
Novel Health Supplement for Recovery of Alcohol Induced Adverse Effects and Associated Disorders: HepG2 Cells Study and Safety Dose Profiling on Rats
*Corresponding Author(s):
Neelesh Kumar NemaNutraceuticals Division, C.V.J. Creative Centre-Bioingredients, Synthite Industries Pvt. Ltd., Synthite Valley, Kadayiruppu, Ernakulam, Kerala, India
Tel:+91 7356480222,
Email:neeleshk@synthite.com
Abstract
The present study objective was to design and develop novel health-supplement formula from plant extracts and was to evaluate the formula for high episodic alcohol toxicities, and associated disorders against alcohol intoxicated and oxidative damaged Human Hepatoma HepG2 cell line. The CC50 of the supplement was determined based on MTT assay. To get the safe dose concentration, an acute toxicity study was performed on Wistar rats as per OECD guidelines 423. The phyto-constituents of the supplement e.g. curcuminoids, piperine, β-caryophyllene, punicalagin, ellagic acid and isoquecetins from respective plant extracts were also analyzed and confirmed via HPLC and GC-FID profiling, which recovered more than 70% HepG2 cells with >75% cell viability. Normalization of oxidative stressed cells were confirmed via biochemical parameter studies. The same was verified via ROS inhibiting properties of the formula to reinforce the claim. In this study, any abnormal clinical symptom was not observed in treated animals at 2000mg/kg BW, hence it was considered as a safe dose for oral consumption. The present studies conclude that this health supplement contains herbal ingredients has a preventing role against alcohol-induced adverse effects and associated disorders.
Keywords
Alcoholism; Alcohol disorder; Alcohol toxicity; Antihangover; Nutraceuticals
Graphical Abstract

Introduction
Alcoholism is an extensive and serious Global Burden of Disease (GBD), interference to psycho-social lifestyle constantly [1,2]. Excessive consumption of alcohol makes undesirable and deleterious consequences to the human body and generates characteristic leading risk factors responsible for disability, morbidity and mortality [3,4]. More than 230 different types of diseases in 2016, where alcohol had a significant role directly or indirectly had affected 237 million men and 46 million women worldwide. The Americas, Europe and Western Pacific are the three regions as per WHO, where alcohol is consumed by more than half of the population. Acute or chronic ingestion of alcohol adversely affects human health especially leading to Alcoholic Liver Disease (ALD) e.g. inflammation, hepatocellular injury, oxidative stress, fat accumulation (steatohepatitis), fibrosis, cirrhosis or hepatocellular carcinoma and hangover [5]. Other secondary dysfunctions are also accompanied in association with some central nervous system complications and behavioral response [6,7].
Alcohol gets eliminated through its metabolic degradation products via multiple enzymatic and non-enzymatic pathways and affects specific organs or systems such as the brain, gastrointestinal tract, liver and immune system (Figure 1) resulting in characteristic symptoms like typical hangover, which may cause electrolyte imbalance, hypoglycemia, dehydration or gastrointestinal disturbances [8,9].
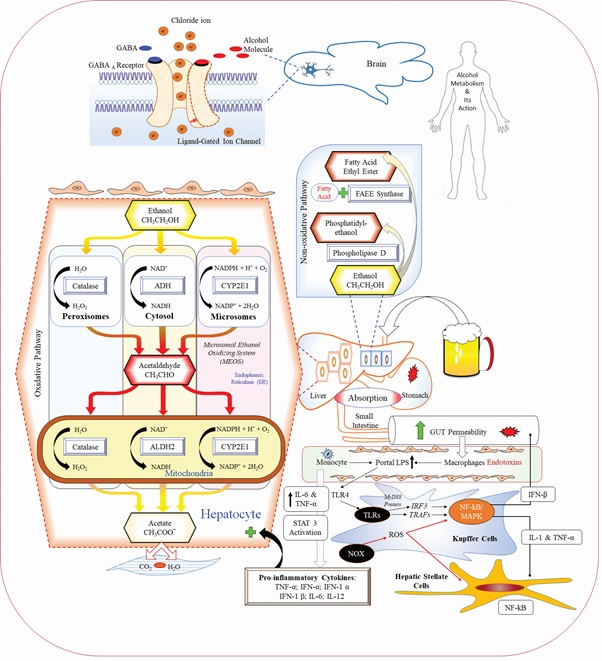 Figure 1: Alcoholic intoxication and its metabolism inside the body.
Figure 1: Alcoholic intoxication and its metabolism inside the body.
In order to reduce acute episodic alcohol and associated disorders, several therapeutic treatments and formulations are available and are already in practice. However, contemporary supporting agents focuses on an instant relief rather deep curing. Another side, synthetic agents may develop further complications after usage. Some of the marketed products, which may contain natural extracts either rapidly eliminate acetaldehyde from the blood by enhancing ALDH activity or they reduce the acetaldehyde formation in the body after the alcohol consumption [9]. Considering all the above mentioned facts and characteristics properties, this study was mainly designed to prepare an health supplement from turmeric, black pepper, pomegranate and moringa extracts based on preliminary screening of antioxidant activities (DPPH and FRAP assays-Data not shown). The work was aimed to evaluate the supplement against alcohol- intoxicated and oxidative damaged HepG2 cell lines. An acute toxicity study was performed on rats to get the knowledge about a safe dose of supplement formula. To the best of our knowledge, this is a unique proprietary combination for treating alcohol-induced adverse effect and associated disorders.
