
Optimistic Stratagems of Machine Based Deep Learning Employed with Automation in Psychiatry and Early Neurodevelopmental Disorders
*Corresponding Author(s):
Anita Margret ADepartment Of Biotechnology And Bioinformatics, Bishop Heber College, Tamilnadu, India
Email:anitamargret5@gmail.com
Abstract
Automation has become a significant module in abetting diagnosis during the contemporary era of medical field. Deep learning is a categorization of machine learning encompassing artificial intelligence. Machine based learning is renowned for its rapid detection of anomalies and has gained immense attention in recent years. The data is catalogued in an amicable manner where the results are presentable based on various images, videos, and sounds with high accuracy through a regularized mechanization using big data. The impact of neurological disorders is felt as the burden that is expanding in due course of global population both in developed and developing countries. Early developmental disabilities are an assemblage of conditions due to impairment in physical, learning, language, or behavior areas which profoundly affect the normal existence of children. The spectrum of neurological ailment is diversified irrespective of demographic parameters and entails significant diagnosis and treatment. Brain is the most complicated organ that requires extensive advances in understanding its development and behavior. Interdisciplinary developments are the becoming inevitable to enhance clinical practice and diagnosis through convolution neural networks. It is necessary to propagate innovative approaches to treat neural disorders and monitor brain development. This chapter proposes a novel framework of research involved with animal models and various detection strategies to perceive neural irregularities. Several visual approaches with biomarkers as neuroimage bestows application during various gestational ages. Conversely, the contents uplift the promise of machine learning at varied levels in neuroscience investigation to discover the working of neural network and brain functioning.
Keywords
Automation; Biomarkers; Convolution neural networks; Early developmental disabilities, Neuroimage; Neurological disorders
Prelude on Neurodevelopment Disorders and Its Global Prevalence
Neurodevelopment Disorders (NDDs) are mental infirmities which affects the foremost functioning of neurological system and brain. They are formed due to neurogical deformities that onset from birth and interfere with the progression of embryological development which produce impairments in the functioning of central nervous system [1]. They include varied neurological conditions that lead to intellectual disabilities and communication like disorders Autism Spectrum Disorder (ASD), Attention-Deficit/Hyperactivity Disorder (ADHD), neurodevelopmental Motor Disorders, and Specific Learning Disorders. The impact of these disorders affects the personal, social, and economic existence of an individual. Deciphering a specific causative source for these infirmities remains a challenge since the reason to decipher the specific reason for the disability is diverse [2] it includes various genetic, nutritional, infectious diseases, traumatic parameters. Globally, there is a sturdy need to devise remedy and combat this mental ailment. Though, neonatal and infant mortality are considered as, a threat to the society, the rise of neurodevelopmental disabilities is alarming and requires a significant curb, since it is considered as second leading cause of fatality [3]. There is a greater prevalence (80%) of NDD in low- and middle-income countries where the maximum global population (90%) of children prevail [4]. Understanding the impact of neurological disabilities the UN formulated sustainable development goals reduce the burden of non-communicable diseases by 2030. There is an immediate necessitate to devise stratagems and plans that would target neurological disorders which would potentially help to achieve the goals of UN.
Threats Of Neurological Disorder And Its Normal Existence
Several neurological disorders confine the normal physiological existence of an individual which affects their regular functioning and activities. Neurological and psychiatric disorders are assumed to be negligent due to the few incidence of direct mortality. The assessment of GBD (The Global Burden of Disease Study) in 1990 reported that noncommunicable ailments such as neurological disorders substantially can hinder health and endure to be a burden worldwide [5]. Further it stated that neurological disabilities were the foremost cause of disrupting healthy life and its threats were underrated by considering only death rate. Hence, based on the need to update the predictions of global mortality WHO prepared a forecast to elucidate the trends for deaths and anomalous impacts of the neurological disabilities that ranges from 2005 to 2030 [6]. The Disability-Adjusted Life Years (DALYs) are a progression that deduces the complete health and expectation of life. It coordinates with the number of years expended on sickness, disability or early death that are prevalent among different countries. The data in table 1 represents the measurement of DALYs in thousands that are related with neurological ailments from 2005, 2015 till 2030 [7]. It is intimidating that the neurological disorders has raised from 2005 (92 million DALYs ) to 103 million in 2030 which contributes an upsurge of nearly 12% conversely, cognitive impairment diseases such as Alzheimer and dementias are predicted to show a profound increase of 66% from 2005 to 2030.
