
Nutritional Evaluation of Isolates from Two (2) Varieties (DAS & BS) of Underutilized Germinated Nigeria Cultivated Solojo-Cowpea [Vigna Unguiculata (L.) Walp.]
*Corresponding Author(s):
Henry O ChibudikeDepartment Of Chemical, Fiber And Environmental Technology, Federal Institute Of Industrial Research, Oshodi, Nigeria
Tel:+234 8023225788,
Email:henry.chibudike@fiiro.gov.ng / henrychibudike@gmail.com
Abstract
Nutritional properties of raw flour and total protein isolates of the two varieties i.e. Dark-Ash Solojo (DAS) and Brown Solojo (BS) of Nigeria cultivated Cowpea (Vigna unguiculata) were investigated before and after dehulling of the germinated seeds for their proximate composition, major mineral elements and carbohydrate fractions. Ungerminated seeds were used as the control. Protein isolates from dehulled defatted solojo Cowpea seeds were prepared using isoelectric (CPIA) procedure. Dehulled samples had a higher protein solubility compared with germinated and control samples. Both varieties of cowpea (DAS and BS) investigated were soaked in distilled water and germinated at varying periods i.e. 0, 6, 24, 36, 48 and 72hrs. Proximate analysis gave 75% crude protein, 2.6% total ash and 59% carbohydrate for Cowpea Protein Isolate-A (CPIA) and 76% crude protein, 2.3% total ash and 13.1% carbohydrate for Cowpea Protein Isolate-B (CPIB). Functional properties were analyzed which include Moisture content, crude protein, crude fat, crude fibre and total ash of DAS ranged from 9.00-11.40, 24.82-31.00, 1.56-2.66, 1.43-1.67, and 3.20- 4.14%, respectively; while those of BS flours ranged from 7.10-9.50, 24.90-30.14, 1.17-2.37, 1.06-1.52 and 3.05-3.93%, respectively. The protein contents for DAS were 81.57±0.53, 86.44±0.84, 89.39±1.51, 90.23±0.53, 91.81±0.77 and 94.85±0.86, while for BS were 84.39±0.39, 85.44±0.56, 90.05±0.10, 90.47±0.89, 92.78±0.28 and 95.81±0.19% for 0, 6, 24, 36, 48 and 72hrs, respectively. Bulk density ranged between 0.69 and 0.80g/dm3. Water and oil absorption capacities ranged between 1.89 and 2.15, and 1.95 and 2.31ml/g, respectively. Swelling power had values varying from 265 to 268% while foam capacity varied from 10.00 to 21.00ml. Surface morphology, functional group and thermal properties were determined for protein isolates by scanning electron microscopy, Fourier Transform Infrared (FTIR) spectrometry and differential scanning calorimetry, respectively. Data were analyzed using design expert software and Analysis of Variance (ANOVA) was carried out at α 0.05. The results indicate that the two varieties of cowpea have great potential as functional agents in the food industry.
Keywords
BS; DAS; Functional; Nutritional composition; Protein isolate; Solojo Cowpea; Under-utilized legumes
Introduction
Determination of the quality of materials, overall acceptability of the product by consumers and nutritional value establishment are all hinged on proximate analysis of the product [1]. The nutritional chemical analysis of both raw and germinated seed flours of full fat and defatted dark-ash and brown solojo cowpea (FFDAS, FFBS, DFDAS and DFBS) varieties, as well as that for DAS and BS isolates are as shown in Tables.
Storage stability of any food is established by determining the moisture content directly or by ascertaining the dry matter quantity of the sample indirectly. Germination caused the moisture content to decrease with time from Raw (Control) to germinated beans, this observation has similarity to that expressed by Rahma et al. [2], for Lima beans, where the amount of moisture ranged between 4 to 13%. Ghavidel and Prakash [3], also reported similar observation for lentil, cowpea and chickpea germinated seed flours with decrease in moisture content after removing the hulls. Desalegn [4], in his study, on chickpea flours, also observed an initial increase in moisture content with soaking which later reduced on germinating from 7.69% to 7.30%. On the contrary, observation of increase in moisture content of two varieties of tiger nut with germination from 7.14 to 9.98% was made by Chinma et al. [5]. Likewise, D’souza [6], made similar observation of increase in moisture for field bean with germination, going from 5.23-11.35 after 60h of germination, this was adduced to increased number of cells for hydration and low dry matter content. Observation of moisture content range from 9.19% to 11.83% for five lima bean varieties was made by Yellavila et al. [7], with ‘Koloenu brown’ having the least moisture content while ‘Nsawam black and white’ recorded uppermost moisture quantity. Moisture contents of the present study fall within the approved range for flours (<14%) [2]. The defatted samples were also noticed to have larger moisture content than the full fat, as was also expressed by [8,9]. In all, low moisture content of less than 14% is recommended for greater resistance to microbial growth, better storability and prevention of the development of hydrolytic rancidity [7,9].
The appreciable increase in protein quantity observed in sprouted Solojo Cowpea could be ascribed to increased formation of some amino acids from protein degradation during sprouting [10]. Chinma et al. [5], also observed a similar increase in protein content of sprouted brown tiger nut from 10.6-12.4% after 48h sprouting. Myrene [11], deduced that significant increase in protein content could be ascribed to improved water activity as a result of activation of hydrolytic enzymes. It could also be due to hormonal changes, according to Nenogaki et al. [12], or a component change resulting from the degradation of other constituents [13]. While Kavitha and Parimalavalli [14], also surmised the increase could be as a result of formation of enzyme proteins.
