
One-Year Postoperative Outcomes following Transfixion of Foldable Intraocular Lens with Polytetrafluoroethylene Suture for Scleral Fixation
*Corresponding Author(s):
Angel Pineda-FernandezCentro Oftalmologico De Valencia CEOVAL, Av. Bolivar Norte, Edificio Torre Venezuela, Piso 2., Valencia, Venezuela
Tel:+58 4122408347,
Fax:+58 2418247090
Email:dr.angelpinedafernandez@gmail.com
Abstract
Purpose: Transfixion of foldable Posterior Chamber Intraocular Lens (PC-IOL) with polytetrafluoroethylene suture forscleral fixation is a new technique that we described in March 2021 in eyes with deficient capsular support. The purpose of this article is to evaluate the 1-year postoperative outcome and complication profile, of eyes operated using this technique.
Methods: Eyes operated in our institution between November 2019 and July 2021 with transfixion of PC- IOL for scleral fixation were included in the study. The preoperative and postoperative Uncorrected Distance Visual Acuity (UDVA), Corrected Distance Visual Acuity (CDVA), manifest refraction, and intraocular pressure (IOP) were evaluated; slit lamp biomicroscopy, and dilated fundus examination were performed. Ultrasound Biomicroscopy (UBM) (Absolu, Quantel Medical) was used in all operated eyes to document the IOL position. The 1-year postoperative complications were analyzed.
Results: We examined 19 eyes of 19 patients. Significant improvement in visual acuity was noted in all cases. In addition, most of the IOLs (84.21%) were well centered, and 3 eyes (15.78%) presented mild inferior decentration. Only one eye (5.26%) developed mild pigment dispersion with no evidence of glaucoma.
Conclusion: One-year results post foldable acrylic PC-IOL transfixion for scleral fixation provided good visual prognosis with minimal complications in eyes with deficient capsular support.
Precis: Evidence on 1-year follow-up after transfixion of foldable acrylic PC-IOL for scleral fixation support the technique, as it provides good visual prognosis with minimal complications in eyes with deficient capsular support.
Introduction
Several surgical techniques for scleral fixation of foldable posterior chamber intraocular lens (PC-IOL) have been described over the years. Many of them use polypropylene suture of varying thickness [1-6], whereas others use polytetrafluoroethylene suture [7-9].
Some techniques for scleral fixation that do not use sutures. The suture-less intrascleral foldable PC-IOL fixation described by Gabor and Pavlidis [10], the fibrin glue-assisted suture-less foldable PC-IOL scleral fixation described by Agarwal et al., [11] and the flanged intrascleral foldable PC-IOL fixation described by Yamane et al., [12] were all developed for 3-piece foldable IOLs and cannot be used for single-piece IOL. Transfixion of foldable PC-IOL forscleral fixation is a technique that we described in March 2021 in eyes with deficient capsular support [13,14]. This surgical technique works effectively with an acrylic foldable PC-IOL, regardless of it being 3-piece or single-piece, using the polytetrafluoroethylene suture (Gore-Tex CV8) and potentially usable with every acrylic foldable PC-IOL. It is performed using a stable 4-point transscleral fixation of the Intraocular Lens (IOL) optic at four points with the polytetrafluoroethylene suture. In the present study we evaluate the 1-year postoperative outcome and complication profile of eyes operated using this technique.
Materials And Methods
In this retrospective study, patients who underwent transfixion of foldable intraocular lens with polytetrafluoroethylene suture for scleral fixation were analyzed 1 year post surgery. All eyes operated from November 2019 to July 2021 in our institution using this procedure were included in the study and followed up for 12 months post-surgery. The selection criteria were eyes with deficient capsular or sulcus support in which implantation of PC-IOL was not possible in previous cataract surgery. Informed consent was obtained from all patients’ prior surgery. The study followed the tenets of the Declaration of Helsinki.
On the 12-month follow-up visit, the following parameters were assessed: uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest refraction, intraocular pressure (IOP) with aplanometry tonometry, slit lamp biomicroscopy, and dilated fundus examination. Ultrasound biomicroscopy (UBM) (Absolu, Quantel Medical) was performed to document the IOL position.