Alcohol Dehydrogenase (ADH), Cytochrome P450 2E1 (CYP2E1), and catalase are bio-transformed alcohol to acetaldehyde, which further transformed to acetate by the mitochondrial Aldehyde Dehydrogenase (ALDH) enzyme and coming out from the liver to circulates into peripheral tissues. Non-oxidative pathway responsible for the esterification of alcohol, where alcohol is metabolized to Fatty Acid Ethyl Esters (FAEE) through enzyme FAEE synthase. Alcohol is also catalyzed by phosphatidylcholine-specific enzyme phospholipase D and form Phosphatidylethanol product through non-oxidative pathway.
Materials and Methods
Chemicals and reagents
MTT reagent 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide Phosphate-Buffered Saline (PBS), Trypsin and Antibiotics were purchased from Hi-media; Fetal Bovine Serum (FBS) and Dulbecco's Modified Eagle Medium-High Glucose (DMEM-HG) were acquired from Gibco (New York, USA). 12-well cell culture cluster, 96-well cell culture cluster, T-25 cm2 cell culture flask were purchased from GBO (Greiner Bio One, Austria). Standards e.g. mixture of curcumin, desmethoxycurcumin and bisdesmethoxycurcumin, ≥95.0% Piperine and Isoquercetin were procured from Sigma Aldrich (Germany). Punicalagin α and β were procured from PhytocomoundsTM, Natural Remedies Pvt. Ltd. (Bangalore, India). Phytoconstituents were standardized on fully automated latest Shimadzu HPLC system (Prominence-i, LC 2030C 3D Plus), equipped with variable wavelength PDA detector. All the chemicals, reagents, and buffers of analytical grade were employed.
Development of extracts and polyherbal mix
Health supplement Re-partyTM further referred as, “PHM” which contains herbal ingredients was prepared by using four herbs with respective their five enriched extracts. Turmeric (Curcuma longa L.) rhizome extract and black pepper (Piper nigrum L.) fruit extracts (Powder and essential oil), were mixed with pomegranate (Punica granatum L.) fruit pericarp extract and moringa (Moringa oleifera Lam) leaves extract. The extracts were prepared using Synthite’s trademarked Cold-Crafting technology. The individual extract was selected based on their screening potentials e.g. DPPH and FRAP assays (Data not shown). Extracts were homogenized in a Silverson L5M-A Laboratory mixer and were subjected to process ultra-fine grinding VextranoTM to arrive at reduced particle size. The resultant solution was spray-dried using maltodextrin as a carrier to yield a fine dry powder, “PHM” which was dissolved in methanol, DMSO and water according to biological and analytical evaluations.
HepG2 cell line and in vitro cells protective studies
Preparation of test solution for cytotoxicity studies: PHP (10mg) was dissolved separately and volume was made up with Dulbecco's Modified Eagle Medium-High Glucose (DMEM-HG) with 2% inactivated Fetal Bovine Serum (FBS) to obtain a stock solution of 1mg/mL concentration and it was sterilized through a 0.22 µmol syringe filter. Six doses of test solutions from 1000-31.25µg/mL were prepared by a two-fold serial dilutions method.
Cell line and culture medium: HepG2 (Human Hepatoma Cells) were procured from National Centre for Cell Sciences (NCCS), Pune, India. Stock cells were cultured in DMEM-HG supplemented with 10% inactivated FBS, penicillin (100IU/ml), streptomycin (100µg/ml) and amphotericin B (5µg/ml) in a humidified atmosphere of 5% CO2 at 37°C until confluent.
Determination of Cytotoxicity by MTT assay: The Microculture Tetrazolium Assays (MTT) assay was performed to determine cellular metabolic activity as it is an indicator of cell viability, proliferation, and cytotoxicity [10]. Briefly, the monolayer cell culture was trypsinized and the cell count was adjusted to 1.0 x 105 cells/ml using DMEM (HG) containing 10% FBS. To each well of 96 well microtiter plate, 0.1ml of the diluted cell suspension (approximately 10,000 cells) was added. After 24 h, once a partial monolayer formed, the supernatant was flicked off; monolayer was washed with medium and finally 100µl of different concentrations of tested PHM was added individually into the partial monolayer of microtiter plates. The plates were incubated for 3 days at 37°C in a 5% CO2 atmosphere, and microscopic observations were carried out at every 24h interval. At the end of incubation period (after 72h), solutions of PHM in the wells were discarded and 50µl of 3-(4, 5-dimethythiazol- 2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) prepared in PBS (yellow colour) was added to each well. The plates were gently shook and incubated for 3 h at 37°C in a 5% CO2 atmosphere. After 3h, the supernatant was removed and 100µl of alcohol was added to solubilize the formed formazan. Resultant plate was incubated for 30min at room temperature with constant shaking. The absorbance [Optical Density (OD)] was measured using a Microplate Auto-Reader (Bio-Tek Instruments, Inc., Winooski, VT) at a wavelength of 540nm. At the end of the period, cytotoxicity was assessed by estimating % growth inhibition of HepG2 cells [11].
% Growth inhibition of cells = (Mean OD of normal control - Mean OD of test group/Mean OD of normal control) × 100
A dose-response curve was generated using % growth inhibition on Y-axis and the extract concentration (μg/ml) on X-axis. The dose-response curve was plotted to generate concentration of test sample, needed to inhibit 50% cell growth (CC50 value).
Cells protective activity against alcohol-intoxicated HepG2 cells
The protective activity of PHM was evaluated by using well-maintained HepG2 cells. Alcohol was used as toxicant with stressed toxic concentration 250μM. The choice of concentrations of PHM were based on the results of the MTT assay. The experiment was performed as per the standard protocol [11,12]. Different non-toxic concentrations of PHM, substances (250 and 500µg/ml) prepared in DMEM and FBS except control one. The plates were incubated for 24h at 37°C with an atmosphere of 5% CO2. After incubation, cell supernatants were collected into micro tubes and were stored at -20°C for biochemicals estimation (AST, ALT, and LDH) by using standard kits. The groups were assigned as follows:
Group I (Control): HepG2 Cells + 100µL culture media as vehicle control.