|
Cause category |
2005 |
2015 |
2030 |
|||
|
No. of DALYs (000) |
Percentage of total DALYs |
No. of DALYs (000) |
Percentage of total DALYs |
No. of DALYs (000) |
Percentage of total DALYs |
|
|
Epilepsy |
7 308 |
0.5 |
7 419 |
0.5 |
7 442 |
0.49 |
|
Alzheimer and other dementias |
11 078 |
0.75 |
13 540 |
0.91 |
18 394 |
1.2 |
|
Parkinson’s disease |
1 617 |
0.11 |
1 762 |
0.12 |
2 015 |
0.13 |
|
Multiple sclerosis |
1 510 |
0.1 |
1 586 |
0.11 |
1 648 |
0.11 |
|
Migraine |
7 660 |
0.52 |
7 736 |
0.52 |
7 596 |
0.5 |
|
Cerebrovascular disease |
50 785 |
3.46 |
53 815 |
3.63 |
60 864 |
3.99 |
|
Poliomyelitis |
115 |
0.01 |
47 |
0 |
13 |
0 |
|
Tetanus |
6 423 |
0.44 |
4 871 |
0.33 |
3 174 |
0.21 |
|
Meningitis |
5 337 |
0.36 |
3 528 |
0.24 |
2 039 |
0.13 |
|
Japanese encephalitis |
561 |
0.04 |
304 |
0.02 |
150 |
0.01 |
|
Total |
92 392 |
6.29 |
94 608 |
6.39 |
103 335 |
6.77 |
Table 1: Presents the data in percentage that projects the futuristic occurrence neurological disorders associated with DALYs (Data from Updated projections of global mortality and burden of disease, 2002-2030: data sources, methods and results Geneva, World Health Organization, 2005) [8].
The estimated reduction of DALYs in poliomyelitis, tetanus, meningitis and Japanese encephalitis (57%) combined console the menace during the fourth coming years [9]. The graphical representation in figure 1 illustrates the significance of death rates during 2005, 2015 and 2030 [6]. The death rate of neurological disorders is influenced by the pathogenesis of other diseases. For instance, cerebrovascular diseases are responsible for 85% of the deaths due to neurological disorders. The GBD data reveals the significance rise of neurological disorders from 2005 to 2030 [10].
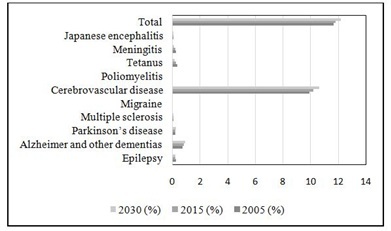 Figure 1: Projected death rate percentage of neurological diseases and its associated diseases (Adapted from GBD 2015 Neurological Disorders Collaborator Group) [6].
Figure 1: Projected death rate percentage of neurological diseases and its associated diseases (Adapted from GBD 2015 Neurological Disorders Collaborator Group) [6].
The projected threats of these ailments would plan futuristic strategies to restrain and find contemporary remedies. Consequently, distinct actions to improve the mental health are required instantly otherwise the neurological burden will remain as a serious threat to public health.
Deep Learning-A Novel Strategy Involved In Monitoring And Screening Of Neural Anomalies
Abnormalities associated with nervous system are based on congenital and environmental attributes. The development of nervous system occurs both during and after birth and hence is influenced by a varied environmental issue which can lead to neural anomalies. Congenital anomalies are up surging and are common among infants. It is estimated that 2%-3% of new born are diagnosed with complicated congenital malformation which can drive towards an increase rate of morbidity and mortality [11]. There is a significant necessitate to curb and devise strategies to understand its etiologies. Digitalization of significant data and resources is the prerequisite of today's era of data science management. Machine Learning (ML) Artificial Intelligence (AI) and deep learning techniques are considered as the essential tools to decipher complications in the contemporary period. Figure 2 depicts the integrated coherence and their individual versatility to resolve intricacies in the field of neuroscience. Nearly 45% of official units are performed with automated technologies where, 80% of its features are based on the capabilities of ML capabilities [12].
 Figure 2: Neural synchrony of automation tools with exclusive uniqueness.
Figure 2: Neural synchrony of automation tools with exclusive uniqueness.
The optimistic ambiances of these technologies in massive scale can benefit health care and could create an economic impact by saving labor-intensive expenses widely. The estimation of the International Data Corporation states that the futuristic activities and daily schedules are dependent on AI and ML which will eventually grow to $79.2 billion by 2022. Holding a multifarious progress there is an expectation of upsurge in the annual economic growth rate of 38% from the year 2018 to 2022 [13]. Machine learning develops methodologies that are fed in the computer as mathematical models and procure significant algorithms that can solve a given problem. Deep involves the concept automation profoundly than the classical system, where the computers represent and features mechanically. It focuses on processing the raw data instinctively without feeding prior experiential data or learning inputs [14]. The enchantment in deep learning networks includes the distinguished pattern and structure involved behind the immense data. The varied computational models known as neural networks support the working mechanisms constituted in deep learning concepts.
Stages of Deep Learning
Deep learning conceptualizes distinct phases to evaluate and decipher a particular query. The initial stage requires defining the opted network and it’s planning which is preceded by the compilation step. This stage constitutes is to configure the specific model and the compile the critical parameters that defines the evaluation procedure [15]. Computing and calculating are the foremost aspects of deep learning where, configuring and fitting the model plays a significant role. This function exercises the model to fix an exact set of iterations on a dataset. Another significant step of deep learning is evaluation where the model is verified for its functioning. The programmed test data, validated with a variable data through random tests can relate an augment the ideal operation of the model [16]. Once the model is created the final phase is to deploy and transmit the program widely. The choice of transmission is very important to gain publicity and can be done by creating websites and apps.