A comparable increase in protein content was also reported in Ugba (African oil bean) [15], Mungbean [16]; Field beans [6], and Australian sweet lupin [17], upon germination. Devi et al. [18], also detected notable rise in crude protein after malting in all the three accessions of cowpea they worked upon. This observed increase in protein quantity may, according to them, be associated with loss in dry matter, especially carbohydrates due to respiration during malting. Similarly, Zhang et al. [19], observed significant increase in protein content of buckwheat after germination for 72h, this is probably due to higher rate of protein synthesis compared to proteolysis [20]. On the contrary, El-Adawy et al. [21], observed a reduction in protein content after germinating for 120 h, mung bean, going from 26.40 to 22.52%; Pea from 34.70 to 30.73%; and lentil from 31.41 to 28.37%. Murugkar et al. [22], alluded the rise in protein observed in the nutrient of their mixes to compensative increment in free amino acids and peptides.
Fat, a major component, which is also an avenue of production of nutritional and biologically active compounds such as fatty acids of the mono- and polyunsaturated class, tocopherols and phytosterols, reduced with germination time for both the flour and the isolate. Several researchers have reported the degradation of fat as a result of germination process. Comparable results were obtained for Soya bean, Mungbean, Sesame and three- genotypes of Cowpea on germination [18,23,24].This reduction in oil content on malting, may be connected to its utilization as a source of energy in malting process [22], energy for germination is obtained through the oxidation of fatty acids to carbon dioxide and water [23]; could also be as a result of enhanced lipolytic enzymes activities during germination [25]. The decrease in fat content is equally very good for shell life stability. The germinated flour and isolate will be able to last longer on the shelf than the ungerminated samples.
Ash content generally reduced with germination, only the 48h protein isolate of DAS and the 6h isolate of BS had values greater than the control. This reduction in ash content was parallel to observation in Soybean; Mung bean and Sesame [6,14,26]. The reduction in content of ash may be as a result of mineral loss in water during washing in order to minimize the acerbic smell produced over the period of sprouting. On the converse, Chinma et al. [5], observed an increment in ash content for varieties of tigernut (Brown and Yellow). Devi et al. [18], also observed weighty rise in the ash content after germination in each varieties of cowpea improved genotype (PL-1, PL-2 and PL-3) used by them, which they surmised as probably due to loss of carbohydrate. The reduction in ash content observed in this project may be as a result of the leaching of both the macro and micro elements as a result of soaking.
The indigestible plant material capable of lowering the level of blood cholesterol, preventing cancer, reducing the hazard of developing hypertension, diabetes and hypercholesterolemia is the crude fiber [27]. The crude fibre of germinated FFDAS, FFBS and DFDAS generally reduced with germination, except for 72h for all of them and 24h FFDAS. While DFBS had its crude fibre increasing with germination except that of 36h which reduced. The experienced reduction is probably due to degradation of fibre into simple sugars brought about by endogenous enzymes. This is collaborated by other research work on chickpea, mungbean, kidney beans [16,28,29]. Enujiugha et al. [15], also observed a reduction in crude fibre with germination, with value going from 47.9% to 38.8%, for African oil bean. Likewise, Ramadan [30], had a similar observation for soybean which was attributed to reduction in indigestible dietary fibre during germination.
On the contrary, Chinma et al. [5], observed increase in crude fibre content with germination for the two tigernut varieties; Rumiyati et al. [17], also observed increase for Australian sweet lupin; and Borijindakul and Phimolsiripol [10], for Lablab. Rusydi et al. [24], during the study of germination effect on crude fibre for four legumes, observed, that the crude fibre of Kidney bean and Mung bean both decreased with germination, while that of soya bean and peanut increased with germination. Thus, it could be concluded that the effect of germination on crude fibre depended on the nature of the legume. Oghbaei and Prakash [31], observed decrease in fiber in soaked peanut, mung bean, wheat, and barley, but contrariwise increased in soaked soy bean and rice. This led to the conclusion that, fiber level is actually affected during the soaking period rather than at the germination proper [32].
The total carbohydrate quantity as Nitrogen free extractive was calculated by difference and was found to reduce with rise in germination time for the DAS flour and isolate, while the NFE of the BS variety of both flour and isolate increased with germination. The observed reduction was similar to the observation of D’souza [6], for field beans, Devi et al. [18], for three genotypes of cowpea. This decrease could be ascribed to the use of carbohydrate to give energy to the growing embryo for germination [11,15]. The complex carbohydrate is fragmented to smaller sugar molecules such as glucose and fructose needed by the growing seed by the increased activity of α-amylase at early stage of germination [11].
Materials And Methods
Two varieties of the underutilized cowpea (V. unguculata) found in Southwest region of Nigeria where it is called ‘solojo’ were used (Figures 1 & 2).
 Figure 1: Brown Solojo Cowpea.
Figure 1: Brown Solojo Cowpea.
 Figure 2: Dark-Ash Solojo Cowpea.
Figure 2: Dark-Ash Solojo Cowpea.
Seeds obtained from Bodija market in Ibadan, Western Nigeria, were screened to get rid of every irrelevant materials and unwholesome seeds. The beans were then portioned into six (6). The solojo seeds for germination were sterilized by soaking in 0.07% sodium hypochlorite for 30min, then, it was rinsed thoroughly. The solojo seeds were then immersed for 6h in distilled water at ambient temperature (1:10 w/v) (~25°), then placed in a colander and germinated under subdued light in an open laboratory for, 24h, 36h, 48h and 72h (Figures 3-5).
 Figure 3: Germinated Dark- ash Solojo Cowpea.
Figure 3: Germinated Dark- ash Solojo Cowpea.