Surgical technique
The foldable PC-IOL was prepared before surgery under the surgical microscope. Using a caliper, four marks were made on the IOL optic, two on either side—1 mm from the haptic base, and 2.5 mm apart—for performing the transfixions (Figure 1A). The needle of the Gore-Tex suture (expanded polytetrafluoroethylene [ePTFE]) CV-8 suture (W.L. Gore & Associates, Inc.) (off label for ophthalmic use) was passed through the first transfixion point from the anterior to the posterior face of the IOL (Figures 1B and 1C). The suture was then passed in front of the haptic (Figure 1D), and the needle was passed through the second transfixion point from the posterior to the anterior face of the IOL (Figure 1E). The same technique was repeated on the opposite side of the IOL (Figure 1F). We used the hydrophilic acrylic foldable single-piece IOL and the hydrophobic acrylic foldable 3-piece IOL.
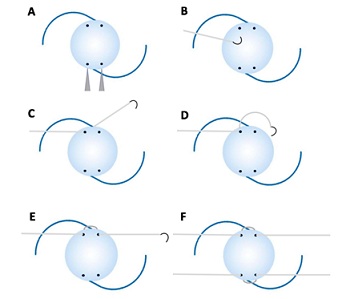 Figure 1: Diagram of the intraocular lens (IOL) transfixion. (A) Four marks are made on the IOL optic, two on either side, 1 mm from the haptic base, and 2.5 mm apart, for the transfixions. (B) and (C): The needle of the polytetrafluoroethylene suture is passed through the first transfixion point, from the anterior to the posterior face of the IOL. (D) The suture is then passed in front of the haptic. (E) The needle is passed through the second transfixion point from the posterior to the anterior face of the IOL. (F) The same technique for IOL transfixion is repeated on the opposite side of the IOL.
Figure 1: Diagram of the intraocular lens (IOL) transfixion. (A) Four marks are made on the IOL optic, two on either side, 1 mm from the haptic base, and 2.5 mm apart, for the transfixions. (B) and (C): The needle of the polytetrafluoroethylene suture is passed through the first transfixion point, from the anterior to the posterior face of the IOL. (D) The suture is then passed in front of the haptic. (E) The needle is passed through the second transfixion point from the posterior to the anterior face of the IOL. (F) The same technique for IOL transfixion is repeated on the opposite side of the IOL.
The intraoperative steps for this procedure comprised making 3 clock-hour conjunctival peritomies, 180º apart, on the nasal and temporal sides. Four sclerotomy sites were marked (2 each on the nasal and temporal side) 2 mm from the limbus and 4 mm apart. A 4-mm vertical, partial-thickness scleral groove was then created between the sclerotomy sites using a 15º blade. A 20-gauge microvitreoretinal blade was used to perform four sclerotomies at the pre-marked scleral spots (Figure 2A). Two 1.2-mm side- paracenteses incisions were made in the peripheral clear cornea with a 15º lance tip to facilitate bimanual irrigation/aspiration, bimanual anterior vitrectomy (if required), and injection of an ophthalmic viscosurgical device (OVD).
A limbal corneal incision of 3.5 mm was created superiorly with a blade. Cohesive OVD was used to maintain the integrity of the globe during the surgery. One end of the needleless polytetrafluoroethylene suture was then placed in the anterior chamber through the main wound (Figure 2B); using a handshake technique, this was then retrieved and externalized through the inferior sclerotomy with micro graspers. The other end of the needleless suture was similarly retrieved and externalized through the opposite scleral opening (Figure 2C). The IOL was then folded on its long axis using forceps and introduced into the posterior chamber through the corneal wound (Figure 2D). The other ends of the needleless sutures were similarly retrieved through the superior scleral openings using the same handshake technique (Figures 2E and 2F). The two sets of sutures on the nasal and temporal sides were then tightened, adjusted for optimum IOL centration (Figure 2G), and tied; the excess sutures were cut. The exposed polytetrafluoroethylene suture was placed within the surgically created vertical scleral groove. The suture knots were buried within the sclerotomies (Figure 2H). The conjunctiva was sutured with 10-0 nylon.