Big Data and Artificial Intelligence in Analyzing Neural Data and Brain Networks
The human brain is a complex organ interconnected with conceptualized hierarchical network, where abundant of neurons are specifically organized into networks which process and transmit information. Brain network are hubs where information are processed from precise patterns through neurons, which brain associate the functioning of brain [17]. This network has a structural, functional progression which is instigated during the early pattern of development and proceeds into adulthood followed by senescence. These biological events are administered by involuntary events and the experiential history of an individual.
Artificial Intelligence and Brain Networks
The application of artificial intelligence reflects profoundly in neuroscience with wide benefits in fields like neuroanatomy, neurodevelopment, electrophysiology, functional brain imaging, and neural basis of cognition [18]. Brain network comparison and characterizations related to anomalies or disorders among a population can be performed using various interface of artificial intelligence like graph theoretical analyses, Magneto Encephalographic (MEG) and neuroimaging studies [19]. There is a tremendous change in the clinical diagnosis among hospitals along with health care systems. They have improved widely to produce data from nervous system in terms of as Medical Imaging which would monitor developmental anomalies and neural deformities.
Neuroimaging Deep Learning Strategies in Psychiatry
Medical experts and neurologist customize these visual imaging technologies to treat brain pathologies, as well as identifying risks and adverse reactions pertaining to therapies. Hence these techniques pave the way for understanding, envisaging diseases and treating, through vital diseases managing techniques. Conversely, the recent developments in neuroimaging are cost-effective and beneficial economically. There are various techniques like Computed Tomography (CT), Positron Emission Tomography (PET), and Magnetic Resonance Imaging (MRI) which are readily amicable with low risk analysis [20]. These imaging techniques revolutionize the study of central nervous system and brain which was earlier very much complicated. It allows the medical professionals to evaluate the structural aspects of brain without damaging it and detect neural abnormalities.
Behavioural And Visual Data Interpretation Using Convolutional Neural Networks (CNNS) As Models
Convolutional Neural Networks (CNNs) are validated models related with the visualization biotic samples and is known as the study of biological vision. They are considered as integrative tools and have accomplished to create of state of art models depicting both the neural activity and behavioral aspects. Convolutional neural networks functions with the principle of automation that links the concepts of artificial intelligence with neuroscience [21]. The progression and activity of a brain can be captured with contemporary tools originated from the fields of computer science and engineering. The foremost resurgence in the field of neuroscience has happened due to the recapitulation of neuronal networks of ventral stream whose, activities are represented through visual information [22]. The finest aspect of CNNs it can predict the visualization of an organism. The observation of an animal can be validated through artificial units and can be forecasted as the activities performed by its real neurons [23]. Figure 3 illustrates a comparative model which represents the neuronal activities between a real and artificial system. The behavioral assessment of humans and animals remains as a challenge. But CNNs outperforms the classical models and relates more with the existing -world image. Hence, can establish itself as a virtuous interface to relate human behavior with its contiguous animal species. A fine study [24] interrelated the behavioral patterns of human and monkeys and by using several features CNN architectures a comparable match score elucidated the relationships between both the species [25].
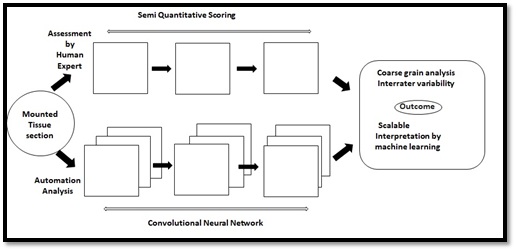 Figure 3: Comparative model representing an image prediction manually and automatedly [26].
Figure 3: Comparative model representing an image prediction manually and automatedly [26].
Automation Techniques Involved In Various Neurological Ailments
Neurological disorders are caused due to the deformities that occur in the peripheral and central nervous systems. The lack of neural coordination will lead several distortions like poor coordination, muscle weakness with pain, paralysis, seizures and loss of consciousness [27]. These symptoms are associated with more than 600 different types of mental ailments where some like brain tumor, Parkinson’s Disease (PD), Alzheimer’s Disease (AD), Multiple Sclerosis (MS), epilepsy, dementia, headache disorders, neuroinfectious, stroke, and traumatic brain injuries are frustrating and requires immediate remedy.
Neuro Developmental Disorders
Attention Deficit Hyperactive Disorder (ADHD) and Autism Spectrum Disorder (ASD) are impulsive developmental neuro disabilities which affect the cognitive progress of an individual. Though both the disorders have some common symptoms they differ in terms of neurological pattern. ASD relates with the learning aspect of the patient along with his communication, language and social behavior. Whereas, ADHD influences the growth patterns of brain and its development that can lead to extreme impulsive hyperactive behavior [28]. The diagnosis and treatment to these disorders depends on the behavioral symptoms, which can be variable and thus uncertain. Hence there is an immense need to comprehend the structural and physiological activities of neurological system to reinforce behavioral patterns of an individual that would elucidate the ambiguity related to the disorder.
