Bos I, Vos S, Verhey F, Scheltens P, Teunissen C, et al. (2019) Cerebrospinal fluid biomarkers of neurodegeneration, synaptic integrity, and astroglial activation across the clinical Alzheimer's disease spectrum. Alzheimers Dement 15: 644-654.
- Camporesi E, Nilsson J, Brinkmalm A, Becker B, Ashton NJ, et al. (2020) Fluid biomarkers for synaptic dysfunction and loss. Biomark Insights 15: 1177271920950319.
- Chatterjee P, Cheong YJ, Bhatnagar A, Goozee K, Wu Y, et al. (2020) Plasma metabolites associated with biomarker evidence of neurodegeneration in cognitively normal older adults. J Neurochem.
- de Wolf F, Ghanbari M, Licher S, McRae-McKee K, Gras L, et al. (2020) Plasma tau, NF-L and amyloid-β levels and risk of dementia; A population-based cohort study. Brain 143: 1220-1232.
- Petersen ME, Rafii MS, Zhang F, Hall J, Julovich D, et al. (2021) Plasma total-tau and neurofilament light chain as diagnostic biomarkers of Alzheimer's disease dementia and mild cognitive impairment in adults with Down syndrome. J Alzheimers Dis 79: 671-681.
- Rosengren LE, Karlsson JE, Karlsson JO, Persson LI., Wikkelsø C (1996) Patients with amyotrophic lateral sclerosis and other neurodegenerative diseases have increased levels of neurofilament protein in CSF. J Neurochem 67: 2013-2018.
- Lycke JN, Karlsson JE, Andersen O, Rosengren LE (1998) NF-L in CSF: A potential marker of activity in multiple sclerosis. J Neurol Neurosurg Psychiatry 64: 402-404.
- Castellanos-Montiel MJ, Chaineau M, Durcan TM (2020) The neglected genes of ALS: Cytoskeletal dynamics im-pact synaptic degeneration in ALS. Front Cell Neurosci 14: 594975.
- Loeffler T, Schilcher I, Flunkert S, Hutter-Paier B (2020) NF-L as biomarker of neurodegenerative and rare diseases with high translational value. Front Neurosci 14: 579.
- Day WA (1977) Solubilization of neurofilaments from central nervous system myelinated nerve. J Ultrastruct Res 60: 362-372.
- Lukiw WJ, Wong L, McLachlan DRC (1990) Cytoskeletal mRNA stability in human neocortex: Studies in normal aging and in AD. Int J Neurosci 55: 81-88.
- Greengard P, Valtorta F, Czernik AJ, Benfenati F (1993) Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 259: 780-785.
- Lukiw WJ (2012) NF-κB-regulated micro RNAs (miRNAs) in primary human brain cells. Exp Neurol 235: 484-490.
- Molinuevo JL, Ayton S, Batrla R, Bednar MM, Bittner T, et al. (2018) Current state of Alzheimer's fluid biomarkers. Acta Neuropathol 136: 821-853.
- Zhao Y, Sharfman NM, Jaber VR, Lukiw WJ (2019) Down-regulation of essential synaptic components by GI-tract microbiome-derived LPS in LPS-treated human neuronal-glial (HNG) cells in primary culture: relevance to AD. Front Cell Neurosci 13: 314.
- Yuan A, Sasaki T, Rao MV, Kumar A, Kanumuri V, et al. (2009) Neurofilaments form a highly stable stationary cytoskeleton after reaching a critical level in axons. J Neurosci 29: 11316-11329.
- Barbieri R, Contestabile A, Ciardo MG, Forte N, Marte A, et al. (2018) Synapsin I and synapsin II regulate neurogenesis in the dentate gyrus of adult mice. Oncotarget 9: 18760-18774.
- Kittur S, Hoh J, Endo H, Tourtellotte W, Weeks BS, et al. (1994) Cytoskeletal NF-L gene expression in brain tissue from AD patients. I. Decrease in NF-L and NF-M message. J Geriatr Psychiatry Neurol 7: 153-158.
- Bundgaard MJ, Regeur L, Gundersen HJ, Pakkenberg B (2001) Size of neocortical neurons in control subjects and in Alzheimer's disease. J Anat 198: 481-489.