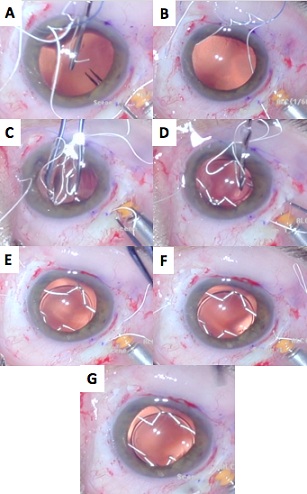 Figure 2: Intraoperative view of the foldable intraocular lens (IOL) scleral fixation using polytetrafluoroethylene suture. (A) A 4-mm vertical, partially thick scleral groove is created between the sclerotomy sites using a 15º blade. (B) One end of the needleless polytetrafluoroethylene suture is then placed in the anterior chamber through the main wound. (C) The end of the needleless suture is then retrieved and externalized through the inferior sclerotomy with micrograspers. The other end of the needleless suture is similarly retrieved and externalized through the opposite scleral opening. (D) The IOL is then folded on its long axis using forceps and introduced into the posterior chamber through the corneal wound. (E) and (F) The other ends of the needleless sutures are similarly retrieved through the superior sclerotomies using the same handshake technique. (G) The two sets of sutures on the nasal and temporal sides are then tightened, adjusted for optimum IOL centration, and tied. (H) The exposed polytetrafluoroethylene suture is placed within the surgically created vertical scleral groove. The suture knots are buried within the sclerotomies.
Figure 2: Intraoperative view of the foldable intraocular lens (IOL) scleral fixation using polytetrafluoroethylene suture. (A) A 4-mm vertical, partially thick scleral groove is created between the sclerotomy sites using a 15º blade. (B) One end of the needleless polytetrafluoroethylene suture is then placed in the anterior chamber through the main wound. (C) The end of the needleless suture is then retrieved and externalized through the inferior sclerotomy with micrograspers. The other end of the needleless suture is similarly retrieved and externalized through the opposite scleral opening. (D) The IOL is then folded on its long axis using forceps and introduced into the posterior chamber through the corneal wound. (E) and (F) The other ends of the needleless sutures are similarly retrieved through the superior sclerotomies using the same handshake technique. (G) The two sets of sutures on the nasal and temporal sides are then tightened, adjusted for optimum IOL centration, and tied. (H) The exposed polytetrafluoroethylene suture is placed within the surgically created vertical scleral groove. The suture knots are buried within the sclerotomies.
Results
The technique was used to perform scleral fixation of the PC-IOL in 19 eyes of 19 patients. Surgeries were performed by one surgeon (A.P). Participants in the study included 15 female and 4 male patients. The mean age + Standard Deviation (SD) was 60.2 + 10.5 years. All eyes underwent surgery as secondary procedure. The indication in all eyes was intraoperative posterior capsular rupture with insufficient capsular support. A hydrophilic acrylic foldable single-peace IOL was implanted in 8 eyes (42.1 %) and a hydrophobic acrylic foldable three-piece IOL was implanted in 11 eyes (57.9 %). SRK II formula was used for IOL power calculation. Postoperative refractive error was targeted for emmetropia.
The LogMar units (expressed as mean ± SD) for UDVA were -1.9 ± 0.18 and -0.67 ± 0.18 preoperative and postoperative, respectively, whereas for CDVA, the LogMar units were -0.21 ± 0.14and -0.12 ± 0.09 preoperative and postoperative, respectively. In regards to the spherical equivalent (EE), the Diopters (D) were +11.57 ± 1.4 preoperative and -0.25 ± 0.61 D postoperative. The postoperative astigmatism was -1.78 ± 0.7 D. No eyes lost lines of CDVA (Figure 3).
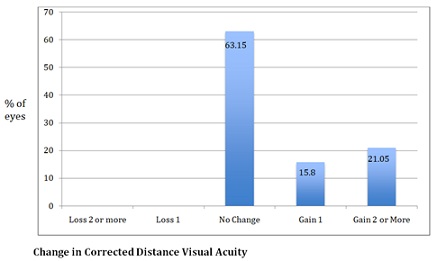 Figure 3: Diagram showing the change in Corrected Distance Visual Acuity (CDVA) at one-year follow-up.