Applications of deep learning has enhanced the cognitive skill management and therapy through neuro imaging which overcomes the risk assessment of classical radiology methods. The diagnostic history of ADHD has incorporated Machine learning concepts in neuroimaging which reveals abnormalities in the regions of brain [29]. Later there was a profound transformation in the degree of automation and emerged an ethiological model of ADHD which primarily focused on the pathophysiology toward dysfunctional interactions within and between distributed networks [30]. Currently, there is a huge advancement in the deep learning skills of automation where the entire brain network is connected and its differential patterns are correlated in terms of low-frequency and spontaneous Blood Oxygenation Level Dependent (BOLD) activity. The functional architecture of the brain is characterized intensely along with its essential neural activities which are evaluated and stated as resting-state functional connectivity (rs-fc) [31]. The advantage of deep learning techniques is intensely used in the prenatal and early diagnosis which relates the functional and structural region of brain. Thus it can improve social skills and resolve the communication problems among ASD affected children. Certain automated tools like Functional Magnetic Resonance Imaging (FMRI) which studies the structure of brain [32] and the Autism Brain Imaging Data Exchange (ABIDE) that deciphers specific biomarker expertise in detecting and treating ASD [33,34] The ASD biomarkers are conceived with multi-channel convolutional neural networks based on a patch-level data-expanding method that involves in early diagnosis and treatment in prenatal phase [35,36] Further, Variational Autoencoder (VAE) intensifies the functional connectivity pattern and Stereotypical Motor Movements (SMM) which facilitates body rocking and complex hand movements in autism patients.
Neural Disability Leading to Impairment of Memory and Cognitive Function
Alzheimer’s is a progressive neural disease that gradually leads to loss of memory and cognitive function. Dementia is the foremost ailment in Alzheimer’s disease where 60-80% of patients suffer with memory loss. Hence both are twinned with each other and require immediate antidote since the prevalence of these ailments is increasing. As per the data of World Alzheimer Report [37], about 50 million people were affected with this mental deformity in 2018, which is forecasted to upsurge thrice by 2050. There is significant need to restrain this condition and deep learning concepts significantly assist to promote mental health. The initial transformation of Mild Cognitive Impairment (MCI), among Alzheimer’s patients is monitored by Magnetic Resonance Images (MRI ) which are actively coordinated by open source databases like ADNI (adni.loni.usc.edu), AIBL (aibl.csiro.au), OASIS (www.oasis-brains.org) and software’s like Statistical Parametric Mapping (SPM) [38]. Machine learning techniques consisting of artificial network and interface like Support Vector Machine (SVM), Artificial Neural Network (ANN), and deep learning [39,40] classifies Alzheimer’s and its various stages. The destructive features of brain leading to memory loss is predicted by several dementia representations like neuropsychological based models, health-based models, multifactorial models and genetic risk scores [41]. The synchronization of multiplex neural networks with the Magnetic Resonance Imaging (MRI), segregates healthy brains from those affected with memory loss. Atrophy of brain leads to progressive Mild Cognitive Impairment (pMCI) which is regularly monitored by Positron Emission Tomography (PET) scans and contemporary machine learning algorithms [42]. Alzheimer’s based dementia diagnosis is enabled [43], by machine learning methodology which has devised novel biomarkers based on Extreme Gradient Boosting XGBoost, Random Forest and Deep Learning concepts that assesses the metabolites in the blood e Cerebrospinal Fluid (CSF).
Chronic Neurodegenerative and Muscular Disorder
Epilepsy is a distressing neurological disease that is characterized by frequent seizures [44]. The sudden change in the functioning of the brain and its connectivity among the neural networks causes varied behaviors like irregular erratic movements, loss of consciousness temporary that affects memory. The intensity of epilepsy and seizure is monitored by Electroencephalography (EEG) which is considered as a significant clinical tool [45]. Computer based models analyses the EEG signals effectively through algorithms which are distinct and classify the EEG signals based on the series of wavelet and its entropy [46]. Exclusive set of neural network and interface like Support Vector Machine (SVM), k-Nearest Neighbour (kNN) and Radial Basis Function Neural Network (RBFNN), Artificial Neural Network (ANN) are used to categorize the signals of EEG [47-49]. Multiple genetic variations play a crucial role in developing neurodegenerative disorder like Cerebellar Ataxia (CA) which mainly affects cerebellum [50]. The dysfuntioning of cerebellum impairs the control and coordination of whole body movements, eye rotation (nystagmus), and affect speech (dysarthria). The single or multimodal imaging is associated with deep learning concepts can link the distinct genetic integrity by producing specific biomarkers. Similarly muscular dystrophy is a inherited disease characterized by muscle weakness and muscular atrophy, which leads to cardiopulmonary failure and eventually can be fatal. Magnetic Resonance Imaging (MRI) has is a noninvasive and expedient technique which can detect a discrete pattern of fatty infiltration [51-54] .The interpretation MRI data is very important and solely depends on the medical expert and incase misdiagnosis it can lead to deleterious effect. In order to avoid these deep learning skills appears to be an ideal option. The Convolutional Neural Networks (CNNs) serves as a comparative media which can interpret informative features based on specific patterns and thus can avoid misinterpretations [55,56].