Group II (Toxicant Control): HepG2 Cells + 100µL culture media containing 250µmol alcohol for induction of toxicity (24h).
Group III (PHM 250µg/mL): HepG2 Cells + 100µL culture media containing 250µmol alcohol + PHM 250μg/mL (100µL).
Group IV (PHM 500µg/mL): HepG2 Cells + 100µL culture media containing 250µmol alcohol + PHM 500μg/mL (100µL).
Antioxidant studies
DPPH free radical scavenging assay: The DPPH free radical scavenging assay was performed in triplicate as per earlier reported method [13]. Ascorbic acid was kept as standard [14].
Finally scavenging activity was determined by using the following equation:
% Inhibition of DPPH radicals = [(Absorbance of Control- Absorbance of Test)/ Absorbance of Control] ×100.
Reducing Power (RP) assay: The assay was performed as per the method described previously [15,16].
The reducing power activity of the blend of plant extracts “PHM” was calculated as follows:
% Reducing Potential = [(OD of Control- OD of Test)/ OD of Control] ×100
In vivo safety dose study
Experimental animals: An experiment was designed according to the standard protocol OECD guidelines 423 [17]. Healthy young adult female (nullifiers and non-pregnant) female albino rats (Wistar strain-total nine) were selected for the study. Three animals were used for initial step. Animals were housed under temperature 22±3°C, relative humidity 50-70%, and 12h light and 12h dark cycle. The diet and drinking water were free from any contaminant and provided ad libitum. All the experimental procedures and protocols used in this study was reviewed and approved by the Institutional Animal Ethics Committee (IAEC- RR/IAEC/65-2019) of Radiant Research Services Pvt. Ltd., Bangalore (Regd no: 1803/PO/RcBi/S/2015/CPCSEA) constituted in accordance with the guidelines of the CPCSEA, Government of India.
Dose levels and acute toxicity study: The test substance PHM was administered in a single dose by oral gavage. Animals were fasted prior to dosing. After administering test substance, food was withheld for 3-4h. The dose level to be used as the starting dose is selected from one of four fixed levels, 5, 50, 300 and 2000mg/kg body weight. Mortality was unlikely at the highest starting dose level (2000mg/kg body weight), and then a limit test was conducted [one dose level of 2000mg/kg body weight carried out with extra six animals (three animals per step)]. All observations were systematically recorded for individual data. Based on overall observation, results were reported.
Statistical analysis
Data were expressed as Mean ± SD of each group (n=3) for antioxidant and acute toxicity analysis using Window-10 (Microsoft Corporation, Redmond, WA, USA). Statistical analysis for hepatoprotective activity was done using software GraphPad Prism latest version 8.3.1 with one-way Analysis of Variance (ANOVA).
Phytochemical analysis
HPLC analysis: Individual botanical extracts of PHM were standardized through HPLC analysis performed on the Shimadzu HPLC system (Prominence-i, LC 2030C 3D Plus) and analyzed by LabSolutionsTM software version 5.96.
Turmeric (Curcuma longa L.) rhizome extract: Chromatographic separations of curcuminoidscurcumin, desmethoxycurcumin, and bisdesmethoxycurcumin were achieved isocratically on Capcell PAK C18, type MG-II column (250mm × 4.6mm i.d., 5μm particle size) referring USP-NF method [18], with slight modification. Reference standard and samples for analysis (1mg/ml) were prepared separately and further diluted with mobile phase to prepare 100ppm solution for the injection. The targeted curcuminoids were identified by observing specific peaks at suited retention times at detection wavelength 420nm.
Black pepper (Piper nigrum L.) fruit extracts: Content of piperine in Black pepper extract was standardized and confirmed through HPLC method described by International Standard Organization [19]. Working standard: 25mg of Piperine was weighed accurately into a 50ml volumetric flask with 5ml of acetonitrile to dissolve it completely. The volume was adjusted with mobile phase. 5ml of this solution was transferred to a 50ml volumetric flask, added acetonitrile, diluted to make up the volume. Sample preparation was done same as like standard with 1g of black pepper extract. The targeted Piperine present in sample was identified by observing specific peaks on retention times 15.13min at detection wavelength 343nm.
Pomegranate (Punica granatum L.) fruit pericarp extract: Punicalagin and ellagic acid were selected for the standardization of punica extract. Chromatographic separations and quantification were achieved by gradient elution on Shimpack C18 column (250mm × 4.6mm i.d., 5μm particle size). Solvent A was prepared with 1mM of anhydrous potassium dihydrogen orthophosphate (KH2PO4) adjusted to pH 3 by 88% orthophosphoric acid. Solvent B was acetonitrile. The both solutions were filtered through 0.45μm membrane filter and sonicated them for 3 minutes. Clear separation of peaks were achieved by using the following gradient elution program (time in min, B in %; 0.01, 10%; 10, 15%; 20, 30%; 25, 50%; 30, 80%; 35, 40%; 40, 10% 45, 10%). Flow rate of the mobile phase was 1ml/min at 25°C. Aqueous and methanol solution of punicalagin and ellagic acid were injected (20μl) in to the system to get the standards chromatograms at the concentration of 100 and 50PPM respectively. Sample solutions (2000PPM) were also prepared similarly, and injected separately to obtained extract chromatograms. Sample peak areas were compared with standards peak areas and quantified at specific detection wavelength λ=254nm.