Figure 3: Diagram showing the change in Corrected Distance Visual Acuity (CDVA) at one-year follow-up.
There were no intraoperative complications. Moreover, no postoperative complications, such as infection, suture erosion, suture breakage, scleral inflammation, suture granuloma formation, glaucoma, iritis, or signs of recurrent uveitis occur. One eye (5.26%) developed mild pigment dispersion (seen 6 months after surgery) with no evidence of glaucoma. No postoperative vitritis or endophthalmitis was detected in any of the patients. No retinal break or retinal detachment was documented on serial fundus examination. Two eyes with cystoid macular edema achieved 20/40 of CDVA and two eyes with age-related macular degeneration achieved 20/30 of CDVA.
In this study, significant improvement in visual acuity was also noted in all cases. In addition to the improved visual acuity, most of the IOLs, 16 eyes (84.21%) were well centered and 3 eyes (15.78%) presented slight inferior decentration (the IOL optic covered the visual axis). These findings were confirmed by UBM (Figure 4). No eyes presented subluxation, tilt, or dislocation during the 1-year follow-up. At the 12-month follow up, there was no sign of optic tears at the transfixion points of all cases, and no signs of suture degradation (Figure 5).
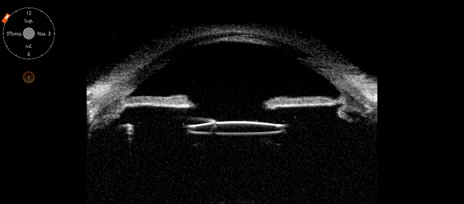 Figure 4: The Ultrasound Biomicroscopy (UBM) showing a well-centered intraocular lens with no tilt.
Figure 4: The Ultrasound Biomicroscopy (UBM) showing a well-centered intraocular lens with no tilt.
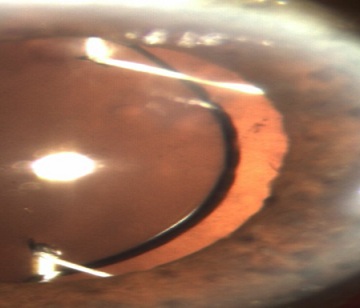 Figure 5: Anterior segment photograph. Under mydriasis, there are no signs of optic tears and suture degradation at the 12-months follow up.
Figure 5: Anterior segment photograph. Under mydriasis, there are no signs of optic tears and suture degradation at the 12-months follow up.
Discussion
IOL transfixion was first described by Parker and Price in 2003 [15]. They sutured a silicone PC-IOL to the iris by passing a 9-0 polypropylene suture through its optic. Canabrava recently described a piercing technique for scleral fixation that involves passing a 6-0 polypropylene suture through two holes created using an IOL punch in the optic zone on each side of the foldable IOL [16]. However, the study does not recommend using this technique because the IOL is fixed at only two points, and the fixation axis subsequently tilts in the opposite direction. Assia and Wong [17] recommend direct suturing through the haptic or optic of practically any foldable IOL, including hydrophilic and hydrophobic IOLs with a one-piece or three-piece design. Other techniques for scleral fixation use 6-0 polypropylene [5] (Canabrava’s four-flanged technique) and polytetrafluoroethylene sutures [7-9].
Although the above techniques are only used for implanting foldable intraocular lenses with four loops, such as the hydrophilic acrylic Akreos IOL (Bausch & Lomb), they enable stable scleral fixation at four points. The main disadvantage of flanged techniques is the possibility of bacterial infection because the flange might serve as a point of microorganisms’entry into the eye [17]. We developed the transfixion technique because it is often difficult for us to obtain foldable PC-IOL with four eyelets at our institution.
Our technique presents various advantages. With the stable 4-point fixation, the IOL tilt in 2-point fixation techniques is avoided [18-20]. Furthermore, the 4-point fixation of the IOL aids in centration, with minor adjustments to the polytetrafluoroethylene suture on both the temporal and nasal sides. Optic decentration is a known complication of transscleral fixation of PC-IOL, inducing lateral shift of focus and radial astigmatism [21].