Research Stratagems Implicated in Deep Learning
Research in neuroscience has established advance stratagems to confine alterations and restrain neurological disabilities. Considering the efficacy of biomolecules and its interference in the nervous system large amount of data in can be produced by using machine learning techniques .The integrity of research findings with machine learning builds up strategies to develop diagnostics and treat mental ailments.
Identifying Biomarkers as Signatures to Construe Neurological Ailments
Many neurological disabilities are categorized as heterogeneous disorder which can imitate other forms of neurological conditions [57]. Certain circumstances can misinterpret the clinical features of neurological ailments and creates a challenge by worsening the condition. [58-60]. Biological components present in blood, neurotransmitters and Cerebrospinal Fluid (CSF) serves as neurological biomarkers. Alzheimer’s disease has clinically significant biomarkers like Amyloid-beta (Aβ), tau phosphorylated (p-tau), and total-tau (t-tau) which are profoundly, applied in clinical research, and drug trials [61,62]. Conversely, there are many neurological ailments like Dementia with Lewy Bodies (DLB) or Frontotemporal Dementia (FTD), which share common symptoms with Alzheimer’s disease and requires specific identification [63,64]. Immunological techniques like Enzyme-Linked Immunosorbent Assay (ELISA) has been associated with CSF AD biomarkers but is widely hindered by the variation in measurement of its analytes and chances of misinterpretations [65,66]. Hence automation techniques holds hands with the neurological ailments to capture the structural and functional changes of central nervous system by using advanced neuroimaging techniques, such as Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET). These dynamic techniques have been developed with deep learning concepts to identify AD-related structural and molecular biomarkers. Computer-aided machine learning approaches can rapidly integrate the biomarkers and finely distinguishes the various neurological ailments.
Reflection of Automation Techniques Using Animal Models
Animals are used as neuro models to develop novel antidotes and identify new therapeutic target against neurological ailments. These models help to evaluate its behavioral, emotional, biochemical and social aspects in association to causative agents and device ideal remedy. Various animal models have been established to mimic human disorders associated with biochemical profiling and behavioral patterns [67] In vivo studies in neuro science uses several animal models for the purpose to augment the functioning and mechanisms of the brain in association with behaviour [68]. Further the clinical trials endorse the detection of potential targets which outcomes to produce neuroprotective and cognition enhancing compounds [69]. Animals such as mice and zebra fish (Danio rerio) are considered as vivacious developmental models. The non-invasive characterization of disease can lead to phenotypical variations in mouse models which associate with disease identification, characterization and progression and eventually will evaluate the criteria for devising effective therapeutic interventions. Live animal imaging is possible with advanced techniques like Positron Emission Tomography (PET)-Magnetic Resonance (MR) and Single Photon Emission Computed Tomography (SPECT)-MR hybrid imaging. The neuro images and motion videos are compared with condition of human being and strategized to combat the ailment [70]. Zebrafish is emerging as an increasingly successful model for translational research on human neurological disorders. It is extremely validated as a powerful vertebrate model for investigating human neurodegenerative diseases [71]. The neuroanatomic and neurochemical pathways of zebrafish brain exhibit a profound resemblance with the human brain. Physiological, emotional and social behavioural pattern similarities between them have also been well established. Fascinatingly, zebrafish models have been used successfully to assess the pathology of Alzheimer’s Disease (AD) as well as Tauopathy. Their relatively simple nervous system and the optical transparency of the embryos permit real-time neurological imaging.
Automated Modelling of Cognitive Behavioral Patterns in vivo Studies
Behavioral patterns of individuals are distinct and based on their categorizations the abnormal functioning of brain can be detected. Detection of neurological disorders is assisted by preclinical animal studies which are further simplified by automation. Behavioral analysis is a significant tool to understand and relate the brain-behavior relationship. The foremost challenge in neuroscience research is to understand and interpret the behavior with fuction and activity of neurons [72]. The development of behavioral analysis requires coordinal neuronal activity which requires precise monitoring and mechanism [73]. Automation tools simplifies the task of interpretating the behavioral patterns effectively. Deep learning skills presents advance techniques to promote automated analysis of images [74]. Comparative image analysis is adapted with various Convolutional Neural Networks (CNN) architecture which results with accurate upshots [75]. Resulting in faster and more accurate outcomes. Implementation of the CNNs has assessed behavioral patterns which analysis the imaging data that focus on body posture among animals [76]. Identification of specific behaviors in association with body positions, are interpretated by algorithms which coordinates body parts. Moreover specific algorithms are devised to analyse the clinical motor function based on subjective scoring of a behavioural activity [77]. The post treated analysis of patients is evaluated by the Kinematic analysis which reveals the specific timing and its related typical movements which differentiates the level recovery [73]. Three Dimensional (3D), marker-less kinematic analysis is contemporary deep learning techniques which assesses the comparative social behavioral interaction between two animals.