Moringa (Moringa oleifera Lam) leaves extract: Standardization of moringa leaf extract was accomplished by considering isoquercetin (3-O-glucoside of quercetin) as standard compound. Quantification was performed by gradient elution on a same column as mentioned. Mobile phase and other conditions were also the same except the elution program, which was as follows (time in min, B in %; 0.01, 5%; 8, 5%; 12, 20%; 15, 20%; 18, 40%; 21, 40%; 25, 70% 30, 70%; 35, 5%; 40, 5%). The flow rate of the mobile phase was set as 1ml/min at 25°C. Separations was carried out in standard conditions and peak was detected in λ=254nm. Standard chromatogram was obtained by injecting (10μl) Isoquercetin in methanol (500PPM). Sample was also prepared (2000PPM) in methanol and injected to get specific peaks area for comparison and quantification against standard chromatogram.
GC analysis of black pepper essential oil: The GC/FID analysis of the black pepper essential oil was analyzed using gas chromatograph 7890 B (Agilent Technologies, USA) equipped with a Flame Ionization Detector (FID). The separation was achieved by using column, HP-5 (5% phenyl methyl siloxane), 30m × 320µm ID, i.e. 0.25μm film thickness. The GC oven temperature was programmed as follows: 80°C for 3min, increased from 80°C to 180°C at 5°C/min for 10min; Run time 33min. The carrier gas was Nitrogen (99.99%) at a flow rate of 1.0ml/min; the injector and detector temperatures were 180°C and 200°C, respectively. Detector gas flows hydrogen, air were 30, and 300ml/min respectively to get makeup flow 13ml/min. The samples 0.2μl was injected using the split ratio 100:1. Identification of components was done based on retention index and by comparison with reference standard.
Macro and Micronutrient analysis: Essential elements including macro and micro elements are present in the human body at very low concentration but they play specific roles and clinical influences significantly on all body functions. Six essential elements were selected for the study. Macro elements namely, Calcium (Ca), Magnesium (Mg), were analysed by Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES) instrument (iCAP 6300 Duo; Thermo Scientific; Mass., United States), whereas micro elements such as Copper (Cu), Iron (Fe), Zinc (Zn), and Selenium (Se) were analysed by using inductively coupled plasma-mass spectrometry (ICP-MS; NexIon 1000; Perkin Elmer; Mass., United States). Presence of essential elements in the PHM were detected according to the standard AOAC Official method 985.01 [20].
Results
Cytotoxicity level of HepG2 Cells
Active metabolites of viable HepG2 Cells convert MTT into a purple colored formazan product with an absorbance maximum near 540nm. After the experiment, absorbance were recorded against different concentrations of test samples. Cytotoxicity concentration CC50 of PHM was recorded as 691.89±2.13μg/mL from linear equation y=0.0676x+3.2208 and regression analysis R²=0.9801 (Figure 2). It indicated that at a lower concentration, 50% cells were viable than control cells and hence, two different nontoxic concentrations (250 and 500μg/mL) were considered for further studies. Cytotoxicity concentration of alcohol (CC50) was found to be 44.86±2.7% at a low concentration level (250µmol) and considered as a toxicant control for the experiments.
 Figure 2: Cytotoxic effect of the PHM on HepG2 Cells.
Figure 2: Cytotoxic effect of the PHM on HepG2 Cells.
Note: A dose-response curve-% growth inhibition on Y-axis and the extract concentration (μg/ml) on X-axis. Cytotoxicity concentration CC50 of PHM was recorded as 691.89±2.13μg/ml from linear equation and regression analysis.
Protective activities against alcohol-intoxicated HepG2 Cell lines
Treatment of test substances were offered dose-dependent HepG2 cells protection 27.95±1.4% and 70.63±8.7% at 250 and 500µg/mL respectively. Viability (%) of cells over the control were found to be 57.4±0.61% and 76.54±3.9% at the concentration of 250 and 500µg/mL respectively. Percent viability over the control of alcohol was found to be 44.86±2.7%. Morphology of control, toxicant, and PHM treated HepG2 cells were examined under an inverted tissue culture microscope at magnifying power 40x and are presented in figure 3.
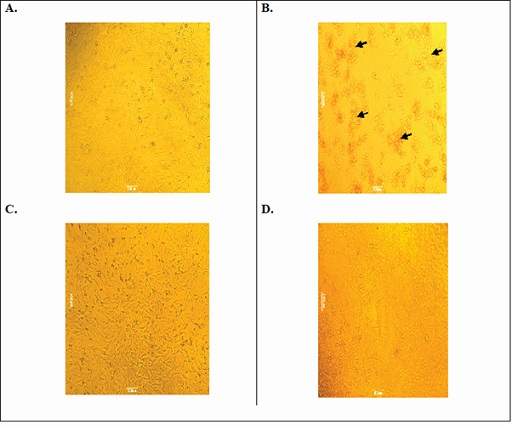 Figure 3: HepG2 cells under an inverted tissue culture microscope (40x). A: Control cell; B: Alcohol toxicant cells-accumulated cells highlighted by black arrow; C: PHM treated (250µg/ml) cells; D: PHM treated (500µg/mL) cells.
Figure 3: HepG2 cells under an inverted tissue culture microscope (40x). A: Control cell; B: Alcohol toxicant cells-accumulated cells highlighted by black arrow; C: PHM treated (250µg/ml) cells; D: PHM treated (500µg/mL) cells.
It’s reported that hepatocytes cells are damaged by toxicants by collapsing the cells structure and thus allowing leakage of cellular enzymes/biomarkers into the serum [21]. Treatment with test substance at two different dose levels normalized the biochemicals release (AST, ALT, and LDH) significantly (Figure 4); 500µg/mL of PHM was selectively more adequate as compared to 250µg/mL.