 Figure 4: Germinated Brown Solojo Cowpea.
Figure 4: Germinated Brown Solojo Cowpea.
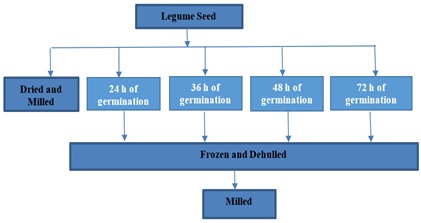
Figure 5: Preparation of Beans Flour/ Schematic representation.
Preparation of Flours
Raw Flour: The grains were segregated to remove the spoilt ones; then dry dehulled with a mechanical dry dehuller (fabricated in FIIRO), dried at 40°C and later milled dry to powder then sifted using 80µm mesh. The flour was stored in flexible bags and preserved at 4°C preceding utilization in a refrigerator freezer.
6h Soaked Flour: The seeds were segregated to remove the unwholesome ones, then immersed for 6h in the ratio (1:10 w/v) (seed/water). The grains were then frozen to prevent germination from setting in, then the hull was removed manually, dried for 48h at 40°C later milled dry to smooth powder prior to sieving using 80µm mesh screen. The resulting flour was packaged in plastic pack and preserved in a fridge freezer at 4°C pending utilization.
Germination of Seed: This was implemented by the method of Mubarak AE with minor adjustment. The seeds for germination were disinfected by soaking in 0.07% sodium hypochlorite for 30 mins, then, it was rinsed painstakingly. The solojo seeds were then immersed for 6 hours at ambient temperature in water in the ratio (1:10 w/v) (seed/water) (~25°C), then placed in a colander and germinated under subdued light in an open laboratory for various hours such as 24h, 36h, 48h and 72h. The process of germination was terminated by freezing; the seeds were manually dehulled, dried in a draught oven at 40°C for 48h, cooled, milled and packaged in an air tight plastic bag in the refrigerator pending analysis (Figure 6).
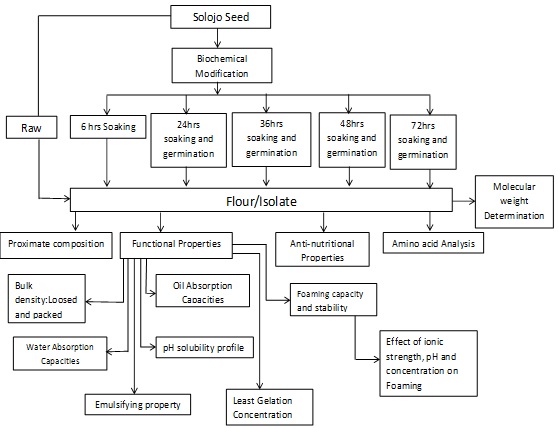 Figure 6: Schematic representation of treatment and analysis on sample flours and isolates carried out.
Figure 6: Schematic representation of treatment and analysis on sample flours and isolates carried out.
Results And Discussion
All over the world, there are different varieties of legume playing important roles in local food production. The greatest attribute of legumes is their high plant protein content which not only have superior nutritional and anti-oxidative properties, they have also been successfully used as nutraceutical ingredient.
Each of the two varieties (brown solojo cowpea and dark-ash solojo cowpea) investigated in this research study can be differentiated by their appearance and their taste. A lot of people turn to them for their source of protein, but then with this, one could get tired of having the same delicacy all the time. So if looking to switch up meal, here are four (4) delicacies that can be derived from them as shown in figures 7-10.
 Figure 7: Beans pudding.
Figure 7: Beans pudding.

Figure 8: Beans cake.

Figure 9: Boiled beans.

Figure 10: Beans porridge.
Figure 7 is beans pudding popularly known as Moin-Moin in Nigeria Yoruba language. This is a delicacy derived from brown cowpea described in figure 1, that has been washed, peeled and grinded to form a pudding. It is usually prepared with onions, fresh pepper and crayfish. Moin-moin is not only delicious and easy to make, it is one meal that can be consumed anytime of the day - breakfast, lunch or dinner.
Figure 8 is beans cake popularly known as Akara in Nigeria Yoruba language. This is another classic Nigerian food derived from figure 1, that is processed by washing, peeling and grinding just like it is done with moin-moin, but this time, the grinded beans is fried to make a delicious fritter. Akara is one savoury and crispy meal that is not only perfect for breakfast (especially Saturday mornings), it is also a delicious snack.
Figure 9 is boiled beans derived from figure 2, popularly known as Ewa woro in Nigeria Yoruba language. The most common delicacy derived from figure 2, is the Nigerian Beans porridge (Figure 10) which those from the southwest of Nigeria (Yoruba language) also call Ewa Riro. This is a delicious meal that includes the beans cooked with onions, pepper, salt and palm oil. This meal can be enjoyed with side dishes such as fried ripe plantain (dodo), pap or even garri (cassava starch).
Germination caused the moisture content to decrease with time from Raw (Control) to germinated beans. Moisture content reduced significantly (p < 0.05) with germination. This might be as a result of utilization of the water for metabolic processes initiated by soaking (Table 1).
|
FFDAS |
% Moisture |
% Protein |
% fat |
% Fibre |
% Ash |
NFE |
|
Raw |
11.40±1.44a |
24.82±2.80c |
2.66±0.08a |
1.67±0.03c |
4.14±0.42ab |
55.31±1.11a |
|
6h |
10.70±0.02a |
28.10±0.17b |
2.16±0.02b |
1.43±0.03e |
3.43±0.03ab |
54.18±0.03b |
|
24h |
9.00±0.30b |
30.40±0.02a |
1.37±0.56c |
1.87±0.03a |
3.37±0.02ab |
53.99±0.02bc |
|
36h |
9.50±0.05b |
30.50±0.03a |
2.12±0.03b |
1.59±0.02d |
3.20±0.02b |
53.09±0.03cd |
|
48h |
9.50±0.03b |
30.80±0.17a |
2.10±0.02b |
1.59±0.03d |
3.27±1.15b |
52.74±0.02d |
|
72h |
9.30±0.02b |
31.00±0.03a |
1.87±0.03b |
1.75±0.02b |
3.61±0.02ab |
52.47±0.01d |
Table 1: Proximate composition analysis of FFDAS flour.