Conclusion
Neuroscientists are acclimatizing to the automation tools that are reliable and interpret the characteristic features of central nervous system. Machine learning, artificial intelligence and deep learning are unique with their specific attributes to combat neurological disorders. Understanding the neurological progression and its association with behavioural conditions can devise remedy to several neurological disabilities. Hence application of deep learning concepts enhances the quality of diagnosis and can instigate effective antidote against mental ailment.
References
- Morris-Rosendahl DJ, Crocq MA (2020) Neurodevelopmental disorders-the history and future of a diagnostic concept. Dialogues Clin Neurosci 22: 65-72.
- Narain JP (2016) Public health challenges in India: Seizing the opportunities. Indian J Community Med 41: 85-88.
- Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, et al. (2006) Disease control priorities in developing countries (2nd edn). The World Bank and Oxford University Press, Washington, DC, USA.
- Murray CJL, Lopez AD (2020) The global burden of disease: A comprehensive assessment of mortality and disability from diseases, injuries and risk factors in 1990 and projected to 2020. World Health Organization, Geneva, Switzerland.
- World Health Organization (1996) Harvard school of public health on behalf of the world health organization and the world bank. World Health Organization, Geneva, Switzerland.
- GBD 2015 Neurological Disorders Collaborator Group (2017) Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the global burden of disease study 2015. Lancet Neurol 16: 877-897.
- Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, et al. (2015) Disability weights for the global burden of disease 2013 study. Lancet Glob Health 3: 712-723.
- Mathers CD, Loncar D (2005) Updated projections of global mortality and burden of disease, 2002-2030: data sources, methods and results. World Health Organization, Geneva, Switzerland.
- GBD 2016 Dementia Collaborators (2019) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: 88-106.
- GBD 2016 Epilepsy Collaborators (2019) Global, regional and national burden of epilepsy, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: 357-375.
- Wojcik MH, Agrawal PB (2020) Deciphering congenital anomalies for the next generation. Cold Spring Harb Mol Case Stud 6: 005504.
- Krizhevsky A, Sutskever I, Hinton GE (2012) ImageNet classification with deep convolutional neural networks. In: Pereira F, Burges CJC, Bottou L,Weinberger KQ (eds.). Advances in neural information processing systems 25. Curran Associates 1097-1105.
- Peters M, Neumann M, Iyyer M, Gardner M, Clark C, et al. (2018) Deep contextualized word representations. In: Proceedings of the 2018 conference of the North American chapter of the association for computational linguistics: Human Language Technologies (long papers) 1: 2227-2237.
- Lee H, Yune S, Mansouri M, Kim M, Tajmir SH, et al. (2018) An explainable deep-learning algorithm for the detection of acute intracranial haemorrhage from small datasets. Nat Biomed Eng 3:173-182 2.
- Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, et al. (2017) A survey on deep learning in medical image analysis. Med Image Anal 42:60-88.
- Esteva A, Chou K, Yeung S, Naik N, Madani A, et al.(2021) Deep learning-enabled medical computer vision. Npj Digit Med 4:
- Bassett DS, Bullmore ET (2017) Small-world brain networks revisited. Neuroscientist 23: 499-516.
- Hosseini SM, Hoeft F, Kesler SR (2012) GAT: A grap theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS one 7: 40709.
- Niso G, Carrasco S, Gudín M, Maestú F, del-Pozo F, et al. (2015) What graph theory actually tells us about resting state interictal MEG epileptic activity. NeuroImage Clin 8: 503-515.
- Kawahara J, Brown CJ, Miller SP, Booth BG, Chau V, et al. (2017) BrainNetCNN: Convolutional neural networks for brain networks; towards predicting neurodevelopment. Neuroimage 146: 1038-1049.
- Kietzmann TC, McClure P, Kriegeskorte N (2019) Deep neural networks in computational neuroscience. In Oxford Research Encyclopedia of Neuroscience, UK.
- Storrs KR, Kriegeskorte N (2019) Deep learning for cognitive neuroscience. arXiv.
- King ML, Groen II, Steel A, Kravitz DJ, Baker CI (2019) Similarity judgments and cortical visual responses reflect different properties of object and scene categories in naturalistic images. NeuroImage 197: 368-382.
- Rajalingham R, Issa EB, Bashivan P, Kar K, Schmidt K, et al. (2018) Large-scale, high-resolution comparison of the core visual object recognition behavior of humans, monkeys, and state-of-the-art deep artificial neural networks. Journal of Neurosciece 38: 7255-7269.
- Jozwik KM, Kriegeskorte N, Storrs KR, Mur M (2017) Deep convolutional neural networks outperform feature-based but not categorical models in explaining object similarity judgments. Front Psychol 8: 1726.