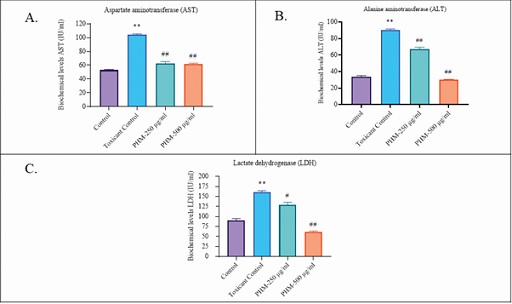 Figure 4: Biochemical levels. (A): Aspartate aminotransferase (AST); (B): Alanine aminotransferase (ALT); (C): Lactate dehydrogenase (LDH).
Figure 4: Biochemical levels. (A): Aspartate aminotransferase (AST); (B): Alanine aminotransferase (ALT); (C): Lactate dehydrogenase (LDH).
Note: One-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparisons test using Prism software (V. 8.3.1; GraphPad Prism, San Diego, CA, USA). Differences between means were considered significant at p<0.01.
*Toxicant control group compared with control group.
#Treated groups compared with toxicant control group.
Antioxidant activities
In the present study of DPPH assay, linear equation y=6864x+5.0226 R2=0.9997 and y=6332x+3.7842 R2=0.9989 were drawn through best-fitting straight line to calculate IC50 value for ascorbic acid and PHM respectively. PHM was exhibited dose-dependent DPPH scavenging activity with a 50% inhibition (IC50), at a concentration of 65.52±1.32µg/ml, which was observed very close to IC50 value of standard ascorbic acid (72.98±2.46 µg/ml) (Figure 5).
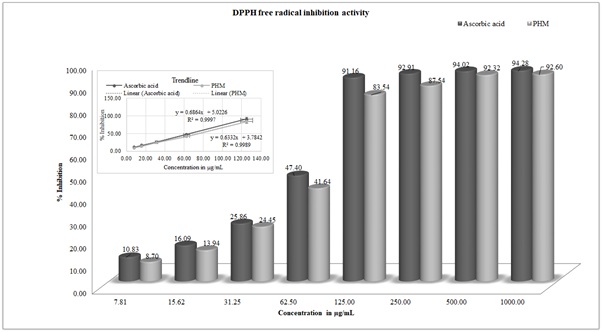 Figure 5: DPPH inhibition activity of Ascorbic acid and PHM at different concentrations.
Figure 5: DPPH inhibition activity of Ascorbic acid and PHM at different concentrations.
Note: Independent variable concentration (7.81 to 1000µg/ml) on X-axis and the percentage of scavenging effect as the dependent variable on Y-axis.
Similarly, in reducing power assay, the yellow color changed to pale green and/or blue color (Fe3+ to Fe2+) depending on the concentration of antioxidants, present in the sample. The antioxidants such as phenolic acids and flavonoids are presented in considerable amount in the blend therefore PHM was shown good reducing potential percentage (86.17%) at low dose 125µg/ml. PHM was followed the same pattern as like ascorbic acid. At very high concentration e.g. 1000µg/ml, both ascorbic acid and PHM were shown almost similar activities 97.75 and 97.16% inhibition respectively, whereas at very low concentration e.g. 31.25µg/ml (5th diluted solution), ascorbic acid was shown 47.62% inhibition in compared to PHM, which was found to be 41.31% inhibition (Figure 6).
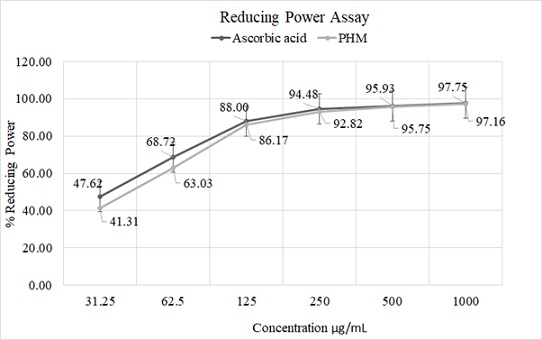 Figure 6: Antioxidant assays.
Figure 6: Antioxidant assays.
Note: Reducing Power (RP) activity of ascorbic acid and PHM; Independent variable concentration on X-axis and the percentage of reducing power (RP assay) as the dependent variable on Y-axis
Safety dose activity
The safety dose study revealed that the test substance did not produce any mortality throughout the study period of 14 days even when the limit dose was maintained at 2000mg/kg body weight with extra six animals. All the animals were appeared normal throughout the experimental period. No groups at a single oral dose of 2000mg/kg body weight were caused any death or clinical symptoms over a period of 14 days treatment (Table 1).
|
Animal Group |
Dose |
Treatment |
||||
|
Before |
After |
|||||
|
Day 0 |
Day 3 |
Day 7 |
Day 14 |
Day 14 |
||
|
RA 01 |
2000 mg/kg |
189.3±0.9 |
192.0±0.6 |
195.7±0.7 |
204.7±0.7 |
No macroscopic alteration occurred |
|
RA 02 |
||||||
|
RA 03 |
||||||
|
RA 04 |
Limit test 2000 mg/kg |
186.3±0.8 |
188.8±1.0 |
192.7±1.1 |
201.8±1.0 |
No macroscopic alteration occurred |
|
RA 05 |
||||||
|
RA 06 |
||||||
|
RA 07 |
||||||
|
RA 08 |
||||||
|
RA 09 |
||||||
Table 1: Body weight of Rats during the study.
Note: Values were expressed as Mean ± SEM.
Phytochemical analysis
HPLC Standardization: Curcuma longa: Total curcuminoids, i.e. curcumin, desmethoxycurcumin, and bisdesmethoxycurcumin were found to be 76.7%, 16.53% and 2.32% respectively (Figure 7(A and B)). Piperine shown a specific peak at retention time 15.13 min and found to be 93.12% w/w in the extract (Figure 7(C and D)). Compared to standard, punicalagin (α and β) in the punica extract were found to be 5.88% with specific peaks at retention time 8.95 and 11.37min respectively (Figure 7(E and F)). Similarly compared to standard, ellagic acid was showed specific peaks at 20.90min and was found to be 2.72% (Figure 7(G and H)). In moringa extract, isoquercetin was shown specific peaks at 21.79min and was found to be 2.25% in the extract (Figure 7(I and J)).