Note: NFE- Nitrogen Free Extractive (Carbohydrate); FFDAS- Full fat dark ask Solojo
Means in columns not followed by same alphabet(s) are significantly different at 5% level (P < 0.05)
The appreciable increase in protein quantity observed in sprouted Solojo Cowpea could be ascribed to increased formation of some amino acids from protein degradation during sprouting (Table 2).
|
DFDAS |
% Moisture |
% Protein |
% Fat |
% Fibre |
% Ash |
NFE |
|
Raw |
12.70±0.07a |
25.86±1.18e |
1.83±0.06a |
1.65±0.04b |
4.05±0.15a |
53.91±0.66b |
|
6h |
12.20±0.36ab |
28.95±0.07d |
1.06±0.03b |
1.36±0.02c |
2.89±0.01c |
53.54±0.05b |
|
24h |
12.50±0.07ab |
31.45±0.04c |
0.32±0.02f |
1.20±0.03d |
2.99±0.02c |
51.54±0.05c |
|
36h |
11.60±0.08bc |
33.69±0.05b |
0.73±0.05c |
1.35±0.02c |
2.92±0.02c |
49.71±0.02d |
|
48h |
12.30±1.18ab |
34.40±0.02ab |
0.41±0.02e |
1.33±0.04c |
3.44±0.02b |
48.12±0.03f |
|
72h |
10.93±0.15c |
34.62±0.03a |
0.61±0.03d |
1.84±0.05a |
3.53±0.03b |
48.47±0.26e |
Table 2: Proximate composition analysis of DFDAS flour.
Note: DFDAS- Defatted dark ash Solojo; NFE- Nitrogen Free Extractive
Means in columns not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
Notable rise in crude protein after malting in both cowpea varieties investigated. This observed increase in protein quantity may be associated with loss in dry matter, especially carbohydrates due to respiration during malting (Table 3).
|
FFBS |
%Moisture |
% Protein |
% Fat |
% Fibre |
% Ash |
NFE |
|
Raw |
9.50±0.30a |
24.90±0.06f |
2.37±0.08a |
1.52±0.08b |
3.93±0.03a |
57.78±0.03e |
|
6h |
8.10±0.10b |
26.43±0.22e |
1.65±0.04b |
1.43±0.04c |
3.24±0.03c |
59.15±0.04a |
|
24h |
7.90±0.03bc |
26.99±0.05d |
1.42±0.03c |
1.21±0.02e |
3.05±0.06e |
58.92±0.03b |
|
36h |
7.90±0.05bc |
27.80±0.04c |
1.36±0.03cd |
1.06±0.01f |
3.15±0.04d |
58.73±0.03c |
|
48h |
7.80±0.01c |
28.20±0.04b |
1.29±0.03e |
1.32±0.02d |
3.32±0.01b |
58.07±0.02d |
|
72h |
7.10±0.01d |
30.14±0.05 |
1.17±0.08f |
2.15±0.05a |
3.24±0.02c |
56.20±0.03f |
Table 3: Proximate composition analysis of FFBS flour.
Note: NFE- Nitrogen Free Extractive (Carbohydrate); FFBS- Full fat brown Solojo
Means in columns not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
The amount of the crude protein of the full fat ranged between 24.82 and 31.00% for FFDAS and 24.90 to 30.14% for FFBS while that of DFDAS and DFBS was between 25.86 to 34.62%, and 25.64 to 31.80% respectively (Table 4).
|
DFBS |
%Moisture |
% Protein |
% Fat |
% Fibre |
% Ash |
NFE |
|
Raw |
12.20±0.56a |
25.64±0.15f |
1.89±0.10a |
1.37±0.09c |
3.93±0.05a |
54.99±0.20d |
|
6h |
10.20±0.04bc |
26.22±0.04e |
1.20±0.01c |
1.58±0.03a |
2.99± 0.02e |
57.81±0.01a |
|
24h |
10.30±0.02b |
29.26±0.04d |
0.10±0.02f |
1.50±0.03b |
3.36± 0.03c |
55.85±0.02b |
|
36h |
10.30±0.02b |
29.52±0.02c |
0.19±0.03e |
1.27±0.03d |
3.33±0.03cd |
55.39±0.03c |
|
48h |
9.80±0.01c |
29.92±0.04b |
0.67±0.02d |
1.34±0.02cd |
3.30±0.03d |
55.39±0.03c |
|
72h |
10.00±0.02bc |
31.80±0.02a |
1.67±0.03b |
1.39±0.03c |
3.42±0.02b |
51.72±0.03e |
Table 4: Proximate composition analysis of DFBS flour.
Note: DFBS- Defatted brown Solojo; NFE- Nitrogen Free Extractive
Means in columns not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
This is expected because the removal of oil due to defatting reduces the competition of the oil with protein in the flour during analysis. The protein content of the isolates too was observed to increase with germination. The NFE of the FFDAS was also found to be higher than that of DFDAS; this was due to the removal of the fat.