- Tang Z, Chuang KV, DeCarli C, Jin L-W, Beckett L, et al. (2019) Interpretable classification of Alzheimer’s disease pathologies with a convolutional neural network pipeline. Nat Commun 10:
- https://www.who.int/mediacentre/news/releases/2007/pr04/e/
- Xue J, Zhang Y, Huang Y (2019) A meta-analytic investigation of the impact of mindfulness-based interventions on ADHD symptoms. Medicine (Baltimore) 98: 15957.
- Valera EM, Faraone SV, Murray KE, Seidman LJ (2007) Meta-analysisofstructural imaging findings inattention-deficit/hyperactivitydisorder. Biol Psychiatry 610:1361-1369.
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, et al. (2008) Cingulate-precuneus interactions: A new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 630: 332-337.
- Yang H, Wu QZ, Guo LT, Li QQ, Long XY, et al. (2011) Abnormal spontaneous brain activity in medication nave adhd children: A resting state fMRI study. Neurosci Lett 502: 89-93.
- Heinsfeld AS, Rosa Franco A, Cameron Craddock R, Buchweitz A, et al. (2018) Identification of autism spectrum disorder using deep learning and the ABIDE dataset. NeuroImage 17: 16-23.
- Kazeminejad A, Sotero R (2019) The importance of anti-correlations in graph theory based classification of autism spectrum disorder. bioRxiv.
- Sharif H, Khan RA (2019) A novel framework for automatic detection of Autism: A study on Corpus Callosum and Intracranial Brain Volume. arXiv.
- Li X, Dvornek NC, Zhuang J, Ventola P, Duncan JS (2018b) Brain biomarker interpretation in ASD using deep learning and fMRI. arXiv.
- Li G, Liu M, Sun Q, Shen D, Wang L (2018a) Early diagnosis of autism disease by multi-channel CNNs. Mach Learn Med Imaging 11046: 303-309.
- Christina Patterson (2018) The state of the art of dementia research: New frontiers. World Alzheimer’s Report 2018, London.
- Veitch DP, Weiner MW, Aisen PS, Beckett LA, Cairns NJ, et al. (2018) Understanding disease progression and improving Alzheimer’s disease clinical trials: Recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s & Dementia 15: 106-152.
- M Termenon, Grana M, Besga A, Echeveste J, Gonzalez-Pinto A ((2013) Lattice independent component analysis feature selection on diffusion weighted imaging for Alzheimer‘s disease classification. Neurocomputing 114: 132-141.
- Pellegrini E, Ballerini L, Hernandez MDeCV, Chappell FM, González-Castro V, et al. (2018) Machine learning of neuroimaging for assisted diagnosis of cognitive impairment and dementia: A systematic review. Alzheimers Dement (Amst) 10: 519-535.
- R Chaves, J Ramírez, JM Górriz, M López, D Salas-Gonzalez, et al. (2009) SVM-based computer-aided diagnosis of the Alzheimer’s disease using t-test NMSE feature selection with feature correlation weighting. Neurosci Lett 461: 293-297.
- Ying Chen,Tuan D Pham (2013) Development of a brain MRI-based hidden Markov model for dementia recognition. Biomedical engineering online 12.
- Cabral C, Morgado PM, Costa DC, Silveira M, Alzheimer‘s Disease Neuroimaging Initiative (2015) Predicting conversion from MCI to AD with FDG-PET brain images at different prodromal stages. Comput Biol Med 58 101-109.
- Ahmadi A, Behroozi M, Shalchyan V, Daliri MR (2018) Classification of epileptic EEG signals by wavelet based CFC. Electr Electro Biomed Eng Comput Sci.
- Acharya UR, Oh SL, Hagiwara Y, Tan JH, Adeli H (2017) Deep convolutional neural network for the automated detection and diagnosis of seizure using EEG signals. Comput Biol Med 100: 270-278.
- Shen CP, Chen CC, Hsieh SL, Chen WH, Chen JM, et al. (2013) High-performance seizure detection system using a wavelet-approximate entropy-fSVM cascade with clinical validation. Clin EEG Neurosci 44: 247-256.
- Siuly S, Li Y, Wen P (2011) EEG signal classification based on simple random sampling technique with least square support vector machines. Int J Biomed Eng Technol 7: 390-409.
- Siuly S, Li Y (2014) A novel statistical framework for multiclass EEG signal classification. Eng Appl Artif Intell 34:154-167.
- Aslan K, Bozdemir H, Sahin C, Noyan Ogulata S, Erol R (2008) A radial basis function neural network model for classification of epilepsy using EEG signals. J Med Syst 32: 403-408.
- Ruano L, Melo C, Silva MC, Coutinho P (2014) The global epidemiology of hereditary ataxia and spastic paraplegia: A systematic review of prevalence studies. Neuroepidemiology 42: 174-183.
- Akima H, Lott D, Senesac C, Deol J, Germain S, et al. (2012) Relationships of thigh muscle contractile and non-contractile tissue with function, strength, and age in boys with Duchenne muscular dystrophy. Neuromuscul Disord 22: 16-25.