- Saviouk V, Moreau MP, Tereshchenko IV, Brzustowicz LM (2007) Association of SYN-2 with schizophrenia in families of northern European ancestry. Schizophr Res 96: 100-111.
- Pospelov VA, Pospelova TV, Julien JP (1994) AP-1 and Krox-24 transcription factors activate the neurofilament light gene promoter in P19 embryonal carcinoma cells. Cell Growth Differ 5: 187-196.
- Petersohn D, Schoch S, Brinkmann DR, Thiel G (1995) The human synapsin II gene promoter. Possible role for the transcription factor zif268/egr-1, polyoma enhancer activator 3, and AP2. J Biol Chem 270: 24361-24369.
- McLachlan DR, Lewis PN, Lukiw WJ, Sima A, et al. (1984) Chromatin structure in dementia. Ann Neurol 15: 329-334.
- Rao MV, Nixon RA (2003) Defective neurofilament transport in mouse models of amyotrophic lateral sclerosis: A review. Neurochem Res 28: 1041-1047.
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, et al. (1999) Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci 354: 269-279.
- Fressinaud C, Berges R, Eyer J (2012) Axon cytoskeleton proteins specifically modulate oligodendrocyte growth and differentiation in vitro. Neurochem Int 60: 78-90.
- Selkoe DJ (1986) Altered structural proteins in plaques and tangles: What do they tell us about the biology of Alzheimer's disease? Neurobiol Aging 7: 425-432.
- Dickson DW, Farlo J, Davies P, Crystal H, Fuld P, et al. (1988) Alzheimer's disease. A double labeling immunohistochemical study of senile plaques. Am J Pathol 132: 86-101.
- Haleem K, Lippa CF, Smith TW, Kowa H, Wu J, et al. (2007) Presenilin-1 C410Y AD plaques contain synaptic proteins. Am J Alzheimers Dis Other Demen 22: 137-144.
- Millecamps S, Gowing G, Corti O, Mallet J, Julien JP (2007) Conditional NF-L transgene expression in mice for in vivo analysis of turnover and transport rate of neurofilaments. J Neurosci 27: 4947-4956.
- Cohen LD, Zuchman R, Sorokina O, Müller A, Dieterich DC, et al. (2013) Metabolic turnover of synaptic proteins: kinetics, interdependencies and implications for synaptic maintenance. PLoS One 8: e63191.
- Zetterberg H, Blennow K (2021) Moving fluid biomarkers for Alzheimer's disease from research tools to routine clinical diagnostics. Mol Neurodegener 16: 10.
- Hoffman PN, Cleveland DW, Griffin JW, Landes PW, Cowan NJ, et al. (1987) Neurofilament gene ex-pression: A major determinant of axonal caliber. Proc Natl Acad Sci USA 84: 3472-3476.
- Uher T, McComb M, Galkin S, Srpova B, Oechtering J, et al. (2020) NF-L levels are associated with blood-brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in MS. Mult Scler 7: 1352458520912379.
- Ma M (2013) Role of calpains in the injury-induced dysfunction and degeneration of the mammalian axon. Neurobiol Dis 60: 61-79.
- Siller N, Kuhle J, Muthuraman M, Barro C, Uphaus T, et al. (2019) Serum NF-L is a biomarker of acute and chronic neuronal damage in early multiple sclerosis MS. Mult Scler 25: 678-686.
- Ashton NJ, Hye A, Rajkumar AP, Leuzy A, Snowden S,et al. (2020) An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat Rev Neurol 16: 265-284.
- Khalil M, Pirpamer L, Hofer E, Voortman MM, Barro C, et al. (2020) Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun 11: 812.
- Ding M, Shen K (2008) The role of the ubiquitin proteasome system in synapse remodeling and neurodegenerative diseases. Bioessays 30: 1075-1083.
- Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D (2019) Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease. J Neurovirol 25: 702-709.
- Lukiw WJ, Pogue AI (2020) Vesicular transport of encapsulated microRNA between glial and neuronal cells. Int J Mol Sci 21: 5078.