Ash content generally reduced with germination, only the 48h protein isolate of DAS and the 6h isolate of BS had values greater than the control.
The reduction in content of ash may be as a result of mineral loss in water during washing in order to minimize the acerbic smell produced over the period of sprouting (Table 5).
|
DAS |
% Moisture |
% Protein |
% Ash |
% Fat |
NFE |
|
Raw |
10.60±0.64a |
81.57±0.53e |
2.52±0.22c |
2.43±0.23a |
2.93±0.05a |
|
6h |
3.25±0.15c |
89.39±1.51c |
3.79±0.29b |
1.94±0.16b |
1.63±0.12de |
|
24h |
1.54±0.06d |
94.85±0.86a |
2.13±0.20d |
0.08±0.02c |
1.40±0.10e |
|
36h |
5.15±0.03b |
91.81±0.77b |
0.73±0.03e |
0.25±0.01c |
2.07±0.02c |
|
48h |
4.80±0.20b |
86.44±0.84d |
5.92±0.22a |
0.23±0.02c |
2.62±0.32b |
|
72h |
5.14±0.11d |
90.23±0.53bc |
2.53±0.08c |
0.30±0.10c |
1.80±0.20cd |
Table 5: Proximate composition analysis of DAS protein isolate.
Note: DAS- Dark-ash protein Isolate; NFE- Nitrogen free extractive
The crude fibre of germinated FFDAS, FFBS and DFDAS generally reduced with germination, except for 72h for all of them and 24h FFDAS. While DFBS had its crude fibre increasing with germination except that of 36h which reduced. The total carbohydrate quantity as Nitrogen free extractive was calculated by difference and was found to reduce with rise in germination time for the DAS flour and isolate, while the NFE of the BS variety of both flour and isolate increased with germination. Reduction is probably due to degradation of fibre into simple sugars brought about by endogenous enzymes. Means in columns not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
This decrease could be ascribed to the use of carbohydrate to give energy to the growing embryo for germination (Table 6).
|
BS |
%Moisture |
%Protein |
%Ash |
%Fat |
NFE |
|
Raw |
9.40±0.20a |
84.39±0.39e |
3.74±0.05b |
1.64±0.14a |
0.71±0.07f |
|
6h |
3.22±0.14d |
90.47±0.89c |
4.80±0.40a |
0.27±0.03bc |
1.25±0.04d |
|
24h |
0.61±0.02e |
95.81±0.19a |
2.26±0.20d |
0.32±0.04b |
1.00±0.10e |
|
36h |
4.92±0.04c |
90.05±0.10c |
2.75±0.08c |
0.20±0.02cd |
2.08±0.04b |
|
48h |
3.28±0.04d |
92.78±0.28b |
1.90±0.30de |
0.18±0.02cd |
1.87±0.03c |
|
72h |
7.58±0.12b |
86.44±0.56d |
1.81±0.17e |
0.12±0.01d |
4.05±0.10a |
Table 6: Proximate composition analysis of BS protein isolate.
Note: BS- Brown protein Isolate; NFE- Nitrogen free extractive
Means in columns not followed by same alphabet(s) are significantly different at 5% level (P < 0.05).
The amino acid configuration of the protein determines the nutritional value of the protein. Tables 7-13, showed the amino acid composition of germinated DAS and BS flour and isolate.
|
EAA |
Raw |
6h |
24h |
36h |
48h |
72h |
FAO/WHO (1991) |
|
Lysine |
6.85 |
6.92 |
7.16 |
7.30 |
7.79 |
7.91 |
5.8 |
|
Histidine |
3.10 |
3.19 |
3.30 |
3.50 |
3.62 |
3.79 |
1.9 |
|
Cysteine |
1.10 |
1.18 |
1.24 |
1.38 |
1.80 |
2.01 |
|
|
Methionine |
0.65 |
0.70 |
0.70 |
0.75 |
0.86 |
0.86 |
|
|
Threonine |
4.75 |
5.01 |
5.20 |
5.27 |
5.61 |
5.80 |
3.4 |
|
Isoleucine |
4.60 |
4.70 |
4.89 |
5.25 |
5.41 |
5.61 |
|
|
Leucine |
7.95 |
8.25 |
8.40 |
8.64 |
8.78 |
8.91 |
6.6 |
|
Tyrosine |
2.22 |
2.98 |
3.31 |
3.31 |
3.64 |
3.81 |
|
|
Phenylalanine |
4.85 |
4.98 |
5.05 |
5.18 |
5.24 |
5.52 |
|
|
Valine |
5.25 |
5.71 |
5.89 |
6.05 |
6.62 |
6.38 |
3.5 |
Table 7: Essential Amino Acid of Dark ash Solojo flour.
Note: EAA: Essential amino acid.
The value of the essential amino acid for the flours and isolate was generally highest for Leucine; this was followed by lysine and then phenylalanine. Sprouting was observed to cause increment in the AA quantity of the samples.
|
EAA |
Raw |
6h |
24h |
36h |
48h |
72h |
|
Arginine |
5.80 |
6.38 |
6.74 |
7.05 |
7.20 |
7.30 |
|
Aspartic acid |
11.40 |
11.52 |
11.66 |
11.85 |
11.93 |
12.08 |
|
Sarine |
6.55 |
6.85 |
7.06 |
7.14 |
7.20 |
7.24 |
|
Glutamic acid |
14.95 |
15.20 |
15.32 |
15.42 |
15.60 |
15.70 |
|
Proline |
4.65 |
4.82 |
4.92 |
5.05 |
8.10 |
8.22 |
|
Glysine |
7.59 |
7.69 |
7.89 |
8.02 |
8.21 |
8.22 |
|
Alanine |
7.65 |
7.80 |
7.89 |
8.02 |
8.21 |
8.42 |
Table 8: Non –essential amino acid for Dark ash Solojo flour.