In our study, the UBM in most of eyes (84.21%) showed a well-centered IOL with no tilt at the 12-month follow-up, and only 3 eyes (15.78%) showed mild inferior decentration of IOL with the IOL optic covering the visual axis. Also, we did not observe pseudophakodonesis in any of the cases, probably due to the stable 4-point fixation of the IOL with this technique. Two eyes with cystoid macular edema achieved 20/40 of CDVA and two eyes with age-related macular degeneration achieved 20/30 of CDVA.
The use of polytetrafluoroethylene suture has gained widespread popularity because of its high tensile strength and resistance to degradation [22-24], but some surgeons avoid using it because of the manufacturer’s label warning against its intraocular usage. Nevertheless, its high tensile strength, high visibility due to its white color, minimal inflammatory response, and minimal memory make it exceptionally easy to manipulate. To date, suture degradation has not been reported in the ophthalmic or non-ophthalmic literature [22]. We had not observed any sign of optic tears at the transfixion points and suture degradation at the 12-month follow up. Moreover, our technique of foldable IOL transfixion can be used with other type of sutures and IOL material, such as polypropylene suture and silicone PC-IOL as we recently reported [25].
In one study [26], a high incidence of pigment dispersion syndrome (PDS) secondary to sulcus transscleral fixation of a single-piece hydrophobic acrylic foldable PC-IOL was found in a Chinese population; the transscleral fixation at 1.5 mm from the limbus was performed. In our study, we performed the transscleral fixation at 2 mm from the limbus, reducing the possibility of iris chafing with rough edges from de IOL surface. We observed only one eye (5.26%) with mild pigment dispersion with no evidence of glaucoma. We did not detect any signs of uveitis in any patient with either the single-piece or the 3-piece IOL at the 12-month follow-up. A long-term follow-up is required to confirm these observations.
This technique is simple, and the learning curve is relatively easy. All ophthalmologists using this technique can benefit patients requiring secondary foldable PC-IOL implantation in the absence of capsular support, because this technique can potentially be used with all available acrylic foldable PC-IOL procedures.
Based on the current study, 1-year follow-up after transfixion of foldable acrylic PC-IOL for scleral fixation provided good visual prognosis with minimal complications in eyes with deficient capsular support.
Large case series with longer follow-ups are necessary to assess the long-term visual outcome, safety, and complications.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
None to disclose.
Funding Support
None to disclose.
References
- Güell JL, Barrera A, Manero F (2004)A review of suturing techniques for posterior chamber lenses. Curr Opin Ophthalmol 15:44-50.
- Kokame GT, Yanagihara RT, Shantha JG, Kaneko KN (2018) Long-term outcome of pars planavitrectomy and sutured scleral-fixated posterior chamber intraocular lens implantation or repositioning. Am J Ophthalmol 189:10-16.
- John T, Tighe S, Hashem O, Sheha H (2018) New use of 8-0 polypropylene suture for four-point scleral fixation of secondary intraocular lenses. J Cataract Refract Surg 44: 1421-1425.
- Wallmann AC, Monson BK, Adelberg DA (2015)Transscleral fixation of a foldable posterior chamber intraocular lens. J Cataract Refract Surg 41: 1804-1809.
- Canabrava S, Andrade N, Rezende PH (2021) Scleral fixation of a 4-Eyelet foldable intraocular lens in patients with aphakia using a 4-flanged technique. J Cataract Refract Surg 47: 265-269.
- Vote BJ, Tranos P, Bunce C, Charteris DG, Cruz L (2006) Long-term outcome of combined pars planavitrectomy and scleral fixated sutured posterior chamber intraocular lens implantation. Am J Ophthalmo l141: 308-312.
- Khan MA, Gerstenblith AT, Dollin ML, Gupta OP, Spirn MJ (2014) Scleral fixation of posterior chamber intraocular lenses using Gore-Tex suture with concurrent 23-gauge pars planavitrectomy. Retina 34: 1477-1480.
- Das S, Nicholson M, Deshpande K, Kummelil MK, Nagappa S, et al. (2016) Scleral fixation of a foldable intraocular lens with polytetrafluoroethylene sutures through a Hoffman pocket. J Cataract Refract Surg 42: 955-960.