- Gafson AR, Barthélemy NR, Bomont P, Carare RO, Durham HD, et al. (2020) Neurofilaments: Neurobiological foundations for bi-omarker applications. Brain 143: 1975-1998.
- Gordon BA (2020) Neurofilaments in disease: What do we know? Curr Opin Neurobiol 61: 105-115.
- Bomont P (2021) The dazzling rise of neurofilaments: Physiological functions and roles as biomarkers. Curr Opin Cell Biol 68: 181-191.
- Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57: 178-201.
- Rossi D, Volanti P, Brambilla L, Colletti T, Spataro R, et al. (2018) CSF neurofilament proteins as diagnostic and prognostic biomarkers for ALS. J Neurol 265: 510-521.
- Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood-brain barrier breakdown in AD and other neurodegenerative disorders. Nat Rev Neurol 14: 133-150.
- Bitetto G, Fonzo DA (2020) Nucleo-cytoplasmic transport defects and protein aggregates in neurodegeneration. Transl Neurodegener 9: 25.
- Hutten S, Dormann D (2020) Nucleocytoplasmic transport defects in neurodegeneration-cause or consequence? Semin Cell Dev Biol 99: 151-162.
- Zhao Z, Zlokovic BV (2020) Therapeutic TVs for crossing barriers in the brain. Cell 182: 267-269.
- Alexandrov PN, Dua P, Hill JM, Bhattacharjee S, Zhao Y, et al. (2012) microRNA speciation in Alzheimer’s disease (AD) CSF and extracellular fluid (ECF). Int. J. Biochem. Mol Biol 3: 365-373.
- Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, et al. (2019) Plasma NFL as a potential biomarker of neurodegeneration in AD. Alzheimers Res Ther 10: 71.
- Antonell A, Tort-Merino A, Ríos J, Balasa M, Borrego-Écija S, et al. (2020) Synaptic, axonal damage and inflammatory cerebrospinal fluid biomarkers in neurodegenerative dementias. Alzheimers Dement 16: 262-272.
- Hoffman PN, Pollock SC, Striph GG (1993) Altered gene expression after optic nerve transection: Reduced NF expression as a general response to axonal injury. Exp Neurol 119: 32-36.
- Uphaus T, Bittner S, Gröschel S, Steffen F, Muthuraman M, et al. (2019) NF-L levels as a predictive marker for long-term outcome after ischemic stroke. Stroke 50: 3077-3084.
- Pereira JB, Westman E, Hansson O (2017) Association between cere-brospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in AD. Neurobiol Aging 58: 14-29.
- Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K (2019) Association between longitudinal plasma NF-L and neurodegeneration in patients with AD. JAMA Neurol 76: 791-799.
- Agren-Wilsson A, Lekman A, Sjöberg W, Rosengren L, Blennow K, et al. (2007) CSF biomarkers in the evaluation of idiopathic normal pressure hydrocephalus. Acta Neurol Scand 116: 333-339.
- Ru Y, Corado C, Soon RK, Melton AC, Harris A, et al. (2019) NF-L is a treatment-responsive biomarker in CLN2 disease. Ann Clin Transl Neurol 6: 2437-2447.
- Lukiw WJ, Vergallo A, Lista S, Hampel H, Zhao Y (2020) Biomarkers for Alzheimer's disease (AD) and the application of precision medicine. J Pers Med 10: 138.
- Steiner JP, Ling E, Bennett V (1987) Nearest neighbor analysis for brain synapsin; Evidence from re-association assays with membrane protein and the NF-L subunit. J Biol Chem 262: 905-914.
- McLachlan DR, Lukiw WJ, Wong L, Bergeron C, Bech-Hansen NT (1988) Selective messenger RNA reduction in AD. Brain Res 427: 255-261.
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV (2019) Blood-brain barrier: From physiology to disease and back. Physiol Rev 99: 21-78.
- Zhao Y, Cong L, Jaber V, Lukiw WJ (2017) Microbiome-derived LPS enriched in the perinuclear region of AD brain. Front Immunol 8: 1064.
- Zhao Y, Cong L, Lukiw WJ (2017) LPS accumulates in neurons of AD brain and impairs transcription in neuronal-glial co-cultures. Front Aging Neurosci 9: 407.