Note: Non - EAA: Non - Essentail amino acid.
The lysine content of germinated DAS flour increased from 6.85 to 7.91g/100g after 72h of germination, while that of BS went from 6.97 to 8.20g/100g. The Isolates of the two variety also increased with germination, the isolate of DAS went from 6.75 to 7.89; that of BSg/100g.
|
g/100g |
Raw |
6h |
24h |
36h |
48h |
72h |
FAO/WHO (1991) |
|
Lysine |
6.97 |
7.08 |
7.20 |
7.35 |
7.88 |
8.20 |
5.8 |
|
Histidine |
3.40 |
3.44 |
3.76 |
3.85 |
3.89 |
4.05 |
1.9 |
|
Threonine |
5.10 |
5.25 |
5.44 |
5.55 |
5.70 |
5.90 |
3.4 |
|
Cysteine |
1.35 |
1.38 |
1.52 |
1.73 |
1.91 |
2.28 |
|
|
Valine |
5.84 |
5.89 |
6.00 |
6.20 |
6.48 |
6.69 |
3.5 |
|
Methionine |
0.65 |
0.70 |
0.75 |
0.80 |
0.86 |
0.91 |
|
|
Isoleucine |
4.65 |
4.69 |
4.85 |
4.98 |
5.01 |
5.40 |
2.8 |
|
Leucine |
8.79 |
8.96 |
9.11 |
9.20 |
9.38 |
9.50 |
6.6 |
|
Tyrosine |
3.29 |
3.31 |
3.31 |
3.48 |
3.48 |
3.97 |
|
|
Phenylalanine |
4.90 |
5.01 |
5.15 |
5.28 |
5.45 |
5.60 |
|
Table 9: Amino assays of Brown Solojo Cowpea Flour (Essential Amino Acid).
Note: EAA- Essential Amino Acid
Frota et al. [33], obtained a value of 6.8 and 6.7g/100g respectively for the flour and protein isolate of their cowpea variety. This is similar in value to our raw samples.
|
g/100g |
Raw |
6h |
24h |
36h |
48h |
72h |
|
Arginine |
6.40 |
6.52 |
6.88 |
7.21 |
7.40 |
7.51 |
|
Aspartic acid |
12.40 |
12.50 |
12.66 |
12.77 |
12.96 |
12.60 |
|
Serine |
7.09 |
7.11 |
7.20 |
7.39 |
7.55 |
7.81 |
|
Glutamic acid |
15.07 |
15.21 |
15.49 |
15.54 |
15.78 |
15.95 |
|
Proline |
4.70 |
4.98 |
5.06 |
5.15 |
5.25 |
5.40 |
|
Glycine |
7.65 |
7.85 |
7.98 |
8.11 |
8.24 |
8.34 |
|
Alanine |
7.77 |
7.98 |
8.06 |
8.28 |
8.37 |
8.51 |
Table 10: Amino assays of Brown Solojo Cowpea Flour (Non-essential amino acid).
The sulphur containing amino acids too also increased with germination, methionine of germinated DAS flour increased maginally from 0.65 to 0.86g/100g, BS flour from 0.65 to 0.91g/100g.
|
g/100g |
Raw |
6h |
24h |
36h |
48h |
72h |
FAO/WHO (1991) |
|
Lysine |
6.90 |
6.98 |
7.28 |
7.40 |
7.89 |
8.19 |
5.8 |
|
Histidine |
3.38 |
3.40 |
3.52 |
3.59 |
3.78 |
3.84 |
1.9 |
|
Threonine |
4.40 |
4.42 |
4.48 |
4.53 |
4.82 |
4.93 |
3.4 |
|
Cysteine |
0.90 |
0.97 |
1.11 |
1.24 |
1.38 |
1.52 |
|
|
Valine |
5.55 |
5.80 |
5.92 |
6.20 |
6.39 |
6.51 |
3.5 |
|
Methionine |
0.75 |
0.88 |
0.97 |
1.05 |
1.12 |
1.15 |
|
|
Isoleucine |
4.50 |
4.56 |
4.69 |
4.82 |
5.17 |
5.65 |
2.8 |
|
Leucine |
9.50 |
9.69 |
9.09 |
10.03 |
10.27 |
10.50 |
6.6 |
|
Tyrosine |
1.80 |
1.99 |
2.07 |
2.16 |
2.32 |
2.40 |
|
|
Phenylalanine |
5.60 |
5.68 |
5.70 |
5.78 |
5.84 |
5.95 |
|
Table 11: Amino assay of Dark ash Solojo Cowpea Isolate (Essential Amino Acid).