- Morkin MI, Patterson M (2019) Scleral-sutured intraocular lenses: Single-surgeon technique for suture-preloaded intraocular lens insertion through a small-incision corneal wound. J Cataract Refract Surg 45: 121-124.
- Gabor SG, Pavlidis MM (2007)Suturelessintrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg 33: 1851-1854.
- Agarwal A, Kumar DA, Jacob S, Baid C, Agarwal A, et al. (2008) Fibrin glue-assisted sutureless posterior chamber intraocular lens implantation in eyes with deficient posterior capsules. J Cataract Refract Surg 34: 1433-1438.
- Yamane S, Sato S, Inoue MM, Kadonosono K (2017) Flanged intrascleral intraocular lens fixation with double-needle technique. Ophthalmology 124: 1136-1142.
- Pineda-Fernández A, Chen Y, Rodriguez L. Transfixion of foldable intraocular lens with polytetrafluoroethylene suture for scleral fixation. J Refract Surg. 2021;37(3):180-185.
- Pineda-Fernández AA, Chen Y. Management of late in-the-bag IOL dislocation with exchange and scleral fixation of IOL with the transfixion technique. JCRS Online Case Rep. 2022;10(1):e00068.
- Parker DS, Price FW (2003) Suture fixation of a posterior chamber intraocular lens in anticoagulated patients. J Cataract Refract Surg 29: 949-954.
- Canabrava S (2019) 4-Flanged technique with a foldable. American Academy of Ophthalmology.
- Assia EI, Wong JXH (2020) Adjustable 6-0 polypropylene flanged technique for scleral fixation, part 2: Repositioning of subluxated IOLs. J Cataract Refract Surg 46: 1392-1396.
- Bergren RL (1994) Four-point fixation technique for sutured posterior chamber intraocular lenses. Arch Ophthalmol 112: 1485-1487.
- Lockington D, Ali NQ, Al-Taie R, Patel DV, McGhee CN (2014) Outcomes of scleral-sutured conventional and aniridia intraocular lens implantation performed in a university hospital setting. J Cataract Refract Surg 40: 609-617.
- Durak A, Oner HF, Koçak N, Kaynak S (2001) Tilt and decentration after primary and secondary transsclerally sutured posterior chamber intraocular lens implantation. J Cataract Refract Surg 27: 227-232.
- Holladay JT (1986) Evaluating the intraocular lens optic. Surv Ophthalmol 30: 385-390.
- Khan MA, Gupta OP, Smith RG, Ayres BD, Raber IM, et al. (2016) Scleral fixation of intraocular lenses using Gore-Tex suture: Clinical outcomes and safety profile. Br J Ophthalmol 100: 638-643.
- Phillips PM, Shah VC, Muthappan V (2016) Endothelial keratoplasty in the setting of a dislocated intraocular lens (IOL). In: Jacob S, ed, Mastering Endothelial Keratoplasty: DSAEK, DMEK, E-DMEK, PDEK, Air Pump-Assisted PDEK and Others;2nd New Delhi, India, Springer; 2016: 15-38.
- Gimbel HV, Condon GP, Kohnen T, Olson RJ, Halkiadakis I (2005) Late in-the-bag intraocular lens dislocation: Incidence, prevention, and management. J Cataract Refract Surg 31: 2193-2204.
- Pineda-Fernández A, Chen Y, Rodriguez L (2022) Transfixion of Silicone Intraocular Lens with 7-0 polypropylene Suture for Scleral Fixation. Clin Surg 7: 3439.
- Tong N, Liu F, Zhang T, Wang L, Zhou Z, et al. (2017) Pigment dispersion syndrome and pigmentary glaucoma after secondary sulcus transscleral fixation of single-piece foldable posterior chamber intraocular lenses in Chinese aphakic patients. J Cataract Refract Surg 43: 639-642.
Citation: Pineda-Fernández A, Chen Y, Rodriguez L (2022) One-Year Postoperative Outcomes following Transfixion of Foldable Intraocular Lens with Polytetrafluoroethylene Suture for Scleral Fixation. J Ophthalmic Clin Res 9: 099.
Copyright: © 2022 Angel Pineda-Fernandez, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