- Zhao Y, Jaber V, Lukiw WJ (2017) Secretory products of the human GI-tract and their potential impact on AD: Detection of LPS. Front Cell Infect Microbiol 7: 318.
- Zhan X, Stamova B, Sharp FR (2018) LPS associates with amyloid plaques, neurons and oligodendrocytes in AD brain. Front Aging Neurosci 10: 42.
- Chen B, Qin C, Tao R, Dong YJ, Ma X, et al. (2020) The clinical value of the albumin quotient in patients with neuromyelitis optica spectrum disorder. Mult Scler Relat Disord 38: 101880.
- Benedet AL, Ashton NJ, Pascoal TA, Leuzy A, Mathotaarachchi S, et al. (2019) Plasma neurofilament light associates with Alzheimer's disease metabolic decline in amyloid-positive individuals. Alzheimers Dement (Amst) 11: 679-689.
- Stolp HB, Dziegielewska KM (2009) Review: Role of developmental inflammation and BBB dysfunction in neuro-developmental and neurodegenerative diseases. Neuropathol Appl Neurobiol 35: 132-146.
- Subramanian ML, Vig V, Chung J, Fiorello MG, Xia W, et al. (2020) NF-L in the vitreous humor of the eye. Alzheimers Res Ther 12: 111.
- Racine AM, Merluzzi AP, Adluru N, Norton D, Koscik RL, et al. (2019) Association of longitudinal white matter degeneration and CSF biomarkers of neurodegeneration, inflammation and AD. Brain Imaging Behav 13: 41-52.
- Sjögren M, Blomberg M, Jonsson M, Wahlund LO, Edman A, et al. (2001) NF-L in CSF: a marker of white matter changes. J Neurosci Res 66: 510-516.
- Lukiw WJ (2013) Alzheimer's disease (AD) as a disorder of the plasma membrane. Front Physiol 4: 24.
- Li D, Mielke MM (2019) An update on blood-based markers of Alzheimer's disease using the SiMoA platform. Neurol Ther 8: 73-82.
- Bridel C, van Wieringen WN, Zetterberg H, Tijms BM, Teunissen CE, et al. (2019) Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: A systematic review and meta-analysis. JAMA Neurol 76: 1035-1048.
- Zhao Y, Jaber V, Alexandrov PN, Vergallo A, Lista S, et al. (2020) microRNA-Based bi-omarkers in Alzheimer's disease (AD). Front Neurosci 14: 585432.
- Karantali E, Kazis D, Chatzikonstantinou S, Petridis F, Mavroudis I (2020) The role of neurofilament light chain in frontotemporal dementia: A meta-analysis. Aging Clin Exp Res 33: 869-881.
- Schlaf G, Salje C, Wetter A, Stuertz K, Felgenhauer K, et al. (1998) Determination of synapsin and synaptophysin in body fluids by two-site enzyme-linked immunosorbent assays. J Immunol Methods 213: 191-199.
- Andrade-Moraes CH, Oliveira-Pinto AV, Castro-Fonseca E, da Silva CG, Guimarães DM, et al. (2013) Cell number changes in Alzheimer's disease relate to dementia, not to plaques and tangles. Brain 136: 3738-3752.
- Lukiw WJ, Cong L, Jaber V, Zhao Y (2018) Microbiome-derived LPS selectively inhibits NF-L gene expression in HNG cells in primary culture. Front Neurosci 12: 896.
- Paterson RW, Slattery CF, Poole T, Nicholas JM, Magdalinou NK, et al. (2018) Cerebrospinal fluid in the differential diagnosis of Alzheimer's disease: Clinical utility of an extended panel of biomarkers in a specialist cognitive clinic. Alzheimers Res Ther 10: 32.
Citation: Zhao Y, Arceneaux L, Culicchia F, Lukiw WJ (2021) Neurofilament Light (NF-L) Chain Protein from a Highly Polymerized Structural Component of the Neuronal Cytoskeleton to a Neurodegenerative Disease Biomarker in the Periphery. J Alzheimers Neurodegener Dis 7: 056.
Copyright: © 2021 Yuhai Zhao, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