The isolate of DAS from 1.15 to 1.37g/100g and BS isolate from 1.25 to 1.56g/100g. The methiones for the isolate was found to be higher than that of our own cowpea; this could be due to varietal differences.
|
g/100g |
Raw |
6h |
24h |
36h |
48h |
72h |
|
Arginine |
7.00 |
7.25 |
7.59 |
7.10 |
7.36 |
7.62 |
|
Aspartic acid |
13.05 |
13.14 |
13.22 |
13.24 |
13.43 |
13.65 |
|
Serine |
6.46 |
6.58 |
6.69 |
6.77 |
6.88 |
6.98 |
|
Glutamic acid |
15.92 |
16.06 |
16.22 |
16.41 |
16.69 |
16.80 |
|
Proline |
4.94 |
5.09 |
5.28 |
5.40 |
5.60 |
5.81 |
Table 12: Amino assay of Dark ash Solojo Cowpea Isolate (Non-essential amino acid).
|
g/100g |
Raw |
6h |
24h |
36h |
48h |
72h |
FAO/WHO (1991) |
|
Lysine |
7.04 |
7.20 |
7.28 |
7.42 |
7.93 |
8.32 |
5.8 |
|
Histidine |
3.58 |
3.65 |
3.71 |
3.78 |
3.84 |
3.90 |
1.9 |
|
Threonine |
4.90 |
5.00 |
5.20 |
5.30 |
5.55 |
5.78 |
3.4 |
|
Cysteine |
1.20 |
1.24 |
1.24 |
1.38 |
1.52 |
1.66 |
|
|
Valine |
6.00 |
6.07 |
6.13 |
6.29 |
6.61 |
6.62 |
3.5 |
|
Methionine |
0.78 |
0.92 |
1.02 |
1.10 |
1.18 |
1.22 |
|
|
Isoleucine |
4.74 |
4.80 |
4.87 |
5.07 |
5.39 |
5.78 |
2.8 |
|
Leucine |
9.63 |
9.78 |
9.98 |
10.18 |
10.29 |
10.38 |
6.6 |
|
Tyrosine |
2.10 |
2.17 |
2.32 |
2.38 |
2.42 |
2.51 |
|
|
Phenylalanine |
5.65 |
5.70 |
5.80 |
5.88 |
6.05 |
6.23 |
|
Table 13: Amino assay of Brown Solojo Cowpea Isolate (Essential Amino Acid).
The non- essential amino acid had the glutamic acid having the greatest value, followed by aspartic acid, then arginine, while the rest followed at various levels. The non-essential amino acids were also improved by germination (Table 14).
|
g/100g |
Raw |
6h |
24h |
36h |
48h |
72h |
Soy |
|
Arginine |
7.19 |
7.35 |
7.75 |
7.83 |
8.04 |
8.12 |
8.93 |
|
Aspartic acid |
13.10 |
13.28 |
13.30 |
13.45 |
13.62 |
13.82 |
13.64 |
|
Serine |
6.75 |
6.82 |
6.98 |
7.08 |
7.18 |
7.29 |
3.70 |
|
Glutamic acid |
16.14 |
16.83 |
16.51 |
16.69 |
16.87 |
17.02 |
21.60 |
|
Proline |
5.05 |
5.12 |
5.35 |
5.55 |
5.76 |
5.98 |
3.02 |
Table 14: Amino assay of Brown Solojo Cowpea Isolate (Non-essential amino acid).
All the samples had their amino acid quantity reaching and surpassing the FAO/WHO requirement for both children and adult especially after germination except for methionine which is slightly short of the standard after germination.
Conclusion and Recommendation
Biochemical modification which involves the activation of the intrinsic enzymes of the Solojo cowpea seed itself by germination was carried out for different hours for the two varieties, i.e. the Dark-Ash and the Brown Solojo beans. Proximate analysis, revealed that biochemical modification improved the nutritional quality of the Solojo beans better, when compared with the obtained result of Chemical modification in the literature. Which means that, biochemically modified flour and protein isolates will do better in food processing than chemically modified isolates. Germination also brought about reduction in indigestible dietary fibre that causes flatulence and discourages people from wanting to consume legumes, as a result of the increase in α-amylase activity which brings about the breakdown of complex carbohydrate, this is majorly taken care off. All these make consumption of germinated seed to be healthier than the ungerminated seed. Germination also improved the bio-availability of the important minerals, this is as a result of breakdown of phytate and other anti-nutrients which chelates to the divalent metals, most especially Fe, Zn and Ca, making them unavailable. The decrease in value of the phytate and other anti-nutrients and increase in value of the micro-nutrients shows that germination actually brought about this break down. With the breakdown of the anti-nutrients, the minerals are made more available for use both in the food product and when consumed. The amino acid was also found to increase with germination, this is attributed to the breakdown of the higher molecular weight storage protein which are broken down to lower molecular weight proteins, thus making it more available to the body when used in production. Germination has also been found to increase the amount of the limiting sulphur amino acids which makes legume protein to be termed as incomplete. This research work shows that germination had a profound and significant (p < 0.05) effect on the nutritional composition of Solojo beans, revealing great improvement and thus making Solojo a potential substitute to other important legumes such as soya beans.
References
- Butt MS, Batool R (2010) Nutritional and functional properties of some promising legumes protein isolates. Pakistan Journal of Nutrition 9: 373-379.
- Rahma EA (2000) Production and Evaluation of Yambean (Hochstex Rich) And Bambara Groundnut (Voandzeia Subterranean L). Journal of Science and Food Agriculture 41: 123-124.
- Ghavidel RA, Prakash J (2007) The impact of germination and dehulling on nutrients, antinutrients in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. Journal of Food Science and Technology 40: 1292-1299.
- Desalegn BB (2015) Effect of soaking and germination on proimate composition, mineral bioactivity and functional properties of chickpea flour. Food and Public Health 5: 108-113.
- Chinma CE, Adewumi O, Abu JO (2009) Effect of germination on the chemical, functional and pastry property of flour from brown and yellow varieties of tiger nut (Cyperus esculentus). Food Research International 42: 1004-1009.
- D’Souza MR (2013) Effect of traditional processing methods on nutritional quality of field bean. Advances in Bioresearch 4: 29-33.