GC chromatogram: GC-FID analysis of black pepper essential oil was revealed the existence of main components β-caryophyllene retention time 17.87min with Area% 31.85 (Figure 7K). Other components were also recognized with their Area % e.g. d-limonene (13.18%), δ-3-carene (8.53%) sabinene (7.51) β-pinene (6.71%) and α-pinene (6.00%).
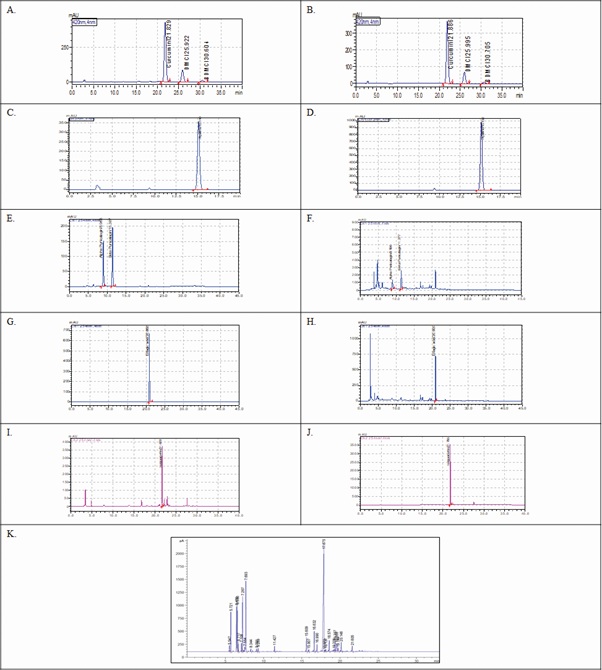 Figure 7: HPLC chromatograms. (A): Standard Curcuminoids; Curcumin (C), Demethoxycurcumin (DMC) and Bis-demethoxycurcumin (BDMC) on 420 nm; (B): Curcuma longa extract Curcuminoids; C, DMC and BDMC showed specific peaks at retention time 21.88, 25.99 and 30.70min respectively; (C): Standard Piperine on 343 nm; (D): Piper nigrum extract; Piperine showed specific peak at retention time 15.13min; (E): Standard Punicalagin α and β on 254nm; (F): Puniuca granatum extract; Punicalagin α and β showed specific peaks at retention time 8.95 and 11.37min respectively; (G): Standard ellagic acid on 254nm; (H): Puniuca granatum extract; ellagic acid showed specific peaks at retention time 20.90 min; (I): Standard Isoquercetin on 254nm; (J): Moringa oleifera extract; Isoquercetin showed specific peaks at retention time 21.79min; GC chromatogram (K): A typical chromatogram of black pepper essential oil.
Figure 7: HPLC chromatograms. (A): Standard Curcuminoids; Curcumin (C), Demethoxycurcumin (DMC) and Bis-demethoxycurcumin (BDMC) on 420 nm; (B): Curcuma longa extract Curcuminoids; C, DMC and BDMC showed specific peaks at retention time 21.88, 25.99 and 30.70min respectively; (C): Standard Piperine on 343 nm; (D): Piper nigrum extract; Piperine showed specific peak at retention time 15.13min; (E): Standard Punicalagin α and β on 254nm; (F): Puniuca granatum extract; Punicalagin α and β showed specific peaks at retention time 8.95 and 11.37min respectively; (G): Standard ellagic acid on 254nm; (H): Puniuca granatum extract; ellagic acid showed specific peaks at retention time 20.90 min; (I): Standard Isoquercetin on 254nm; (J): Moringa oleifera extract; Isoquercetin showed specific peaks at retention time 21.79min; GC chromatogram (K): A typical chromatogram of black pepper essential oil.
Micronutrient detection
The quantitative determinations were carried out using standard calibration curve, drawn by the AAS data obtained from the standard solution of metals having optimal detectable concentration ranges. Concentration of the metals were expressed in terms of parts per million; ppm [22] and presented in table 2.
|
Type |
Name |
Symbol |
Unit of Measurement in |
|
|
PPM |
% |
|||
|
Macro- Elements |
Calcium |
Ca |
6190 |
0.619 |
|
Magnesium |
Mg |
2782 |
0.278 |
|
|
Micro- Elements |
Copper |
Cu |
<10 |
<0.001 |
|
Iron |
Fe |
<40 |
<0.004 |
|
|
Zinc |
Zn |
<10 |
<0.001 |
|
|
Selenium |
Se |
<2 |
<0.0002 |
|
Table 2: Essential metal contents in PHM.
Note: *AHPA-The American Herbal Products Association.
**FSSAI-Food Safety and Standards Authority of India; (Contaminants, toxins and residue Regulation).
Discussion
In the designed MTT assay, CC50 was found to be 691.89±2.13μg/mL safe for the studies and hence two different doses were selected. 500μg/mL dose was shown >75% hepatoprotection and the same dose was potent enough to normalize the biochemicals release (AST, ALT, and LDH). It specified that the PHM has potency to protect liver cells. All these findings strongly were supported that PHM can alter morphological changes and reversing the structure of affected HepG2 cells. PHM was exhibited dose-dependent DPPH inhibition activity. The value was very close to that of ascorbic acid, which indicated that the PHM would be a good formula for the proposed claim. More than 90% of DPPH inhibition activities was observed after the concentration of 125µg/mL. Likewise, PHM was also displayed significant reducing potential at a low dose of 125µg/mL. All these promising results were substantiated the use of PHM for treating ROS induced cells toxicity. Acute toxicity study revealed a safe dose of PHM. All the phytochemicals present in PHM were standardized and were quantified using HPLC system and oil was quantified by using GC-FID system.