- Leung DG (2017) Magnetic resonance imaging patterns of muscle involvement in genetic muscle diseases: a systematic review. Journal of neurology 264: 1320-1333.
- Kinali M, Arechavala-Gomeza V, Cirak S, Glover A, Guglieri M, et al. (2011) Muscle histology vs MRI in Duchenne muscular dystrophy. Neurology 76: 346-353.
- Tasca G, Iannaccone E, Monforte M, Masciullo M, Bianco F, et al. (2012) Muscle MRI in Becker muscular dystrophy. Neuromuscul Disord 22: 100-106.
- Dodge S, Karam L (2017) A study and comparison of human and deep learning recognition performance under visual distortions. 2017 26th international conference on computer communication and networks ICCCN, Canada.
- He K, Zhang X, Ren S, Sun J (2015) Delving deep into rectifiers: Surpassing human level performance on image net classification. Proceedings of the IEEE international conference on computer vision.
- Di Fede G, Catania M, Maderna E, Ghidoni R, Benussi L, et al. (2018) Molecular subtypes of Alzheimer’s disease. Sci Rep8: 3269.
- Sawyer RP, Rodriguez-Porcel F, Hagen M, Shatz R, Espay AJ (2017) Diagnosing the frontal variant of Alzheimer’s disease: A clinician’s yellow brick road. J Clin Mov Disord 4: 2.
- de Souza LC, Mariano LI, de Moraes RF, Caramelli P (2019) Behavioral variant of frontotemporal dementia or frontal variant of Alzheimer’s disease? A case study. Dement Neuropsychol 13: 356-360.
- Villain N, Dubois B (2019) Alzheimer’s disease including focal presentations. Semin Neurol 39: 213-226.
- Paterson RW, Slattery CF, Poole T, Nicholas JM, Magdalinou NK, et al. (2018) Cerebrospinal fluid in the differential diagnosis of Alzheimer’s disease: Clinical utility of an extended panel of biomarkers in a specialist cognitive clinic. Alzheimers Res Ther 10: 32.
- Gaetani L, Paoletti FP, Bellomo G, Mancini A, Simoni S, et al. (2020) CSF and blood biomarkers in neuroinflammatory and neurodegenerative diseases: Implications for treatment. Trends Pharmacol Sci 41: 1023-1037.
- Bartlett JW, Frost C, Mattsson N, Skillbäck T, Blennow K, et al. (2012) Determining cut-points for Alzheimer’s disease biomarkers: Statistical issues, methods and challenges. Biomark Med6: 391-400.
- Molinuevo JL, Blennow K, Dubois B, Engelborghs S, Lewczuk P, et al. (2014) The clinical use of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimer’s Dement10: 808-817.
- Mattsson-Carlgren N, Palmqvist S, Blennow K, Hansson O (2020) Increasing the reproducibility of fluid biomarker studies in neurodegenerative studies. Nat Commun11: 6252.
- Le Bastard N, De Deyn PP, Engelborghs S (2015) Importance and impact of preanalytical variables on Alzheimer disease biomarker concentrations in cerebrospinal fluid. Clin Chem61: 734-743.
- Matthews DJ, Kopczynski J (2001) Using model-system genetics for drug-based target discovery. Drug Discov Today 6: 141-149.
- Porges SW (2006) Asserting the role of biobehavioral sciences intranslational research: The behavioral neurobiology revolution. Dev Psychopathol 18: 923-933.
- Snaith MR, Törnell J (2002) The use of transgenic systems in pharmaceutical research. Brief Funct Genomic Proteomic 1: 119-130.
- Cryan JF, Slattery DA (2007) Animal models of mood disorders: Recent developments. Curr Opin Psychiatry 20: 1-7.
- Whiteside GT, Adedoyin A, Leventhal L (2008) Predictive validity of animal pain models? A comparison of the pharmacokinetic-pharnacodynamic relationship for pain drugs in rats and humans. Neuropharmacology 54: 767-775.
- National Institute of Health (2014) BRAIN 2025 Report. National Institute of Health, Maryland, USA.
- Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, Poeppel D (2017) Neuroscience needs behavior: correcting a reductionist bias. Neuron 93: 480-490.
- LeCun Y, Bengio Y, Hinton G (2015) Deep learning. Nature 521: 436-444.
- Szegedy C, Vanhoucke V, Ioffe S, Shlens J, Wojna Z (2016) Rethinking the inception architecture for computer vision. 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), California, USA.
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, et al. (2018) DeepLabCut: Markerless pose estimation of userdefined body parts with deep learning. Nat Neurosci 21: 1281-1289.
- Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, et al. (2017) Agreed definitions and a shared vision for new standards in stroke recovery research: The stroke recovery and rehabilitation roundtable taskforce. Neurorehabil Neural Repair 31: 793-799.
Citation: Anita MA, Aishwarya S (2023) Optimistic Stratagems of Machine Based Deep Learning Employed with Automation in Psychiatry and Early Neurodevelopmental Disorders. J Psychiatry Depression Anxiety 9: 047.
Copyright: © 2023 Anita Margret A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