- Yellavila SB, Agbenorhevi JK, Asibuo JY, Sampson GO (2015) Proximate composition, minerals content and functional properties of five lima bean accessions. Journal of Food Security 3: 69-74.
- Iheanacho KME (2010) Comparative studies of the nutritional composition of soy bean (Glycine Max) and lima bean (Phaseolus Lunatus). Scientia Africana 9: 29-35.
- Moses O, Olawuni I, Iwouno JO (2012) The proximate composition and functional properties of full-fat flour, and protein isolate of lima bean (Phaseolus Lunatus). Open Access Scientific Reports 1: 349.
- Borijindakul L, Phimolsiripol Y (2013) Physicochemical and functional properties of starch and germinated flours from Dolichos lablab. Food and Applied Bioscience Journal 1: 69-80.
- Myrene RD, Souza (2013) Effect of traditional processing methods in nutritional quality of field bean. Advances in Bioresearch 4: 3.29-33.
- Nenogaki H, Bassel GW, Bewley JW (2010) Germination still a mystery. Plant Science 179: 574-581.
- Agugo, Afam Anene OC, Agugo UA, Anyaegbu, EC (2016) Effect of germination on the nutritional and anti-nutritional contents of mungbean (Vigna radiata). African Journal of Agricultural Science and Technology (AJAST) 4: 801-805.
- Kavitha S, Parimalavalli R (2014) Effect of processing methods on proximate composition of cereal and legume flours. Journal of Human Nutrition and Food Science 2: 1051.
- Enujiugha VN, Badejo AA, Iyiola SO, Oluwamukomi MO (2003) Effect of germination on the nutritional and functional properties of African Oil bean (Pentaclethra macrophylla Benth) seed flour. Food Agriculture and Environment 1: 72-75.
- Mubarak AE (2005) Nutritional composition and antinutritional factors of Mung bean seeds (Phaseolus auneus) as affected by some home traditional processes. Food Chemistry 89: 489-495.
- Rumiyati AP, James VJ (2012) Effect of germination on the nutritional and protein profile of Australian Sweet Lupin (Lupinus angustifolius) Food and Nutrition Science 3: 621-626.
- Devi CB, Kushwaha A, Kumar A (2015) Sprouting characteristics and associated changes in nutritional composition of cowpea (Vigna unguiculata) Journal Food Science and Technology 52: 6821-6827.
- Zhang S, Xia C, Dong Y, Yan Y, Li J, et al. (2016) Soy protein isolate-based films reinforced by surface modified cellulose nanocrystal. Industrial Crops and Products 80: 207-213.
- Nkhata SG, Ayua E, Kamau EH, Shingiro JB (2018) Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Science and Nutrition 6: 2446-2458.
- El-Adawy TA, Rahma EH, El-Bedawey AA, El-Beltagy AE (2004) Nutritional potential and functional properties of germinated mung bean, pea and lentil seeds. Plant Foods for Human Nutrition 58: 1-13.
- Murugkar DA, Paridhi G, Chetan G (2013) Effect of sprouting on physical properties and functional and nutritional components of multinutrient mixes. International Journal of Food and Nutritional Sciences 155: 1-15.
- Hahm TS, Park SJ, Lo YM (2009) Effects of germination on chemical composition and functional properties of sesame (Sesamum indicum) seeds. Bioresource Technology 100: 1643-1647.
- Rusydi MR, Noraliza CW, Azrina A, Zulkhairi A (2011) Nutritional changes in germinated legumes and rice varieties. International food Research Journal. 18: 705-713.
- Nwosu JN (2013) Evaluation of the proximate composition and antinutritional properties of African yam beans (Sphenostylis sternocarpa) using malting treatment. International Journal of basic and applied sciences 2: 157-169.
- Ahmad SJ, Pathak DK (2000) Nutritional changes in soya bean during germination: Journal of Food Science and Technology 37: 665-666.
- Oboh, HA, Omofoma CO (2008) The effects of heat-treated lima beans (Phaseolus lunatus) on plasma lipids in hypercholesterolemic rats. Pakistan Journal of Nutrition 7: 636-639.
- Dida D (2010) Study on the effect of traditional processing on proximate composition and bioavailability of minerals in chickpea (Cicer arietinum) grown in Ethiopia. Food Engineering Project Topics.
- Berhanu A, Amare G (2013) Effects of salt (NaCl) concentration on the functional properties of defatted brebra (Millettia ferruginea) seed flour. Middle-East Journal of Scientific Research 13: 889-897.
- Ramadan EA (2012) Effect of processing and cooking methods on the chemical composition, sugars and phytic acid of soybeans. Food and Public Health 2: 11-15.
- Oghbaei M, Prakash J (2016) Effect of primary processing of cereals and legumes on its nutritional quality: A comprehensive review. Cogent Food and Agriculture 2: 1-29.
- Warle BM, Riar CS, Gaikwad SS, Mane VA (2015) Effect of germination on nutritional quality of barley. International Journal of Food and Nutritional Sciences 4: 59-63.
- Frota KMG, Lopes LAR, Silva ICV, Arêas JAG (2017) Nutritional quality of the protein of Vigna unguiculata L. Walp and its protein isolate. Revista Ciência Agronômica 48: 792-798.
Citation: Adeyoju OA, Chibudike HO, Chibudike CE, Adebowale KO, Olu-Owolabi BI, (2025) Nutritional Evaluation of Isolates from Two (2) Varieties (DAS & BS) of Underutilized Germinated Nigeria Cultivated Solojo-Cowpea [Vigna Unguiculata (L.) Walp.]. HSOA J Food Sci Nutr 11: 220.
Copyright: © 2025 Olubamike A Adeyoju, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