Phytochemical point of view, Turmeric is one among, the most popular and explored herb, used as a spice, food colouring agent, and traditional medicine with a wide range of pharmacological activities due to its potential to act on a number of biological targets with no major side effects. The therapeutic attributes of turmeric are mainly due to the presence of curcuminoids, a group of phenolic compounds namely curcumin, demethoxycurcumin and bisdemethoxycurcumin (Figure 8A). These molecules are well recognized for their anti-inflammatory activities by altering the mechanism NF-kB and MAPK pathways. Black pepper, the Indian spice has been used as a culinary ingredient from ancient times and is extensively used in the traditional practice of medicines [24]. The major phytochemical, piperine an alkaloid (Figure 8B) is proven to improve the serum bioavailability of therapeutic agents by inhibiting intestinal and hepatic glucuronidation [25]. Another effective molecule β-Caryophyllene (Figure 8C) is having high potential for treating or preventing hepatic injury associated with oxidative stress, inflammation and steatosis. It is having the ability to act on CB2 receptors and thereby relieving the physiological and mental stress like anxiety and depressive disorders, which are closely associated with alcohol intoxication and accompanying complications [26]. Pomegranate peels extract, a novel source of bioactive phytochemicals was considered in the blend since it is having a significant amount of phenolic contents compared to other parts of the fruit. Peel is a rich source of ellagitannins and derivatives such as punicalagin, ellagic acid (Figure 8(D and E)), etc. All these phytochemicals are identified for its anti-inflammatory, and gastro-protective activities [27]. Miracle tree “Moringa” leaves are universally known for its micronutrient properties and potential to maintain electrolyte concentration. Supplementing with micronutrients from moringa extract is key to maintain a balanced serum osmolarity. It was considered in our formula to replenish and to prevent excess loss of micronutrients due to alcohol-induced diuresis [28]. Moringa leaves are having polyphenol isoqurcetin (Figure 8F), which also possess liver protective potential [29]. The results from all these studies are supported physio-pharmacological characteristics of the phytochemicals present in our proprietary formula.
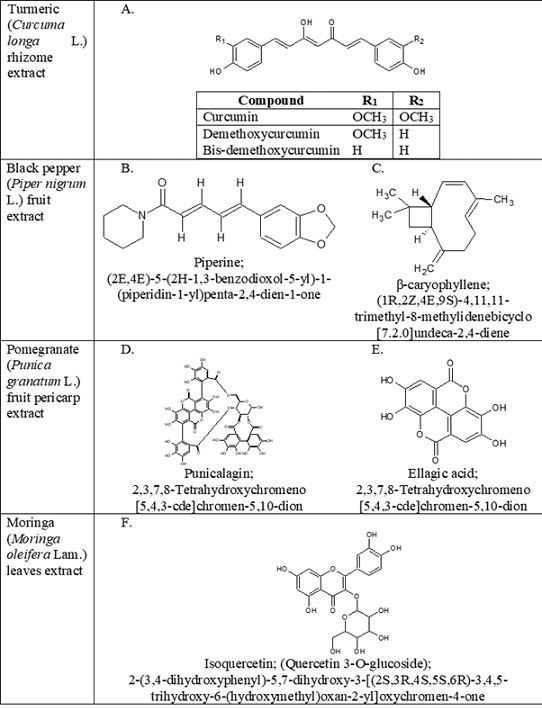 Figure 8: Chemical structures of phyto-constituents. (A): Curcuminoids; (B): Piperine; (C): β-caryophyllene; (D): Punicalagin (α and β); (E): Ellagic acid (F): Isoquercetin.
Figure 8: Chemical structures of phyto-constituents. (A): Curcuminoids; (B): Piperine; (C): β-caryophyllene; (D): Punicalagin (α and β); (E): Ellagic acid (F): Isoquercetin.
Conclusion
The outcome of our studies elucidates that herbal supplement, comprising five standardized extracts has immense protective potential on alcohol-intoxicated HepG2 cells. In addition, its free radical scavenging activities along with a considerable amount of reducing potential as well as the ability to normalize hepatic biochemicals make the supplement a suited solution for treating alcohol-induced toxicity and associated disorders like hepatotoxicity, hangover, and nutrient balance. Promising results from acute toxicity studies on animals ensures that the developed poly herbal mix extracts Re-partyTM is a safe formulation for oral consumption. However, alcohol and aldehyde dehydrogenase activities and clinical investigation to be needed to support and endorse a complete mechanism of action to prove the above in the near future.
Acknowledgment
The authors wish to express their gratitude to the Radiant Research Services Pvt. Ltd., Bangalore, India for conducting the acute toxicity study. The authors also acknowledge the Management of Synthite Industries Pvt. Ltd. for their support and inspiration. The authors also gratitude to the QC and CVJ Creative Centre, Instrumentation team for their analytical support and coordination.
Author Disclosure Statement
The authors declare that there was no conflict of interest with any commercial associations.
Ethical Statements
All the experimental procedures and protocols of this study were reviewed and were approved by the Institutional Animal Ethics Committee (IAEC- RR/IAEC/65-2019) of Radiant Research Services Pvt. Ltd., Bangalore (Regd. no: 1803/PO/RcBi/S/2015/CPCSEA) constituted in accordance with the guidelines of the CPCSEA, Government of India. The experiment procedures were followed in accordance with the OECD guideline 423 published in 2002.
References
- Murray CL, Lopez AD, Jamison DT (1994) The global burden of disease in 1990: Summary results, sensitivity analysis and future directions. Bull World Health Organization. 72: 495-509.
- Harburg E, Gunn R, Gleiberman L, DiFranceisco W, Schork A (1993) Psychosocial factors, alcohol use, and hangover signs among social drinkers: A reappraisal. J Clin Epidemiol 46: 413-422.
- Rehm J (2011) The risks associated with alcohol use and alcoholism. Alcohol Res Health 34: 135-143.
- Rehm J, Samokhvalov AV, Neuman MG, Room R, Parry CD, et al. (2009) The association between alcohol use, alcohol use disorders and Tuberculosis (TB). A systematic review. BMC Public Health 9: 450.
- Kawaratani H, Tsujimoto T, Douhara A, Takaya H, Moriya K, et al. (2013) The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm 2013: 495156.
- Hammer JH, Parent MC, Spiker DA (2018) Mental Help Seeking Attitudes Scale (MHSAS): Development, reliability, validity, and comparison with the ATSPPH-SF and IASMHS-PO. Journal of Counseling Psychology 65: 74-85.
- Li S, Gan L, Li S, Zheng J, Xu D, et al. (2014) Effects of herbal infusions, tea and carbonated beverages on alcohol dehydrogenase and aldehyde dehydrogenase activity. Food and Function 5: 42-49.
- Swift R, Davidson D (1998) Alcohol hangover: Mechanisms and mediators. Alcohol Health Res World 22: 54-60.
- Srinivasan S, Dubey KK, Singhal RS (2019) Influence of food commodities on hangover based on alcohol dehydrogenase and aldehyde dehydrogenase activities, Current Research in Food Science 1: 8-16.
- Denizot F, Lang R (1986) Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability, Journal of Immunological Methods. 89: 271-277.
- Pareek A, Godavarthi A, Issarani R, Nagori BP (2013) Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) Hadidi extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. J Ethnopharmacol 150: 973-981.
- Sathaye S, Bagul Y, Gupta S, Kaur H, Redkar R (2011) Hepatoprotective effects of aqueous leaf extract and crude isolates of Murraya koenigii against in vitro ethanol-induced hepatotoxicity model. Exp Toxicol Pathol 63: 587-591.
- Alam MN, Bristi NJ, Rafiquzzaman M (2013) Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J 21: 143-152.
- Kumar KE, Harsha KN, Sudheer V, Giribabu N (2013) In vitro antioxidant activity and in vivo hepatoprotective activity of aqueous extract of Allium cepa bulb in ethanol induced liver damage in Wistar rats. Food Science and Human Wellness 2: 132-138.
- Jayaprakasha GK, Singh RP, Sakariah KK (2001) Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chemistry 73: 285-290.
- Oyaizu M (1986) Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. The Japanese Journal of Nutrition and Dietetics 44: 307-315.
- OECD Guidelines (2002) OECD Guidelines for the Testing of Chemicals, Section 4. OECD Publishing, Paris, France.
- USP-NF, USP-NF (2013) Pharmacopeia and National Formulary (USP 36-NF 31).
- ISO (1993) ISO 11027: 1993, Pepper and pepper oleoresins-Determination of piperine content- Method using high-performance liquid chromatography. International Standard.
- Pacquette LH, Thompson JJ, Malaviole I, Zywicki R, Woltjes F, et al. (2018) Minerals and trace elements in milk, milk products, infant formula, and adult/pediatric nutritional formula, ICP-MS method: collaborative study, AOAC final action 2015.06, ISO/DIS 21424, IDF 243. Journal of AOAC International 101: 536-561.
- Nikam P, Nikam S, Sontakke A, Khanwelkar C (2009) Biochemical changes in alcoholic hepatitis with phyllanthus amarus therapy: A study. Biomedical Research 20: 192.
- Nema NK, Maity N, Sarkar BK, PK (2014) Mukherjee, determination of trace and heavy metals in some commonly used medicinal herbs in Ayurveda. Toxicology and Industrial Health 30: 964-968.
- Holford NH (1987) Clinical pharmacokinetics of ethanol. Clin Pharmacokinet 13: 273-292.
- Tomeh MA, Hadianamrei R, Zhao X (2019) A review of curcumin and its derivatives as anticancer agents. Int J Mol Sci 20: 1033.
- Atal CK, Dubey RK, Singh J (1985) Biochemical basis of enhanced drug bioavailability by piperine: Evidence that piperine is a potent inhibitor of drug metabolism. J Pharmacol Exp Ther 232: 258-262.
- Varga ZV, Matyas C, Erdelyi K, Cinar R, Nieri D, et al. (2018) β-Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. Br J Pharmacol 175: 320-334.
- Danesi F, Kroon P, Saha S, de Biase D, D’Antuono L, et al. (2014) Mixed pro- and anti-oxidative effects of pomegranate polyphenols in cultured cells. Int J Mol Sci 15: 19458-19471.
- Adinoyi SS (2017) Role of Moringa oleifera on electrolytes levels and cardiovascular function in human. Thera-Peutic Advances in Cardiology 1: 76-82.
- Huang XL, He Y, Ji LL, Wang KY, Wang YL, et al. (2017) Hepatoprotective potential of isoquercitrin against type 2 diabetes-induced hepatic injury in rats. Oncotarget 8: 101545-101559.
Citation: Nema NK, Sarojam S, Khamborkar SD, Sajan LC, Sabu S, et al. (2021) Novel Health Supplement for Recovery of Alcohol Induced Adverse Effects and Associated Disorders: HepG2 Cells Study and Safety Dose Profiling on Rats. J Food Sci Nutr 7: 121.
Copyright: © 2021 Neelesh Kumar Nema, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

